Abstract
Grapevine trunk fungal pathogens, such as Diplodia seriata and Phaeomoniella chlamydospora, can infect plants through pruning wounds. They cause grapevine trunk diseases and are involved in grapevine decline. Accordingly, the protection of pruning wounds is crucial for the management of grapevine trunk diseases. The efficacy of different natural antifungals in inhibiting the growth of several fungi causing grapevine trunk diseases was evaluated in vitro. The fungi showing greater in vitro efficacy were tested on autoclaved grape wood assays against D. seriata and P. chlamydospora. Based on results from these assays, chitosan oligosaccharide, vanillin, and garlic extract were selected for further evaluation on pruning wounds inoculated with D. seriata and P. chlamydospora in field trials. A significant decrease in plant mortality was observed after 2 years of growth in the plants treated with the different natural antifungals compared to the mortality rate observed in infected plants that were not treated with antifungals. Also, the infection rate for the inoculated pathogens was significantly reduced in plants treated with the selected natural antifungals. Therefore, natural antifungals represent a promising alternative for disease control and could provide significant economic benefits for the grape-growing industry.
INTRODUCTION
Grapevine trunk diseases are a major threat to the wine and grape industry. They have been reported in most grapevine-producing regions worldwide, causing serious economic losses due to a significant reduction of both yields and the quality of the grapes (1–3). The diseases include Botryosphaeria dieback, esca, Eutypa dieback, Petri disease, black foot, and Phomopsis dieback as the most relevant pathologies.
Planting material produced in nurseries is frequently infected with fungal pathogens, especially those involved in Petri and black foot diseases (4, 5). In mature vineyards, pathogens such as Ilyonectria sp. mainly penetrate through the root system (6). However, it is widely accepted that for many pathogens, the annually produced pruning wounds are the main route of entry into young and adult plants (7–9). Consequently, numerous studies have been conducted to assess the susceptibility of pruning wounds to fungal pathogens causing trunk diseases. Susceptibility to Botryosphaeriaceae species has been studied by several authors (8–10). Eutypa lata infection has been extensively analyzed by authors like Munkvold and Marois (11), Chapuis et al. (12), Lecomte and Bailey (13), Van Niekerk et al. (8), and Gramaje et al. (14). Pruning wound susceptibility to Phaeoacremonium aleophilum (15) and Phaeomoniella chlamydospora (8, 15) has been widely tested. Finally, Van Niekerk et al. (8) also studied susceptibility to Phomopsis viticola infection. All of these studies support the hypothesis that pruning wounds are the main route of entry of these pathogens in the plant, and, therefore, the development of measures to protect pruning wounds would be an essential tool in the management of vineyards with better sanitation. Accordingly, numerous tests treating pruning wounds with chemical fungicides and/or biological control agents (BCA) have been carried out. Munkvold and Marois (16) checked the efficacy of natural epiphytes and colonizers of pruning wounds for biological control of Eutypa dieback. McMahan et al. (17) reported that a fungal strain, Fusarium lateritium, was effective in controlling the development of E. lata on grapevine segments. The potential of different bacterial strains, including Bacillus subtilis, Erwinia herbicola, and an actinomycete in controlling E. lata was also analyzed (18). There have also been many studies conducted using different strains of Trichoderma, such as Trichoderma atroviride or Trichoderma harzianum (19–22), as pruning wound protectants. These studies reported different degrees of protection, in some cases with an efficacy similar to or higher than that produced by a chemical compound like benomyl (21), although wound protection by Trichoderma strains proved to be mostly dependent on the grapevine cultivar and the timing of application since greatest efficacy was obtained when Trichoderma had been applied at least 3 to 4 days before the pathogen (22).
The use of chemical fungicides to protect pruning wounds from E. lata infections has also been thoroughly tested. Sosnowski et al. (23, 24) evaluated several different chemical antifungals and found that carbendazim was the most effective in reducing fungal colonization. Field trials performed by Halleen et al. (20) indicated that benomyl and flusilazole were the most effective synthetic fungicides. Similarly, when Rolshausen et al. (7) evaluated the efficacy of different fungicides in controlling both Petri disease pathogens and infections from several Botryosphaeriaceae species, they found that thiophanate-methyl was most effective. More recently, Amponsah et al. (25) reported that 9 out of the 16 fungicides tested were effective in inhibiting the growth of several Botryosphaeriaceae species, reducing mycelial growth and/or conidial germination. Assays on potted and field grapevines showed various degrees of protection; flusilazole and carbendazim were the most effective. When Diaz and Latorre (26) analyzed the ability of different chemical fungicides to protect pruning wounds infected by Diplodia seriata, P. chlamydospora, and Inocutis sp., they found that benzimidazole performed best.
Unfortunately, many chemical fungicides have various drawbacks in terms of toxicity and efficacy, and concerns have been raised about both their environmental impact and the potential health risk associated with their use (27). Accordingly, in recent years, public pressure to reduce the use of synthetic fungicides in agriculture has increased. In this context, the use of natural antifungals (NA) is emerging as a possible tool for controlling fungal crop infections (28, 29). Little information is available about the use of NA in controlling grapevine trunk diseases. Nascimento et al. (30) reported the antifungal effect of chitosan on several fungal species involved in grapevine decline. Greenhouse experiments using foliar sprays of chitosan on potted grapevine plants growing in a substrate artificially infected with P. chlamydospora or Ilyonectria liriodendri demonstrated that chitosan significantly improved plant growth and decreased disease incidence. More recently, Sosnowski et al. (24) evaluated the efficacy of garlic and lactoferrin for the control of E. lata in vitro and in field assays. Both treatments reduced the infection rate by 25 and 21% when applied to pruning wounds at a 1% concentration. This study aimed to test the efficacy of different NA in preventing grapevine pruning wound infections by fungi that cause trunk diseases. Initially, NA were selected based on in vitro and autoclaved grape wood assays. The best-performing ones were tested in two field trials with potted vines between 2009 and 2013.
MATERIALS AND METHODS
Phytopathogenic fungi.
Phytopathogenic fungi used were Botryosphaeria dothidea CBS113190, Diplodia seriata CBS 112555, Eutypa lata CBS 101932, Ilyonectria macrodidyma CBS 120170, Phaeoacremonium aleophilum CBS 631.94, Phaeomoniella chlamydospora CBS 239.74, and Phomopsis viticola CBS 267.80 strains.
Conservation of microorganisms.
Microorganisms were maintained at 4°C on potato dextrose agar (PDA) plates (Scharlau, Barcelona, Spain) for 1 to 3 months. For long-term storage, mycelia or spore suspensions were preserved at −80°C in 40% glycerol.
Preparation of natural antifungals (NA).
High-, medium-, and low-molecular-weight (HMW, MMW, and LMW, respectively) chitosan (Sigma-Aldrich, St. Louis, MO, USA) and chitosan oligosaccharide (MW, <3,000) (Nicechem Co., Ltd., Shangai, China) were dissolved at 100 mg ml−1 in hot (50°C) MilliQ water and preserved at 4°C until used. Garlic (Allium sativum L.) extract was prepared by grinding 30 g of fresh garlic cloves at 4°C for 1 min in 100 ml of 70% (vol/vol) ethanol using an Omni Mixer homogenizer (Cole Parmer, Vernon Hills, IL, USA). Lemon (Citrus lemon L.) peel and green coffee (Soria Natural; Soria, Spain) extracts were obtained from 50 g of fresh material that was ground at 4°C for 1 min in 100 ml of 70% ethanol. Extracts were filtered through Whatman filter paper (number 1) and preserved at 4°C until used. Propolis (a resinous mixture that honey bees collect from tree buds, sap flows, or other botanical sources) was collected from hives from Garrafe de Torio (León, Spain; 42°44′20″N, 5°31′ 48″W) and kept desiccated at −20°C until processing. An aliquot of crude propolis (40 g) was dissolved in 70% ethanol with shaking at room temperature for 3 days protected from light. The resulting extract was filtered twice through filter paper to remove insoluble waxes and resins and concentrated by evaporation of the solvent at 40°C in a Labconco CentriVap concentrator (Kansas City, MO, USA). The resin obtained was dissolved in 70% ethanol to a final concentration of 100 mg/ml and was kept at 4°C until used. Lichen extract (Evernia prunastri L.) was obtained as reported by Halama and Van Haluwin (31). Lichens were collected in the autumn of 2008 from oak tree bark (Quercus pyrenaica L.) in the city of Garrafe de Torío (León, Spain). Lichens were immediately frozen in liquid nitrogen and reduced to a fine powder with a mortar. Extracts were produced by extraction of 30 g of lichen powder with 100 ml of cold (−20°C) acetone and incubation with slow rotation at 4°C for 12 h. The extracts were filtered through filter paper and then through 0.45-μm-pore-size cellulose acetate filters. They were preserved at 4°C until used. Vanillin (Sigma-Aldrich) was dissolved at 300 mg ml−1 in methanol and preserved at 4°C.
In vitro antifungal assay.
In vitro antifungal activity of different NA against B. dothidea, D. seriata, E. lata, I. macrodidyma, Phaeoacremonium aleophilum, P. chlamydospora, and P. viticola strains was assayed on 90-mm-diameter PDA plates (containing 25 ml of agar medium) using the classic radial growth method. Briefly, appropriate quantities of stock solutions of each NA were added to and thoroughly mixed with PDA medium to achieve the desired concentration for each NA: chitosan (1 mg ml−1) (30, 32), vanillin (1 mg ml−1) (33), propolis (1 mg ml−1) (34), garlic (4%) (35), green coffee (4%) (36), lemon peel (37), and Evernia prunastri lichen (4%) (31) extracts. Negative controls were performed in parallel by the addition of the corresponding amounts of 70% ethanol, methanol, acetone, or MilliQ water to PDA plates to exactly match the amount of each of these compounds present in the PDA plates of the NA tested. Mycelial discs (5-mm diameter) were taken from 7-day-old fungal cultures with a sterilized cork borer and placed in the center of each petri dish. Two orthogonal axes, passing through the center of the disc, were marked on the base of each dish to indicate disc positions for use as references for recording growth. Plates were incubated at 25°C for 3 to 21 days (depending on the growth rate of each fungal strain). Radial growth along each line was recorded at exactly 24-h intervals, and 5 mm was subtracted to account for the original agar plug. Each experiment consisted of three replicates and was repeated three times. Statistical analysis was carried out as described below.
Antifungal assays on autoclaved grape wood.
The NA showing the best performance in the in vitro assays were tested in assays on autoclaved grape wood to check their behavior on plant material. We also included in this test (and in field trials) a mixture of chitosan oligosaccharide at 25 mg ml−1, garlic extract at 10%, and vanillin at 4 mg ml−1 (CGV). The assays were performed as reported by Munkvold and Marois (16) with some modifications. One-year-old dormant canes (Vitis vinifera cv. Tempranillo) were collected from a 15-year-old vineyard in Bodegas Vega Sicilia (Valbuena de Duero, Valladolid, Spain). Canes were cut into 8-cm-length segments and autoclaved twice at 121°C (20 min) to eliminate any microorganisms. A small piece of the bark, located approximately 1 cm from one end of the cane, was lifted without being totally removed, and a small hole was made by using a scalpel. The NA (100 μl) were applied to the hole and air dried in a laminar flow cabinet until all the NA had been completely adsorbed. Next, a small agar plug (3-mm diameter) containing 7-day-old mycelium of D. seriata or P. chlamydospora was applied to the cane in the hole where the NA had been previously loaded. The hole was covered by the bark, and the injury was then sealed with Parafilm. Each cane segment was individually incubated in a sterile 30-ml tube that was lined with a small amount of water-soaked sterile cotton to maintain adequate moisture conditions for fungal development. Tubes were closed and incubated for 10 days (D. seriata) or 25 days (P. chlamydospora) at 25°C. Segments were recovered from the tubes, and a small 1-cm-long wooden cylinder was cut 1 cm below the point of inoculation by using a scalpel. Seven pieces of tissue (approximately 1 by 0.2 cm in size) were extracted from each cylinder and inoculated on PDA plates supplemented with chloramphenicol (0.2 g liter−1). After 5 days of incubation (D. seriata) or 15 days of incubation (P. chlamydospora), the growth inhibition rate of the pathogen by the NA was expressed as the ratio (percent) of the number of wood chips from which mycelium of the pathogen emerged for canes treated with NA to the number of non-NA-treated canes. A total of three cane segments were used for each NA assayed, and the experiment was performed in triplicate.
Field evaluation.
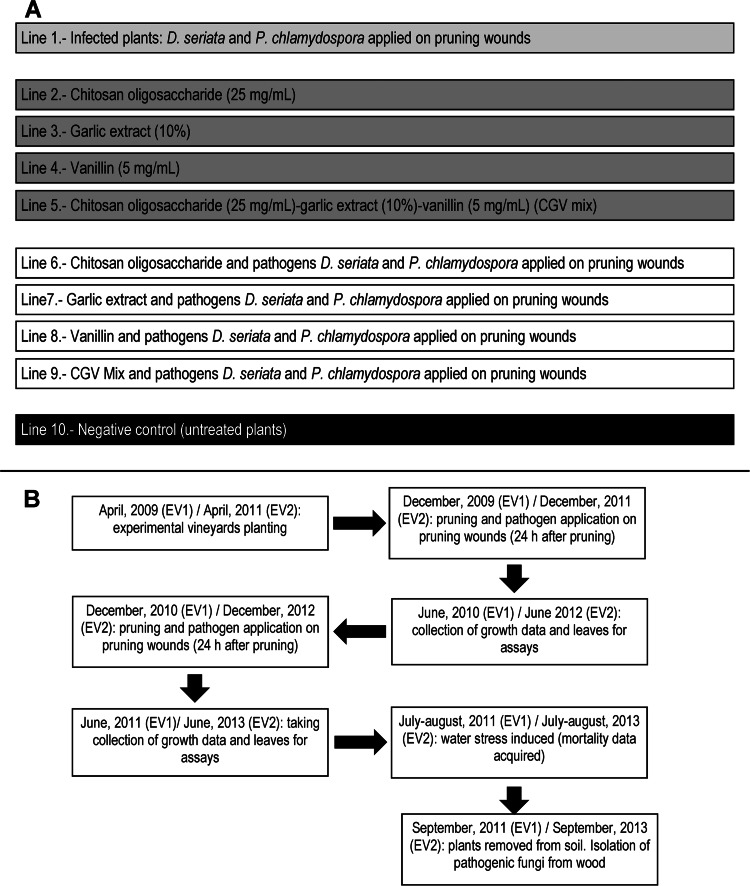
Field assays were conducted in two independent experimental vineyards (experimental vineyard 1 [EV1] and EV2) of 250 grapevine-grafted plants (cv. Tempranillo) each. The plants corresponded to a clonal selection performed by Bodegas Vega Sicilia (Valbuena de Duero, Spain; 41°37′48″N, 4°17′42″W) and were obtained from a French nursery (Pépinierès Guillaume, Charcenne, France). The sanitary status of the batches of grafted plants used for the experimental vineyards was checked prior to planting by isolating fungal pathogens from 25 plants per batch from two wood stubs corresponding to regions 10 and 30 mm below the location of buds, as indicated below. Two different batches of 250 grafted plants were planted (April 2009 for EV1 and April 2011 for EV2) in plastic pots containing 100 liters of a Compo Sana 20-20-30 commercial soil mixture, which is suitable for grapevines (Compo Sana, Barcelona, Spain). Potted vines were grown outdoors at Bodegas Vega Sicilia and regularly drip irrigated. The experimental vineyards were organized in 10 lines of 25 plants, as is shown in Fig. 1A. The vines were pruned to two buds in December 2009 (EV1) and December 2011 (EV2). All pruning wounds were made in 1-year-old wood. Treatments were applied with a paintbrush (within 1 h of pruning) to all the wounds on each vine. At 24 h after NA application, two treated wounds in every vine were drop inoculated (50 μl) using a micropipette with 103 D. seriata and 104 P. chlamydospora spores. These numbers of spores ensured a correct infection of the pruning wound (38). Pruning and applications of NA and pathogens on pruning wounds were repeated in December 2010 (EV1) and December 2012 (EV2) (Fig. 1B). In June 2010 (EV1) and June 2012 (EV2) the plants were measured (total height and length of the 10th internode) to obtain growth data. Simultaneously, three leaves from 10 plants (randomly chosen and collected from the lower, medium, and upper parts of the plant) of every line were collected to perform enzyme assays. This protocol was repeated in June 2011 (EV1) and June 2013 (EV2). In July and August 2011(EV1) and in July and August 2013 (EV2) drip irrigation was severely restricted to induce water stress in order to accelerate development of symptoms in the affected plants. In September 2011 (EV1) and September 2013 (EV2) plants were removed from pots and analyzed for the presence of pathogens.
FIG 1.
General diagram of the experimental vineyard organized in 10 lines of 25 plants each, indicating the treatments applied to each line (A) and the general diagram indicating the timing of the work and treatments performed on the experimental vineyards (B).
The putative positive or harmful effects of the NA or pathogen applications on plants were estimated by measuring growth characteristics (total plant length and elongation of the 10th internode), performing biochemical tests on leaves indicating an alleged oxidative stress (lipid peroxidation levels), and estimating the induction of defensive mechanisms by the plant (catalase, guaiacol peroxidase [GPX], glutathione S-transferase [GST], and superoxide dismutase [SOD] enzyme activity levels), as described below.
Leaf protein extract preparation.
Three leaves, collected from the basal, medium, and upper parts of the plant, were used to create protein extracts. Upon collection, leaves were immediately frozen in liquid nitrogen and preserved at −80°C until used. Leaves were crushed in a mortar in the presence of liquid nitrogen and reduced to a fine powder. Proteins were extracted by gentle rotary shaking (20 min at 4°C) in ice-cold extraction buffer (4 ml g−1 of powder) (50 mM Tris-HCl, pH 7.5, 10% [vol/vol] glycerol, 0.1 mM EDTA, 0.1% [vol/vol] Triton X-100, and 2 mM dithiothreitol [DTT]). Extracts were centrifuged for 10 min at 10,000 × g at 4°C. Supernatants were recovered, and protein content was determined by the Bradford method (39). Protein extracts were stored at −80°C until used.
Enzyme assays.
Lipid peroxide determination was carried out by measuring the amount of malondialdehyde (MDA) produced by thiobarbituric acid reactions, as described by Beuge and Aust (40). Basically, 200 μl of protein extract was mixed with 1 ml of reagent (0.375% thiobarbituric acid, 15% trichloroacetic acid, and 0.01% butylated hydroxytoluene, dissolved in 0.25N HCl) and incubated at 100°C for 15 min in a water bath. The mixture was quickly cooled in an ice bath and centrifuged at 10,000 × g for 20 min. The supernatant's absorbance was measured at 532 nm. The quantity of MDA formed was calculated against a calibration curve made with an authentic standard. Guaiacol peroxidase (GPX) activity was measured by following the H2O2-dependent oxidation of guaiacol at 470 nm, using an extinction coefficient of 26.6 mM−1 cm−1. Assays (carried out at 25°C) were recorded with a Pharma Spec 1700 Shimadzu UV-visible spectrophotometer (Kyoto, Japan). Protein extract (20 μl) was added to a reaction mix containing 12.5 mM phosphate buffer (pH 6.1), 6.25 mM guaiacol, and 25 μM H2O2. Activity was calculated based on the difference in absorbance between two measurements taken at intervals of 90 s. One unit of GPX activity was defined as the amount of enzyme that caused the formation of 1 μM tetraguaiacol per minute. Catalase activity was determined by measuring the initial rates of H2O2 decomposition at 240 nm in a solution containing 10.6 mM H2O2 in 50 mM potassium phosphate buffer (pH 7.0). The assay volume was 1 ml, and the reaction was carried out at room temperature (22 to 25°C) for 2 min. Catalase activity, expressed as micromoles of H2O2 per minute per milliliter, was calculated using an extinction coefficient for H2O2 of 39.58 M−1 cm−1 (41). Glutathione S-transferase (GST) activity was measured using a GST assay kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. Enzyme activity was measured at 340 nm over a 1-min interval. GST specific activity was defined as the amount of enzyme catalyzing the formation of 1 nmol of S-(2,4-dinitrophenyl)glutathione min−1 mg of protein−1. Superoxide dismutase (SOD) activity was estimated using a SOD determination kit (Sigma-Aldrich).
Fungal isolation from wood and identification of pathogens.
Plants were removed from soil and analyzed (two stubs corresponding to regions located 10 and 30 mm below the inoculation point) for the presence of pathogenic fungi. Wood was surface sterilized by immersion in 70% ethanol (1 min), 4% sodium hypochlorite (2 min), and finally in 70% ethanol (1 min). Stubs were split longitudinally, and isolations were made from seven tissue sections (wood chips of 3 by 2 by 2 mm obtained by using a sterile scalpel), which were then plated on PDA plates supplemented with chloramphenicol (150 μg ml−1) to prevent bacterial growth. Plates were incubated at 25°C for up to 4 weeks, with subculturing performed when necessary. Fungi were identified by their cultural-morphological traits as P. chlamydospora (42) or D. seriata (43) and confirmed by sequencing their ITS1-5.8S-ITS2 (where ITS is internally transcribed spacer) regions using primers ITS1 and ITS4 and the PCR conditions described by White et al. (44). Other isolates were identified by sequencing the same region. DNA from wood samples was isolated using a REDExtract-N-Amp (XNAP) kit (Sigma-Aldrich).
Statistical data analysis.
In vitro inhibition of fungal growth by NA data were analyzed using a weighted least-square analysis of variance (ANOVA) test to determine if there were significant differences. When F ratios were statistically significant, post hoc tests (Tukey's honestly significant difference test) were performed to establish where the differences between groups were. Growth and enzyme assay data were tested for univariate normality by the Shapiro-Wilk test and then subjected to univariate analysis of variance using the general linear means procedure. In addition, in the case of mortality data, we tested the null hypothesis of no differences in survival rates against a two-sided alternative hypothesis that the difference in survival rates differs from zero using a chi-square test. When the expected frequencies were less than 5, Fisher's exact test was used. A similar test was carried out for the analysis of the rates of isolation of fungal pathogens from plants of the experimental vineyards. Statistical analyses were performed using R Core Team (3.0.1.) software (http://www.R-project.org). Error bars in graphs indicate standard deviations (SD).
RESULTS
In vitro growth inhibition by natural antifungals.
As shown in Table 1, all of the NA tested inhibited growth of the different pathogens to various degrees. The antifungal activity of green coffee extract was poor since it inhibited mycelial growth of all fungi by less than 37%, and so it was discarded for further testing. Chitosan oligosaccharide (1 mg ml−1) was the most effective NA since it completely inhibited the growth of all the fungi assayed. As a rule, chitosan antifungal activity decreased in inverse proportion to its molecular weight. In the univariate analyses of variance (ANOVAs) with NA as a factor, there were highly significant effects on B. dothidea [F (10, 88) = 2,137, P < 0.001], D. seriata [F (10, 88) = 2,744, P < 0.001], E. lata [F (10, 88) = 2,809, P < 0.001], I. macrodidyma [F (10, 88) = 892.7, P < 0.001], P. chlamydospora [F (10, 88) = 972.2, P < 0.001], P. aleophilum [F (10, 88) = 937.2, P < 0.001], and P. viticola [F (10, 88) = 4,007, P < 0.001]. All F ratios were statistically significant, so post hoc tests (Tukey's honestly significant difference test) were made to determine where the differences between groups lay (Table 1).
TABLE 1.
Efficacy of NA tested in in vitro tests for the inhibition of mycelial growth against several pathogens causing grapevine trunk diseases
| Natural antifungal (concn) | % growth inhibition (time point)a |
||||||
|---|---|---|---|---|---|---|---|
| B. dothidea (2 days) | D. seriata (2 days) | E. lata (7 days) | I. macrodidyma (7 days) | P. chlamydospora (12 days) | P. aleophilum (12 days) | P. viticola (7 days) | |
| HMW chitosan (1 mg/ml) | 24.2 B | 6.2 A | 64.4 B | 62.4 D | 9.5 AB | 60.0 D | 61.5 D |
| MMW chitosan (1 mg/ml) | 7.0 A | 14.8 B | 65.9 B | 50.0 B | 13.6 B | 65.0 DE | 47.1 C |
| LMW chitosan (1 mg/ml) | 88.4 F | 84.9 F | 73.3 C | 68.1 E | 25.8 C | 67.5 E | 90.7 FG |
| Chitosan oligosaccharide (1 mg/ml) | 100 G | 100 H | 100 F | 100 G | 100 F | 100 G | 100 I |
| Evernia prunastri lichen extract (4%) | 86.7 F | 71.1 D | 53.3 A | 56.4 C | 80.3 DE | 41.0 C | 88.4 F |
| Garlic extract (4%) | 89.8 F | 96.0 G | 74.4 C | 92.6 F | 100 F | 100 G | 92.4 GH |
| Green coffee extract (4%) | 10.2 A | 22.7 C | 0.0 A | 16.1 A | 5.4 A | 26.0 B | 36.3 B |
| Lemon peel extract (4%) | 71.4 D | 72.0 D | 73.3 C | 66.4 DE | 76.2 D | 16.0 A | 30.7 A |
| Propolis (1 mg/ml) | 82.1 E | 73.6 DE | 77.6 D | 65.4 DE | 80.3 DE | 87.5 F | 93.7 H |
| Vanillin (1 mg/ml) | 61.9 C | 75.3 E | 81.1 E | 55.7 C | 86.4 E | 91.0 F | 66.0 E |
Data shown correspond to three different experiments performed in triplicate after the indicated number of days of growth. Means in each column followed by the same letter do not differ significantly (P = 0.05).
Effectiveness of NA on autoclaved grape wood assays.
Prior to field trials, several NA were screened in the laboratory for their ability to inhibit D. seriata and P. chlamydospora colonization of excised grapevine stems. HMW, MMW, and LMW chitosan were not tested since their activity was much lower than that of chitosan oligosaccharide. Similarly, green coffee extract was discarded for this assay since it showed the lowest level of growth inhibition in the in vitro tests performed previously against several pathogens like B. dothidea, D. seriata, E. lata, I. macrodidyma, and P. chlamydospora, and its activity was the second lowest against P. aleophilum and P. viticola. The different NA tested had a highly significant effect on the inhibition percentage of fungal growth [F (7, 64) = 102.1, P < 0.001]. Since the F ratio was statistically significant, post hoc tests (Tukey's honestly significant difference test) were performed to measure where the differences between treatments lay. As shown in Table 2, a significant inhibitory effect was detected for all the NA tested although the best performances were exhibited by the CGV mix. Accordingly, they were selected for field assays.
TABLE 2.
Efficacy of natural antifungals to inhibit the proliferation of Diplodia seriata and Phaeomoniella chlamydospora inside sterilized vine shoots
| NA treatment (concn) | % growth inhibitiona |
|
|---|---|---|
| D. seriata | P. chlamydospora | |
| Chitosan oligosaccharide (25 mg ml−1) | 96.8 A | 98.0 A |
| Evernia prunastri lichen extract (4%) | 20.6 E | 5.75 F |
| Garlic extract (10%) | 90.5 AB | 88.6 AB |
| Lemon peel extract (10%) | 47.7 D | 24.0 D |
| Propolis (10 mg/ml) | 68.2 C | 72.0 C |
| Vanillin (5 mg/ml) | 77.8 BC | 90.5 AB |
| CGV mix | 100 A | 100 A |
| Control | 0 F | 0 F |
Data correspond to the average of three independent experiments performed in triplicate. The NA inhibition rate of pathogen development is expressed as the ratio (%) of wood chips that allowed development of the pathogen, in the case of canes treated with NA, versus the number of chips that allowed growth of the pathogen in infected, but non-NA-treated segments. Values within a column with the same letter are not significantly different (P = 0.05).
Effect of the selected NA and pathogens on young grapevine plants.
The effect of each selected NA and pathogen on young grapevine plants was assayed by its application to pruning wounds (Fig. 1).
Growth data.
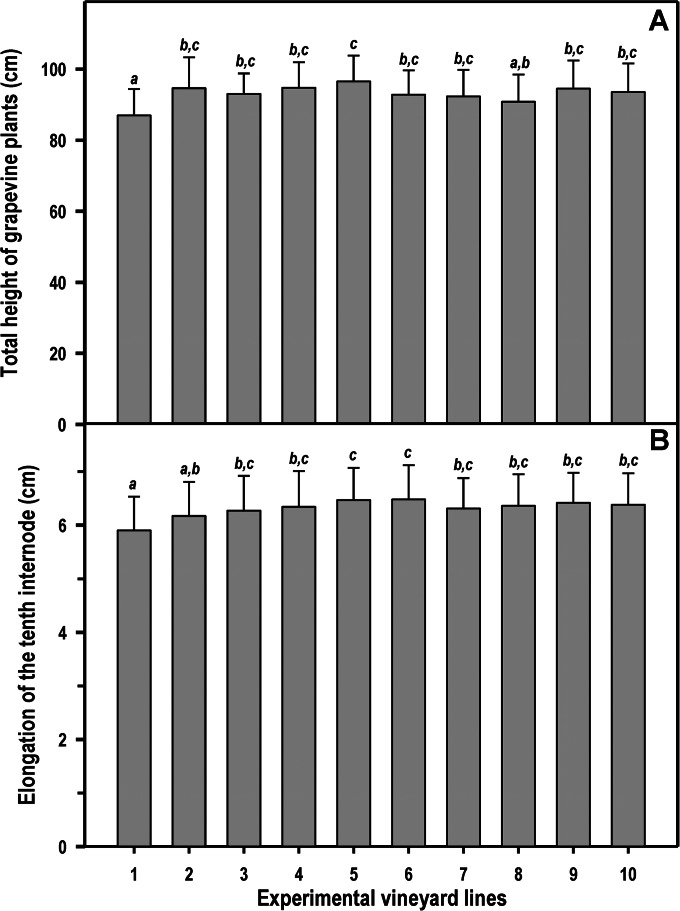
There was a highly significant effect of treatment on both total height of grapevine plants [F (9, 490) = 6.3, P < 0.001] and elongation of the 10th internode [F (9, 990) = 7.7, P < 0.001]. In particular, when infected and nontreated plants (line 1) were compared with the rest (Fig. 2), a significant reduction in both the total height of the plants (7.1%) and length of the 10th internode (7.5%) was detected compared to values for the control plants (line 10). However, the growth was not affected when the infected plants had been previously protected by treatment with NA.
FIG 2.
Plant growth in the experimental vineyards (EV1 and EV2) as determined by total height (A) or length of the 10th internode (B). Line 1, plants infected on pruning wounds; lines 2 to 5, plants protected in pruning wounds with chitosan oligosaccharide, garlic extract, vanillin, and CGV mix, respectively; lines 6 to 9, plants infected on pruning wounds that have been previously protected by the application of chitosan, garlic extract, vanillin, and CGV mix, respectively; line 10, control plants (not infected and not treated with NA). Each bar corresponds to the arithmetic mean of the values determined for the 25 plants of each line and their corresponding SD. The values shown correspond to the average obtained for four consecutive measurements (years 2010 and 2011 for EV1 and years 2012 and 2013 for EV2). Bars marked with the same letter do not differ at a P value of 0.05.
Enzyme assay data.
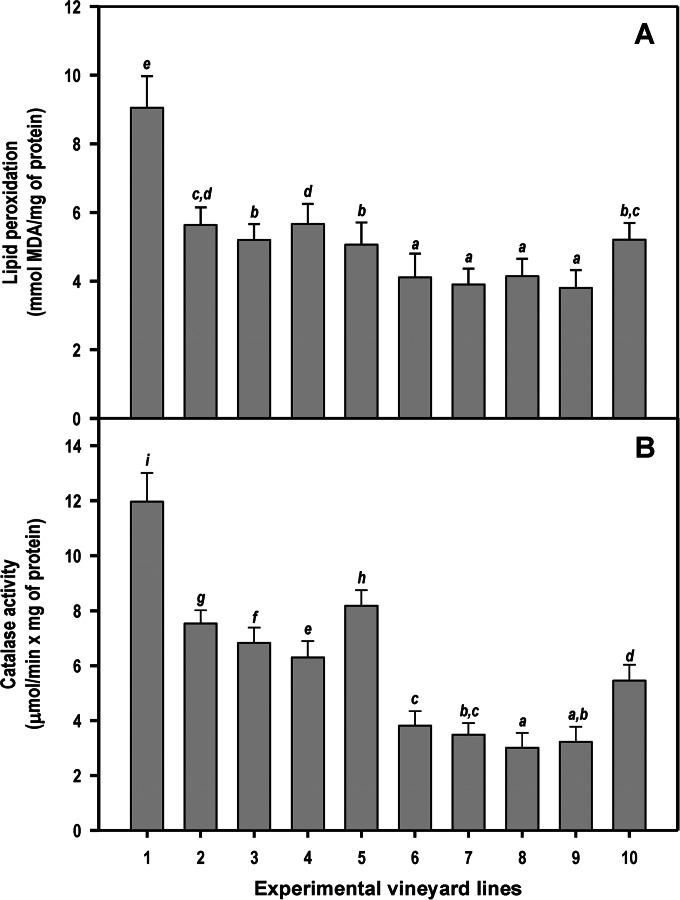
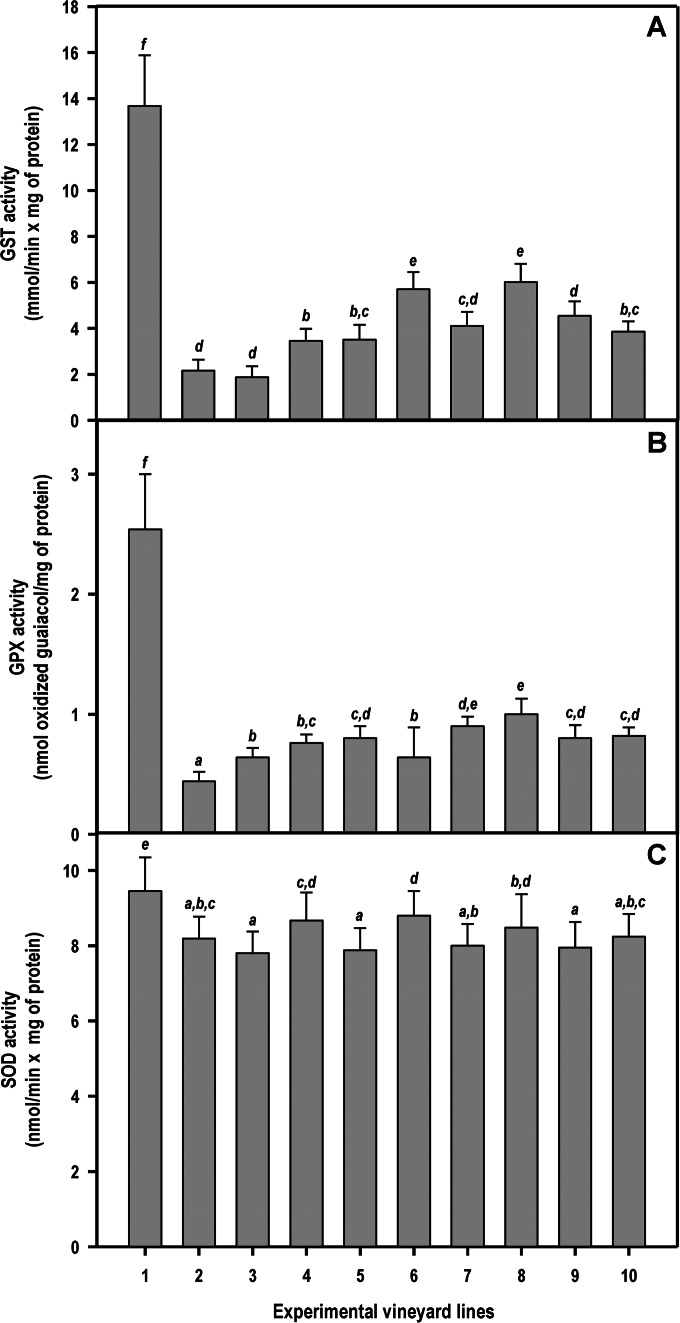
In the univariate analysis of variance with treatment as a factor, there were highly significant effects on lipid peroxidation [F (9, 390) = 266.9, P < 0.001], catalase activity [F (9, 390) = 855.5, P < 0.001], GST activity [F (9, 390) = 549.5, P < 0.001], GPX activity [F (9, 390) = 395.8, P < 0.001], and SOD activity [F (9, 390) = 22.3, P < 0.001]. Analysis of lipid peroxidation levels clearly showed that plants infected through pruning wounds (line 1) had extensive damage to cell membranes (Fig. 3A). The lipid peroxidation levels decreased significantly (P < 0.05) when NA were applied on infected plants (compare line 1 to lines 6 to 9 in Fig. 3A). Plants infected through pruning wounds (line 1) also exhibited the highest levels of the defensive catalase activity (Fig. 3B) (consistent with high intracellular levels of H2O2), the antioxidant enzyme GST (Fig. 4A), GPX activity (Fig. 4B), and SOD (Fig. 4C) compared with levels in control plants, NA-treated plants, and infected plants previously treated with NA.
FIG 3.
Lipid peroxidation levels (indicating damage to lipids of cell membranes) (A) and catalase activity (B), as determined by enzyme assays using leaf protein extracts. Line 1, plants infected on pruning wounds; lines 2 to 5, plants protected in pruning wounds with chitosan oligosaccharide, garlic extract, vanillin, and CGV mix, respectively; lines 6 to 9, plants infected on pruning wounds that have been previously protected by the application of chitosan, garlic extract, vanillin, and CGV mix, respectively; line 10, untreated and noninoculated control plants. Each bar corresponds to the arithmetic mean of the assays (conducted in duplicate) determined for the 10 plants selected from each line and their corresponding standard deviations (SD). Values correspond to the average obtained for four consecutive measurements (years 2010 and 2011 for EV1 and years 2012 and 2013 for EV2). Bars marked with the same letter do not differ at a P value of 0.05.
FIG 4.
Levels of different protective enzymes against reactive oxygen species (ROS) as determined by enzyme assays using leaf protein extracts (GST, glutathione S-transferase; GPX, guaiacol peroxidase, SOD, superoxide dismutase). Line 1, plants infected on pruning wounds; lines 2 to 5, plants protected in pruning wounds with chitosan oligosaccharide, garlic extract, vanillin, and CGV mix, respectively; lines 6 to 9, plants infected on pruning wounds that have been previously protected by the application of chitosan, garlic extract, vanillin, and CGV mix, respectively; line 10, untreated and noninoculated control plants. Each bar corresponds to the arithmetic mean of the assays (conducted in duplicate) calculated for the 5 plants selected from each line and their corresponding standard deviations (SD). The values shown correspond to the average obtained for four consecutive measurements (years 2010 and 2011 for EV1 and years 2012 and 2013 for EV2). Bars marked with the same letter do not differ at a P value of 0.05.
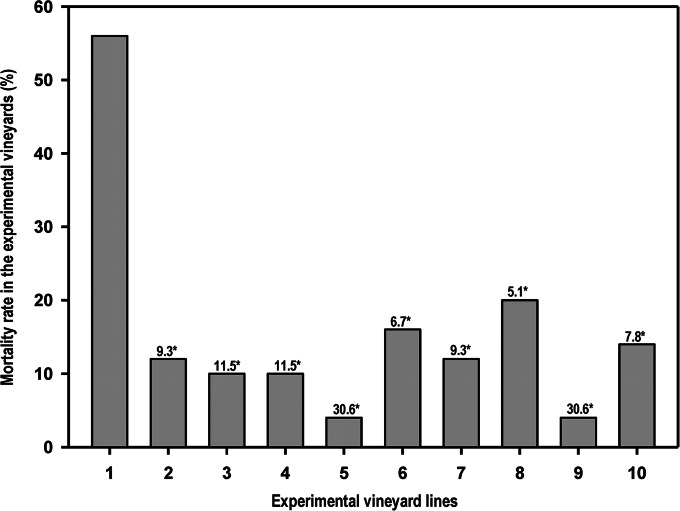
Mortality rate.
In July and August 2011, 2 years after planting, the grapevine plants were subjected to water stress by drastically reducing irrigation. The purpose of this treatment was to hasten the onset of symptoms. Significant differences in mortality rate were observed in the different lines of the experimental vineyards (Fig. 5). The mortality rate of noninoculated untreated (line 10) control plants (14.0%) was significantly lower [χ2 (1) = 19.4, P < 0.001] than that (56.0%) of inoculated untreated (line 1) plants. This seems to represent the fact that, based on the odds ratio, the chances of plants surviving were 7.8 times higher for control plants than for plants of line 1. The mortality rate significantly dropped (P < 0.001) when the infected plants had previously been protected by the chitosan oligosaccharide (16.0%), garlic extract (12.0%), vanillin (20.0%), and CGV mix (4%) applications (Fig. 5). In fact, when infected plants and infected chitosan-treated plants were compared [χ2 (1) = 17.4, P < 0.001], the chances of infected chitosan-treated plants surviving were 6.7 times higher; analogously, survival rates were 9.3 times higher for infected vines treated with garlic extract [χ2 (1) = 21.6, P < 0.001], 5.1 times higher for infected vanillin-treated plants [χ2 (1) = 13.8, P < 0.001], and 30.6 times higher for infected CGV-treated vines (line 9).
FIG 5.
Average mortality rate (%) of grapevine plants observed in the experimental vineyards after a hydric stress was produced by restricted watering. Line 1, plants infected on pruning wounds; lines 2 to 5, plants protected in pruning wounds with chitosan oligosaccharide, garlic extract, vanillin, and CGV mix, respectively; lines 6 to 9, plants infected on pruning wounds that have been previously protected by the application of chitosan, garlic extract, vanillin, and CGV mix, respectively; line 10, untreated and noninoculated control plants. Numbers marked with an asterisk represent the odds of plants in every line of surviving in comparison to the possibilities of survival for infected and nontreated plants from line 1 (P < 0.001, chi-square test).
Isolation of pathogenic fungi.
In September 2011 (EV1) and September 2013 (EV2) the plants were uprooted, and the incidence of grapevine trunk pathogens was determined by isolation from wood material. Higher infection levels were detected in untreated vines inoculated with D. seriata (46%) and P. chlamydospora (74%) (Table 3). In fact, five additional trunk-associated pathogens were also isolated, and their frequency of isolation was higher than that detected in control plants (line 10). The incidence of recovery for D. seriata was significantly reduced when the pruning wounds were treated with the different NA tested. Of note, D. seriata was not recovered from vines treated with garlic extract or CGV mix [χ2 (1) = 29.9, P < 0.001], and plants treated with chitosan and vanillin showed a reduced recovery rate to 8% [χ2 (1) = 18.3, P < 0.001]. In a similar way, the recovery of P. chlamydospora significantly decreased for plants treated with chitosan and garlic extract [χ2 (1) = 48.2, P < 0.001] or vanillin [χ2 (1) = 42.0, P < 0.001], whereas this pathogen was not recovered from CGV-treated vines [χ2 (1) = 58.7, P < 0.001].
TABLE 3.
Percentage of fungal pathogens isolated from plants in the experimental vineyards
| Pathogen | % pathogens isolateda |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stubs before plantingb | Stubs from infected plantsc |
||||||||||
| Line 1 | Line 2 | Line 3 | Line 4 | Line 5 | Line 6 | Line 7 | Line 8 | Line 9 | Line 10 | ||
| Botryosphaeria dothidea | 0 | 14.0 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.0 |
| Cadophora luteo-olivacea | 14.0 | 12.0 | 0 | 4.0 | 4.0 | 0 | 4.0 | 0 | 0 | 4.0 | 6.0 |
| Cylindrocarpon olidum | 0 | 0 | 0 | 0 | 2.0 | 2.0 | 0 | 0 | 0 | 0 | 0 |
| Cryptovalsa ampelina | 4.0 | 2.0 | 0 | 0 | 0 | 0 | 0 | 2.0 | 0 | 0 | 0 |
| Diplodia seriata | 2.0* | 46.0* | 0* | 0* | 0* | 0* | 8.0* | 0* | 8.0* | 0* | 0* |
| Ilyonectria macrodidyma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.0 | 0 | 0 | 0 |
| Phaeoacremonium aleophilum | 0 | 6.0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.0 | 0 | 0 |
| Phaeomoniella chlamydospora | 4.0* | 74.0* | 4.0* | 0* | 0* | 2.0* | 6.0* | 6.0* | 10.0* | 0* | 4.0* |
| Phomopsis sp. | 0 | 8.0 | 0 | 2.0 | 0 | 2.0 | 0 | 0 | 0 | 0 | 0 |
Values in the same row marked with an asterisk indicate significant differences in the pathogen reisolation percentage detected relative to the value for line 1.
Data corresponding of to the average of 2 batches of 25 plants analyzed in February 2009 and February 2011 prior to planting experimental vineyards. Two stubs per plant were analyzed corresponding to regions 10 and 30 mm below the location of the buds.
Total number of wood segments (2 stubs per plant and 25 grapevine plants analyzed per line) that were infected by fungi expressed as a percentage with respect to the total number of segments analyzed per line. Samples were taken 10 and 30 mm below the inoculation point. Data correspond to the average of two batches (EV1 and EV2) of 25 plants analyzed. Line 1, plants infected on pruning wounds; lines 2 to 5, plants protected in pruning wounds with chitosan oligosaccharide, garlic extract, vanillin, and CGV mix respectively; lines 6 to 9, plants infected on pruning wounds that have been previously protected by the application of chitosan, garlic extract, vanillin, and CGV mix, respectively; line 10, control plants (not infected and not treated with NA).
DISCUSSION
Many different studies have been carried out to monitor pruning wound protection with chemical antifungals such as benomyl, carbendazim, fosetyl, tebuconazole, or thiophanate-methyl, among others (7, 20, 23, 25, 26, 45), to cite only the latest. Some of these chemical fungicides have proven to be effective at different rates in the protection of pruning wounds. However, their field application clashes frontally against the competent authorities (at least in Europe, where authorities are trying to severely restrict their use in the field). Also, there is a growing trend among many grape growers to avoid treatments with chemical pesticides in order to produce more ecologic and environmentally friendly wines. Consequently, in recent years, political and social concerns have led many countries to reorient toward the replacement of chemical pesticides for crop protection (including vineyard management) with other control systems that are less toxic and have a lower environmental impact. In this context, the possible use of NA has become an interesting alternative. There are many reports describing the antifungal properties of countless NA, including plant extracts, essential oils, or substances from different sources, such as chitosan or propolis (27–29). All of the NA tested in this work exhibited, although to various degrees, in vitro antifungal activity against the pathogens tested. These data suggest that some other NA, not tested in this work, could also be effective in controlling grapevine trunk pathogens. The search for other effective combinations of NA presents an interesting opportunity.
The NA exhibiting the best performance in the in vitro assays were then checked on autoclaved grape wood tests of D. seriata- and P. chlamydospora-infected canes. This kind of assay was used as a filter prior to the later, more time-consuming field assays. The assumption was that the NA which performed poorly in this assay would exhibit even worse performance under the more strict and harsh conditions of a field trial. From the selected NA, chitosan had previously been tested on grapevines and applied as a foliar spray on potted grapevines growing in a greenhouse. The effect of chitosan against Neonectria liriodendri was similar to that achieved with some chemical fungicides, and it also reduced the disease incidence caused by P. chlamydospora (30).
This study demonstrates that infections caused by D. seriata and P. chlamydospora through pruning wounds can be significantly reduced using an application of chitosan oligosaccharide, garlic extract, or vanillin immediately after pruning although the best results were obtained with the application of CGV mix. To our knowledge, there is no report of chitosan or vanillin being applied to pruning wounds, whereas garlic extract has been reported to have a protective effect against E. lata in vitro and when applied to grapevine pruning wounds (24). The protective effect of these NA was noted in several ways. First, even though the infected plants showed significantly reduced growth (total height and length of the 10th internode) compared with that of control plants, growth parameters returned to normal when infected plants had been previously protected by NA application. Second, the level of lipid peroxidation has been widely used as an indicator of reactive oxygen species (ROS)-mediated damage to cell membranes under stressful conditions (46), and the infected plants were clearly stressed, as deduced from the high lipid peroxidation levels detected in untreated and inoculated vines. However, to our knowledge, this is the first report indicating that fungal pathogens causing trunk diseases are able to increase lipid damage to cells as a possible consequence of high intracellular ROS levels. Third, lipid peroxidation levels significantly decreased when NA were applied on infected plants. If we take into account that ROS are a major source of damage, not only to cellular lipids but also to proteins and DNA, it seems clear that any reduction in their levels means healthier plants. These data were a clear indication that the application of NA on pruning wounds prior to infection had a protective effect on plants. We must also remark that lipid peroxidation levels in noninoculated vines treated with NA were similar to those observed in control plants, which suggested that the application of NA was not producing an apparent stress on the vines, nor did it have any detrimental effects on the growth of plants. Fourth, infected plants also showed significantly higher levels of antioxidant defensive systems like catalase, GST, SOD, and GPX, which are recognized as stress markers (46). Particularly, GPX activity is indicative of intracellular defensive peroxidase levels against ROS, and it is widely recognized as a stress marker (46). GPX levels in infected and NA-treated plants were very similar to those observed in control plants but significantly lower than those exhibited by infected vines. This fact indicates that NA treatment was able to reverse the stressed condition of infected plants. Variations in SOD activity among all treatments were not as evident although, again, a small but significant difference could be noted by comparing SOD levels of infected plants with levels of the rest of the plants. We also must remark that, in spite of the high levels of defensive enzymes detected in infected plants, their activity was not high enough to counteract the lipid oxidation levels encountered as a result of infection by the pathogens.
The analysis of the mortality rate observed, after drastic watering restriction, in the EVs yielded very interesting results. The purpose of this treatment was to hasten the onset of symptoms since one of the biggest disadvantages in the analysis of these types of diseases is that the detection of symptoms under real field conditions is erratic and can vary significantly from year to year depending upon environmental conditions and other factors (47). Interestingly, there was a significant association between pathogen application on pruning wounds (line 1) and increased mortality compared to results in untreated control plants (line 10) [χ2 (1) = 19.4, P < 0.001]. This seems to represent the fact that, based on the odds ratio, the chances of plants surviving were 7.8 times higher for control plants than for plants of line 1 (infected through pruning wounds). In a similar way the odds of infected plants treated with NA were clearly higher than the survival chances of infected plants not treated with NA (line 1). Thus, the mortality rate significantly dropped (P < 0.001) when the infected plants had previously been protected by the chitosan oligosaccharide (16.0%), garlic extract (12.0%), vanillin (20.0%), and CGV mix (4%) applications. These data indicate a significant protective effect of the tested NA on grapevine plants which suffer from pruning wound infections.
Finally, the application of chitosan, garlic extract, vanillin, or the CGV mix effectively controlled the infection rate of the fungal pathogens inoculated. Several issues are worth highlighting. First, the higher infection levels were detected in untreated and inoculated vines, consistent with the high mortality rate observed. The rates of reisolation for D. seriata (46.0%) and P. chlamydospora (74.0%) from plants coinoculated in pruning wounds were high, indicating that the infection protocol had worked well. Also, it should be noted that P. chlamydospora was reisolated at a higher rate than D. seriata even though the species is a slow-growing fungus compared to D. seriata. These data suggested that we can discard any putative inhibitory effect of D. seriata against P. chlamydospora. Second, the application of NA controlled the fungal pathogens inoculated. Thus, whereas the isolation rate for D. seriata in infected plants (line 1) was 46.0%, this pathogen could not be isolated from vines treated with garlic extract and CGV mix, and the recovery rate was as low as 8.0% in plants treated with chitosan and vanillin. A similarly significant reduction in the isolation of P. chlamydospora was detected: the values significantly decreased for plants protected with chitosan (6.0%), garlic extract (6.0%), and vanillin (10.0) compared to the high rates (74.0%) of isolation in infected plants (line 1). Remarkably, again the CGV mix yielded the best results since neither D. seriata nor P. chlamydospora was isolated from CGV-treated plants. In fact, the only pathogen reisolated was Cadophora luteo-olivacea. Note that this fungal strain had been detected at a low rate in the vines analyzed prior to their planting (Table 3), which seemed to suggest that this particular species was infecting the vegetable starting material. Finally, it appears that the infection through pruning wounds weakened the plants, which were then more easily infected by other noninoculated pathogens such as Botryosphaeria dothidea, Phaeoacremonium aleophilum, or Phomopsis viticola (which were hardly ever isolated from other plants). In fact, up to seven different pathogens could be isolated, and their frequency of isolation was also higher in infected nontreated plants. It could be of great interest to analyze in further studies whether the primary infection by one or more pathogens makes them more susceptible to suffer secondary infections.
Although chitosan oligosaccharide, garlic extract, and vanillin exhibited high efficacy in both the autoclaved stem tests and field trials, we should emphasize that the beneficial effect of the CGV mixture seems to be higher than that of some of its individual components. Indeed, the mortality rate observed in CGV-treated plants was clearly lower than that detected when chitosan [χ2 (1) = 4.0, P < 0.05] or vanillin [χ2 (1) = 6.1, P < 0.05] was applied individually. Also, the reisolation rate of pathogens from CGV-treated plants was lower than that of vines treated with a single NA. This improved effect could be due to the combination of different mechanisms of action. We know that chitosan has the ability to penetrate fungal conidia, causing membrane disorganization which leads to loss of the cellular content (48). It has also been reported that low-molecular-weight chitosan causes significant morphological changes on fungal surfaces. These changes are mediated by chitosan interaction with the exposed anionic component of the fungal plasma membrane, which induces leakage of cellular content (32). Unfortunately, not much is known about vanillin's mechanism of antifungal activity, but it has been suggested that its aldehyde group plays an important role in its antifungal activity (49). Garlic contains at least two potent antifungal compounds: ajoene and allicin. Allicin appears to be able to block lipid synthesis in fungal yeast while inhibiting both protein and nucleic acid synthesis (50). The antifungal effect of ajoene remains unclear although it has been suggested that it may damage the fungal cell wall (51).
In conclusion, and considering the pathogen reisolation data shown in Table 3, we can state that chitosan oligosaccharide, garlic extract, and vanillin were able to significantly reduce infection in pruning wounds by D. seriata and P. chlamydospora, especially when they were applied as a CGV mix, since none of these pathogens were detected in CGV-treated vines. The use of NA has several important advantages for grape growers. Since NA are prepared as an aqueous solution, they can be easily applied by spray application although their efficacy perhaps could be improved by inclusion in a paste, as demonstrated for some chemical fungicides (26). However, spray application could be a preferred method of application by grape growers since it is a less time-consuming and cheaper method (7, 24, 52). Also, the efficacy of the three NA (alone or in different combinations) may allow for their use in a rotation system in order to avoid the development of putative resistant fungal strains. In this context, use of the CGV mix could be highly attractive since the mixture of different NA with putative diverse modes of action could greatly hinder the development of resistances. Finally, these NA can be easily prepared and are affordably priced.
ACKNOWLEDGMENT
This work was financed by Bodegas Vega Sicilia S.A. (Valbuena de Duero, Valladolid, Spain).
We thank Alberto Alonso-Monroy for technical assistance.
REFERENCES
- 1.Fourie PH, Halleen F, Van der Vyver J, Schreuder W. 2001. Effect of Trichoderma treatments on the occurrence of decline pathogens in the roots and rootstocks of nursery grapevines. Phytopathol Mediterr 40:S473–S478. [Google Scholar]
- 2.Gubler WD, Rolshausen PE, Trouillas FP, Urbez JR, Voegel T, Leavitt GM, Weber EA. 2005. Grapevine trunk diseases in California. Pract Winery Vineyard Mag 27(1):6–25. [Google Scholar]
- 3.Siebert JB. 2001. Eutypa: the economic toll in vineyards. Wines Vines 82:50–56. [Google Scholar]
- 4.Gramaje D, Armengol J. 2011. Fungal trunk pathogens in the grapevine propagation process: potential inoculum sources, detection, identification and management strategies. Plant Dis 95:1040–1055. doi: 10.1094/PDIS-01-11-0025. [DOI] [PubMed] [Google Scholar]
- 5.Agustí-Brisach C, Gramaje D, García-Jiménez J, Armengol J. 2013. Detection of black-foot disease pathogens in the grapevine nursery propagation process in Spain. Eur J Plant Pathol 137:103–112. doi: 10.1007/s10658-013-0221-8. [DOI] [Google Scholar]
- 6.Halleen F, Fourie PH, Crous PW. 2006. A review of black foot disease of grapevine. Phytopathol Mediterr 45:S55–S67. [Google Scholar]
- 7.Rolshausen PE, Urbez-Torres JR, Rooney-Latham S, Eskalen A, Smith RJ, Gubler WD. 2010. Evaluation of pruning wounds susceptibility and protection against fungi associated with grapevine trunk diseases. Am J Enol Vitic 61:113–119. [Google Scholar]
- 8.Van Niekerk JM, Halleen F, Fourie PH. 2011. Temporal susceptibility of grapevine pruning wounds to trunk pathogen infections in South African grapevines. Phytopathol Mediterr 50:139–150. [Google Scholar]
- 9.Luque J, Elena G, García-Figueres F, Reyes J, Barrios G, Legorburu FJ. 2014. Natural infections of pruning wounds by fungal trunk pathogens in mature grapevines in Catalonia (Northeast Spain). Aust J Grape Wine R 20:134–143. doi: 10.1111/ajgw.12046. [DOI] [Google Scholar]
- 10.Úrbez-Torres JR, Gubler WD. 2011. Susceptibility of grapevine pruning wounds to infection by Lasiodiplodia theobromae and Neofusicoccum parvum. Plant Pathol 60:261–270. doi: 10.1111/j.1365-3059.2010.02381.x. [DOI] [Google Scholar]
- 11.Munkvold GP, Marois JJ. 1995. Factors associated with variation in susceptibility of grapevine pruning wounds to infection by Eutypa lata. Phytopathology 85:249–256. doi: 10.1094/Phyto-85-249. [DOI] [Google Scholar]
- 12.Chapuis L, Richard L, Dubos B. 1998. Variation in susceptibility of grapevine pruning wounds to infection by Eutypa lata in south-western France. Plant Pathol 47:463–472. doi: 10.1046/j.1365-3059.1998.00258.x. [DOI] [Google Scholar]
- 13.Lecomte P, Bailey DJ. 2011. Studies on infestation by Eutypa lata of grapevine spring wounds. Vitis 50:35–41. [Google Scholar]
- 14.Gramaje D, Ayres MR, Trouillas FP, Sosnowski MR. 2012. Efficacy of fungicides on mycelial growth of diatrypaceous fungi associated with grapevine trunk disease. Australas Plant Pathol 41:295–300. doi: 10.1007/s13313-011-0111-5. [DOI] [Google Scholar]
- 15.Eskalen A, Feliciano J, Gubler WD. 2007. Susceptibility of grapevine pruning wounds and symptom development in response to infection by Phaeoacremonium aleophilum and Phaeomoniella chlamydospora. Plant Dis 91:1100–1104. doi: 10.1094/PDIS-91-9-1100. [DOI] [PubMed] [Google Scholar]
- 16.Munkvold GP, Marois JJ. 1993. Efficacy of natural epiphytes and colonizers of grapevine pruning wounds for biological control of Eutypa dieback. Phytopathology 83:624–629. doi: 10.1094/Phyto-83-624. [DOI] [Google Scholar]
- 17.McMahan G, Yeh W, Marshall MN, Olsen M, Sananikone S, Wu JY, Block DE, VanderGheysnt JS. 2001. Characterizing the production of a wild-type and benomyl-resistant Fusarium lateritium for biocontrol of Eutypa lata on grapevine. J Ind Microbiol Biotechnol 26:151–155. doi: 10.1038/sj.jim.7000099. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt CS, Lorenz D, Wolf GA. 2001. Biological control of the grapevine dieback fungus Eutypa lata I: screening of bacterial antagonist. J Phytopathol 149:427–435. doi: 10.1046/j.1439-0434.2001.00658.x. [DOI] [Google Scholar]
- 19.Di Marco S, Osti F. 2007. Applications of Trichoderma to prevent Phaeomoniella chlamydospora infections in organic nurseries. Phytopathol Mediterr 46:73–83. [Google Scholar]
- 20.Halleen F, Fourie PH, Lombard PJ. 2010. Protection of grapevine pruning wounds against Eutypa lata by biological and chemical methods. S Afr J Enol Vitic 31:125–132. [Google Scholar]
- 21.Kotze Ç, Van Niekerk J, Mostert L, Halleen F, Fourie P. 2011. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol Mediterr 50:S247–S263. [Google Scholar]
- 22.Mutawila C, Fourie PH, Halleen F, Mostert L. 2011. Grapevine cultivar variation to pruning wounds protection by Trichoderma species against trunk pathogens. Phytopathol Mediterr 50:S264–S276. [Google Scholar]
- 23.Sosnowski MR, Creaser ML, Wicks TJ, Lardner R, Scott ES. 2008. Protection of grapevine pruning wounds from infection by Eutypa lata. Aust J Grape Wine Res 14:134–142. doi: 10.1111/j.1755-0238.2008.00015.x. [DOI] [Google Scholar]
- 24.Sosnowski MR, Loschiavo AP, Wicks TJ, Scott ES. 2013. Evaluating treatments and spray application for the protection of grapevine pruning wounds from infection by Eutypa lata. Plant Dis 97:1599–1604. doi: 10.1094/PDIS-02-13-0201-RE. [DOI] [PubMed] [Google Scholar]
- 25.Amponsah NT, Jones E, Ridgway HJ, Jaspers MV. 2012. Evaluation of fungicides for the management of Botryosphaeria dieback diseases of grapevines. Pest Manage Sci 68:676–683. doi: 10.1002/ps.2309. [DOI] [PubMed] [Google Scholar]
- 26.Díaz GA, Latorre BA. 2013. Efficacy of paste and liquid fungicide formulation to protect pruning wounds against pathogens associated with grapevine trunk diseases in Chile. Crop Prot 46:106–112. doi: 10.1016/j.cropro.2013.01.001. [DOI] [Google Scholar]
- 27.Castillo F, Hernández D, Gallegos G, Rodríguez R, Aguilar CN. 2012. Antifungal properties of bioactive compounds from plants, p 81–106. In Dhanasekaran D. (ed), Fungicides for plant and animal diseases. InTech Europe, Rijeka, Croatia. [Google Scholar]
- 28.Lang G, Buchbauer G. 2012. A review on recent research results (2008-2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr J 27:13–39. doi: 10.1002/ffj.2082. [DOI] [Google Scholar]
- 29.Yanar Y, Kadioglu I, Gökçe A, Demirtas B, Gören N, Cam H, Whalon M. 2011. In vitro antifungal activities of 26 plant extracts on mycelial growth on Phytophthora infestans (Mont.) de Bary. Afr J Biotechnol 10:2625–2629. [Google Scholar]
- 30.Nascimento T, Rego C, Oliveira H. 2007. Potential use of chitosan in the control of grapevine trunk diseases. Phytopathol Mediterr 46:218–224. [Google Scholar]
- 31.Halama P, Van Haluwin C. 2004. Antifungal activity of lichen extracts and lichenic acids. BioControl 49:95–107. doi: 10.1023/B:BICO.0000009378.31023.ba. [DOI] [Google Scholar]
- 32.Park Y, Kim M-H, Park S-C, Cheong H, Jang M-K, Nah J-W, Hahm K-S. 2008. Investigation of the antifungal activity and mechanism of action of LMWS-chitosan. J Microbiol Biotechnol 18:1729–1734. [PubMed] [Google Scholar]
- 33.Rattanapitigorn P, Arakawa M, Tsuro M. 2006. Vanillin enhances the antifungal effect of plant essential oils against Botrytis cinerea. Int J Aromather 16:193–198. doi: 10.1016/j.ijat.2006.09.003. [DOI] [Google Scholar]
- 34.Fokt H, Pereira A, Ferreira AM, Cunha A, Aguiar C. 2010. How do bees prevent hive infections? The antimicrobial properties of propolis, p 481–493. In Ḿendez-Vilas A (ed), Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex, Badajoz, Spain. [Google Scholar]
- 35.Harris JC, Cottrell SL, Plummer S, Lloyd D. 2001. Antimicrobial properties of Allium sativum (garlic). Appl Microbiol Biotechnol 57:282–286. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- 36.Arora DS, Ohlan D. 1997. In vitro studies on antifungal activity of tea (Camellia sinensis) and coffee (Coffea arabica) against wood-rotting fungi. J Basic Microbiol 37:159–165. doi: 10.1002/jobm.3620370302. [DOI] [Google Scholar]
- 37.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez J. 2008. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 19:1130–1138. doi: 10.1016/j.foodcont.2007.12.003. [DOI] [Google Scholar]
- 38.Elena G, García-Figueres F, Luque J. 2012. The minimum inoculum potential of Diplodia seriata and Phaeomoniella chlamydospora needed for the artificial infection of grapevine pruning wounds. Phytopathol Mediterr 51:434. [Google Scholar]
- 39.Bradford MM. 1976. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Buege JA, Aust SD. 1978. Microsomal lipid peroxidation. Methods Enzymol 52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 41.Del Río LA, Ortega MG, Lopez AL, Gorgé JL. 1977. A more sensitive modification of the catalase assay with Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal Biochem 80:409–415. [DOI] [PubMed] [Google Scholar]
- 42.Crous PW, Gams W. 2000. Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathol Mediterr 39:112–118. [Google Scholar]
- 43.van Niekerk JM, Crous PW, Groenewald JZ, Fourie PH, Halleen F. 2004. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96:781–798. doi: 10.2307/3762112. [DOI] [PubMed] [Google Scholar]
- 44.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, New York, NY. [Google Scholar]
- 45.Pitt WM, Sosnowski MR, Huang R, Qiu Y, Steel CC, Savocchia S. 2012. Evaluation of fungicides for the management of Botryosphaeria canker of grapevines. Plant Dis 96:1303–1308. doi: 10.1094/PDIS-11-11-0998-RE. [DOI] [PubMed] [Google Scholar]
- 46.Sharma P, Jha AB, Dubey RS, Pessarakli M. 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Botany 2012:217037. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 47.Martín MT, Cobos R. 2007. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathol Mediterr 46:18–25. [Google Scholar]
- 48.Palma-Guerrero J, Jansson H-B, Salinas J, Lopez-Llorca LV. 2008. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J Appl Microbiol 104:541–553. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald DJ, Stratford M, Gasson MJ, Narbad A. 2005. Structure-function analysis of the vanillin molecule and its antifungal properties. J Agric Food Chem 53:1769–1775. doi: 10.1021/jf048575t. [DOI] [PubMed] [Google Scholar]
- 50.Adetumbi M, Javor GT, Lau BH. 1986. Allium sativum (garlic) inhibits lipid synthesis by Candida albicans. Antimicrob Agents Chemother 30:499–501. doi: 10.1128/AAC.30.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida S, Kasuga S, Hayashi N, Ushiroguchi T, Matsuura H, Nakagawa S. 1987. Antifungal activity of Ajoene Derived from Garlic. Appl Environ Microbiol 53:615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herche R, Gubler WD. 2010. Control strategies for trunk diseases of grapevine (Vitis vinifera). Phytopathol Mediterr 49:125. [Google Scholar]