Abstract
OBJECTIVE
The aim of this study was to investigate the presence and correlates of clinically relevant cognitive impairment in middle-aged adults with childhood-onset type 1 diabetes (T1D).
RESEARCH DESIGN AND METHODS
During 2010–2013, 97 adults diagnosed with T1D and aged <18 years (age and duration 49 ± 7 and 41 ± 6 years, respectively; 51% female) and 138 similarly aged adults without T1D (age 49 ± 7 years; 55% female) completed extensive neuropsychological testing. Biomedical data on participants with T1D were collected periodically since 1986–1988. Cognitive impairment status was based on the number of test scores ≥1.5 SD worse than demographically appropriate published norms: none, mild (only one test), or clinically relevant (two or more tests).
RESULTS
The prevalence of clinically relevant cognitive impairment was five times higher among participants with than without T1D (28% vs. 5%; P < 0.0001), independent of education, age, or blood pressure. Effect sizes were large (Cohen d 0.6–0.9; P < 0.0001) for psychomotor speed and visuoconstruction tasks and were modest (d 0.3–0.6; P < 0.05) for measures of executive function. Among participants with T1D, prevalent cognitive impairment was related to 14-year average A1c >7.5% (58 mmol/mol) (odds ratio [OR] 3.0; P = 0.009), proliferative retinopathy (OR 2.8; P = 0.01), and distal symmetric polyneuropathy (OR 2.6; P = 0.03) measured 5 years earlier; higher BMI (OR 1.1; P = 0.03); and ankle-brachial index ≥1.3 (OR 4.2; P = 0.01) measured 20 years earlier, independent of education.
CONCLUSIONS
Clinically relevant cognitive impairment is highly prevalent among these middle-aged adults with childhood-onset T1D. In this aging cohort, chronic hyperglycemia and prevalent microvascular disease were associated with cognitive impairment, relationships shown previously in younger populations with T1D. Two additional potentially modifiable risk factors for T1D-related cognitive impairment, vascular health and BMI, deserve further study.
Introduction
Modest cognitive dysfunction is consistently reported in children and young adults with type 1 diabetes (T1D) (1). Mental efficiency, psychomotor speed, executive functioning, and intelligence quotient appear to be most affected (2); studies report effect sizes between 0.2 and 0.5 (small to modest) in children and adolescents (3) and between 0.4 and 0.8 (modest to large) in adults (2). Whether effect sizes continue to increase as those with T1D age, however, remains unknown.
A key issue not yet addressed is whether aging individuals with T1D have an increased risk of manifesting “clinically relevant cognitive impairment,” defined by comparing individual cognitive test scores to demographically appropriate normative means, as opposed to the more commonly investigated “cognitive dysfunction,” or between-group differences in cognitive test scores. Unlike the extensive literature examining cognitive impairment in type 2 diabetes, we know of only one prior study examining cognitive impairment in T1D (4). This early study reported a higher rate of clinically relevant cognitive impairment among children (10–18 years of age) diagnosed before compared with after age 6 years (24% vs. 6%, respectively) or a non-T1D cohort (6%).
Cognitive impairment is important to study in an aging T1D population because these individuals are concurrently exposed to the consequences of long-term T1D and the negative effects of advancing age on cognition. Furthermore, several risk factors for age-related cognitive impairment, such as hypertension (5) and impaired renal function (6), are highly prevalent in T1D, yet the contribution of these factors to worsening cognitive function in an aging T1D cohort remains unknown. Characterizing cognitive impairment in aging participants with T1D is warranted based on findings from cognitive studies of aging populations without T1D: cognitive impairment is related to poor self-care (7), high costs (8), and disability (9). Cognitive impairment in T1D likely has similar adverse effects.
This study tests the hypothesis that childhood-onset T1D is associated with an increased risk of developing clinically relevant cognitive impairment detectable by middle age. We compared cognitive test results between adults with and without T1D and used demographically appropriate published norms (10–12) to determine whether participants met criteria for impairment for each test; aging and dementia studies have selected a score ≥1.5 SD worse than the norm on that test, corresponding to performance at or below the seventh percentile (13). We also assessed relationships between T1D-specific variables and cognitive impairment; based on the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study’s 18-year cognitive follow-up results, we hypothesized that cognitive impairment would be associated with smoking history, high blood pressure, prevalent microvascular complications, and poor metabolic control (14).
Research Design and Methods
Participants
Middle-aged adults with T1D (n = 97; mean age and duration of diabetes 49 and 41 years, respectively) were recruited from the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, an ongoing prospective study of individuals diagnosed before age 18 years and listed in the Children’s Hospital of Pittsburgh’s diabetes registry. The first EDC clinical assessment occurred in 1986–1988 (N = 658; mean age and duration of diabetes 28 and 19 years, respectively). Biennial physical exams and questionnaires occurred through 1996–1998, with an additional exam in 2004–2006 (for details see ref. 15). All locally dwelling EDC participants as of 1 January 2010 (n = 263) were invited to participate in an ancillary neuroimaging/neurocognitive study. Of those, 81 refused, 26 never responded, and 2 were lost to follow-up. Of the 154 interested, 37 were ineligible for MRI (e.g., metallic implants, claustrophobic) and 5 had scheduling conflicts, leaving 112 eligible and scheduled for neuroimaging/neurocognitive testing. Of these, three failed to show for their scheduled visit. On their scheduled testing date, another three refused the MRI and nine refused the cognitive test battery, yielding an analytic sample of n = 97 (Supplementary Fig. 1).
Adults without T1D who were participating in an observational study of the effects of prehypertension (blood pressure higher than “normal” but not high enough to qualify as hypertensive) on cerebral structure and function served as a comparison group. Inclusion criteria were age 35–60 years, local to Pittsburgh, and blood pressure within the values of 120–139/80–89 mmHg. Full exclusion criteria are provided in Supplementary Appendix 1. Of the 414 who responded to the mailing/advertisement and were screened to participate, 110 were ineligible for MRI, 60 changed their mind, and 14 withdrew, leaving 230 enrolled (mean age 46 years). To mirror the EDC’s primarily Caucasian racial distribution, only Caucasian participants with complete neuroimaging/neurocognitive data as of August 2013 (n = 138) were included.
All study procedures received local institutional review board approval, and all participants provided informed consent before any research procedures were initiated.
Measures
For all participants, blood pressure, BMI, and serum glucose were measured using standardized techniques at the time of cognitive testing. Participants with serum glucose ≤70 mg/dL were given a snack then retested after 15 min; no cognitive testing was performed if serum glucose was ≤70 mg/dL. Weekly caloric expenditure (kcal) was estimated using the self-report Paffenbarger questionnaire.
As part of EDC, metabolic control and blood pressure were assessed biennially from baseline through 1996–1998, again in 2004–2006, and at the time of neurocognitive testing (2010–2013); this allowed calculations of long-term average blood pressure and glycemic control (HbA1c and “A1c months,” a calculated measure assessing the degree and duration of hyperglycemia [for details see ref. 16]). In 1990–1992, arterial health was assessed via the ankle-brachial index (ABI); two readings were averaged, then dichotomized as <1.3 or >1.3/noncompressible. An ABI >1.3 is correlated with medial arterial calcification (17), which is associated with arterial stiffening (18). Prevalence of T1D complications (nephropathy, coronary artery disease, distal symmetric polyneuropathy, cardiac autonomic neuropathy, retinopathy) was assessed biennially from baseline through 1996–1998, then again in 2004–2006, using highly standardized methods (for details see ref. 15).
Cognitive Assessment
Cognitive measures included estimated verbal intelligence (North American Adult Reading Test [NAART]); psychomotor efficiency (Digit Symbol Substitution Test [DSST] and Grooved Pegboard [GP]); executive function (Trail Making Test, Part B [TMTB], Verbal Fluency [VF: animal naming and FAS], Stroop Color-Word and Letter/Number Sequencing [LN Sequence]); learning and working memory (Rey Auditory Verbal Learning Tests, Four-Word Short-Term Memory [4WSTM], and Rey-Osterrieth Complex Figure [ROCF] delayed task); and visuoconstruction skills (ROCF copy task [ROCF-copy]). Details are provided by Strauss et al. (10).
Statistical Analyses
Participant characteristics were compared using the t test, Fisher exact test, and Wilcoxon rank sum test, as applicable (Table 1).
Table 1.
Comparison of characteristics of participants without T1D (“no T1D”) and with T1D (“T1D”; EDC) and by degree of cognitive impairment* among participants with T1D
| No T1D (n = 138) | T1D (n = 97) | P value | Degree of cognitive impairment* in participants with T1D |
||||
|---|---|---|---|---|---|---|---|
| 0 (n = 38, 39%) | 1 (n = 32, 33%) | 2 (n = 27, 28%) | P value | ||||
| Demographic factors | |||||||
| Age (years) | 48.7 ± 7.2 | 49.1 ± 6.6 | 0.72 | 48.2 ± 6.8 | 48.5 ± 6.7 | 50.8 ± 6.3 | 0.26 |
| Female sex | 76 (55) | 49 (51) | 0.51 | 19 (50) | 16 (50) | 14 (52) | 0.93 |
| Education (years) | 16 ± 3 | 15 ± 3 | 0.003† | 16 ± 3 | 15 ± 2 | 14 ± 3 | 0.004† |
| Biological factors | |||||||
| Serum glucose (mg/dL) | 91.3 ± 16.3 | 174.5 ± 86.3 | <0.0001† | 169.7 ± 93.8 | 174.5 ± 89.5 | 181.0 ± 74.5 | 0.97 |
| SBP (mmHg) | 119.4 ± 9.9 | 119.6 ± 15.6 | 0.91 | 117.1 ± 15.1 | 122.5 ± 15.9 | 119.8 ± 15.9 | 0.47 |
| DBP (mmHg) | 80 (74–84) | 66 (60–70) | <0.0001† | 65.1 ± 8.4 | 68.5 ± 10.2 | 63.6 ± 10.7 | 0.15 |
| Ever had high blood pressure‡ | 11 (8) | 37 (38) | <0.0001† | 13 (34) | 12 (38) | 12 (44) | 0.38 |
| ApoE4 status (24, 34, or 44) | 27 (21) | 29 (30) | 0.12 | 10 (26) | 12 (38) | 7 (26) | 0.96 |
| Behavioral factors | |||||||
| BMI (kg/m2) | 28.5 ± 5.6 | 27.4 ± 4.7 | 0.11 | 25.8 ± 4.3 | 28.5 ± 4.7 | 28.3 ± 4.9 | 0.05† |
| Physical activity, past week (kcal)§ | 1,628 (616–3,008) | 1,092 (420–1,981) | 0.03† | 1,412 (784–2,618) | 980 (420–1,676) | 448 (140–1,966) | 0.29 |
| Diabetes factors | |||||||
| T1D duration (years) | – | 41.0 ± 6.2 | – | 40.3 ± 6.4 | 41.0 ± 6.4 | 42.0 ± 5.6 | 0.61 |
| Age at T1D diagnosis (years) | – | 8.0 ± 4.2 | – | 7.9 ± 3.7 | 7.5 ± 4.3 | 8.9 ± 4.6 | 0.50 |
| Coronary artery disease§ | – | 15 (15) | – | 5 (13) | 3 (9) | 7 (26) | 0.18 |
| Cardiac autonomic neuropathy§ | – | 41 (47) | – | 13 (37) | 15 (54) | 13 (52) | 0.37 |
| Distal symmetric polyneuropathy§ | – | 46 (51) | – | 13 (36) | 15 (56) | 18 (67) | 0.03† |
| Proliferative retinopathy§ | – | 46 (47) | – | 13 (34) | 16 (50) | 17 (63) | 0.03† |
| Estimated GFR (mL/min/1.73 m2)§ | – | 83.1 ± 23.6 | – | 87.2 ± 23.1 | 82.2 ± 25.7 | 78.6 ± 22.0 | 0.15 |
| 25-year average A1c months (AU) | – | 1,116 ± 431 | – | 962 ± 433 | 1,258 ± 403 | 1,164 ± 405 | 0.06† |
| 14-year A1c >7.5% (>58 mmol/mol) | – | 63 (65) | – | 18 (47) | 23 (72) | 22 (81) | 0.01† |
| A1c >7.5% (>58 mmol/mol) | – | 58 (61) | – | 16 (44) | 22 (69) | 20 (74) | 0.01† |
| Average ABI >1.3‖ | 16 (19) | – | 3 (8) | 6 (24) | 7 (28) | 0.05† | |
All measures were collected at the time of neurocognitive assessment (2010–2013) unless otherwise noted. Data are n (%), mean ± SD, or median (interquartile range). *Degree of cognitive impairment: “0” = no cognitive impairment, no test scores ≥1.5 SD worse than published norms; “1” = mild, only one test score ≥1.5 SD worse than the published norm; “2” = clinically relevant, two or more test scores ≥1.5 SD worse than published norms.
†Statistically significant using an FDR of 0.20. ‡For participants with T1D, systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg or self-report of ever using antihypertensive medication at any EDC physical exam from baseline (1986–1988) through the time of neurocognitive assessment (2010–2013); for participants without T1D, a history of high blood pressure or use of antihypertensive medication based on self-report at the time of neurocognitive assessment. §For participants with T1D, the measure was taken in 2004–2006. ‖Measure was taken in 1990–1992.
Raw cognitive test scores were compared between groups using ANCOVA, adjusted for education (Table 2). Standardized effect sizes for between-group differences in test scores were computed using Cohen d t(n1 + n2)/√df(n1 × n2) and were classified as small (d < 0.30), moderate (d 0.30–0.60), or large (d ≥ 0.60) (19) (Supplementary Fig. 2).
Table 2.
Raw cognitive test scores for participants with T1D (EDC) and participants without T1D
| Domain | Test | Raw score |
Effect size (d)† | ||||
|---|---|---|---|---|---|---|---|
| T1D (mean ± SD) | No T1D (mean ± SD) | Difference (no T1D – T1D) |
|||||
| Mean | % | P value* | |||||
| IQ | NAART | ||||||
| Number correct | 37.1 ± 9.4 | 43.3 ± 10.3 | 6.2 | −15.0 | 0.0005‡ | 0.62 | |
| Estimated verbal IQ (scaled)§ | 108 ± 8 | 113 ± 9 | 5.0 | −4.5 | 0.0012‡ | 0.59 | |
| Psychomotor speed | DSST | 54.5 ± 13.5 | 63.4 ± 11.8 | 8.9 | −15.0 | <0.0001‡ | 0.72 |
| GP‖ | 88.8 ± 33.2 | 69.0 ± 17.6 | 19.8 | 25.1 | <0.0001‡ | 1.02 | |
| Executive function | Stroop Color-Word | 41.4 ± 9.5 | 44.2 ± 8.8 | 3.2 | −7.5 | 0.06‡ | 0.58 |
| TMT | |||||||
| Part B‖ | 65.4 ± 38.5 | 53.4 ± 22.3 | 12.0 | 16.5 | 0.02‡ | 0.47 | |
| Ratio B to A‖ | 2.5 ± 1.4 | 2.0 ± 0.7 | 0.5 | 21.2 | 0.002‡ | 0.54 | |
| VF | |||||||
| FAS | 44.4 ± 13.8 | 48.2 ± 11.9 | 3.7 | −7.4 | 0.10‡ | 0.30 | |
| LN sequence | 11.0 ± 2.9 | 11.9 ± 2.8 | 1.0 | −8.2 | 0.05‡ | 0.34 | |
| Memory | Rey Auditory Verbal Learning | ||||||
| Sum of trials 1–5 | 54.1 ± 8.9 | 56.0 ± 9.0 | 2.0 | −3.7 | 0.44 | 0.22 | |
| Interference | 6.4 ± 2.6 | 6.9 ± 1.9 | 0.5 | −7.5 | 0.34 | 0.24 | |
| Delayed recall | 11.0 ± 3.0 | 11.4 ± 3.0 | 0.4 | −4.0 | 0.65 | 0.15 | |
| ROCF-delayed | 18.6 ± 6.7 | 19.9 ± 6.6 | 1.3 | −6.6 | 0.41 | 0.20 | |
| 4WSTM | |||||||
| 5-s list | 15.2 ± 3.1 | 16.3 ± 3.2 | 1.1 | −7.0 | 0.10‡ | 0.35 | |
| 15-s list | 12.3 ± 4.1 | 13.4 ± 3.8 | 1.2 | −9.2 | 0.17 | 0.31 | |
| 30-s list | 10.5 ± 4.5 | 11.1 ± 4.7 | 0.6 | −5.8 | 0.76 | 0.14 | |
| Visuospatial |
ROCF-copy |
30.7 ± 5.4 |
33.9 ± 3.4 |
3.2 |
−9.9 |
<0.0001‡ |
0.88 |
| Semantic fluency | VF Animals | 21.7 ± 5.3 | 23.3 ± 5.5 | 1.5 | −6.5 | 0.14 | 0.28 |
*P value for mean difference in test score, adjusted for years of education. †Effect size (Cohen d) calculated as t(n1 + n2)/√df(n1 × n2) to account for unequal sample sizes and unequal variances (source: http://www.uccs.edu/lbecker/effect-size.html, accessed 11 December 2014). ‡Statistically significant using a FDR of 0.20. §Estimated verbal IQ calculated as 128.7 − 0.89 (NAART incorrect). ǁTasks in which a higher score indicates worse performance; in all other tasks, higher scores indicate better performance.
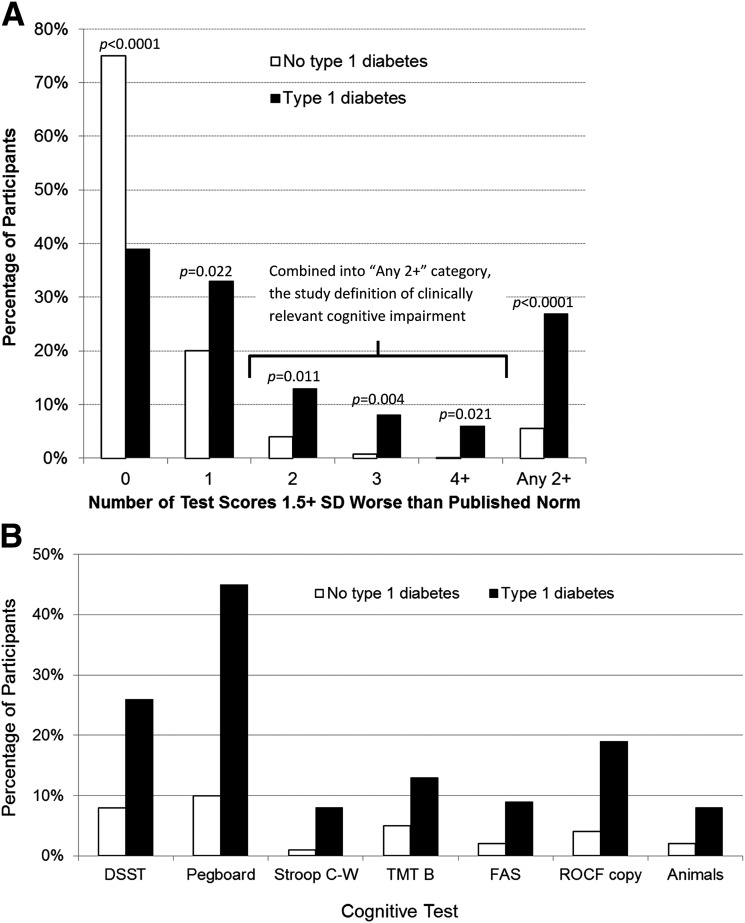
Impairment indicator variables were created for each test. Individual raw test scores ≥1.5 SD worse than published norms (10–12) were considered “impaired.” Between-group differences on impairment for each cognitive test were compared using the Fisher exact test (Fig. 1).
Figure 1.
A: Percentage of participants scoring ≥1.5 SD worse than published normative data in no, one, two, three, or four or more tests. White bars indicate participants without T1D; black bars represent participants with T1D. “Any 2+” indicates the percentage of participants scoring ≥1.5 SD worse than published normative data on two or more tests, thereby meeting the study definition of clinically relevant cognitive impairment. B: Percentage of participants with T1D (black bars) and without T1D (white bars) scoring ≥1.5 SD worse than published normative data, by test. Only tasks with statistically significant (P < 0.05) between-group differences are shown. C-W, Color-Word.
The seven tasks with statistically significant between-group differences in impairment were used to determine levels of cognitive impairment: none = no test scores “impaired”; mild = only one test score impaired; clinically relevant = two or more test scores impaired. This classification scheme is validated and used in epidemiologic studies to assess cognitive impairment in community-dwelling adults aged ≥75 years (13). Between-group differences in the level of cognitive impairment were assessed using the Fisher exact test, controlling for education (Fig. 1).
To examine factors related to between-group differences in cognitive impairment, characteristics that differed between groups (false discovery rate [FDR] = 0.20; Table 1) were entered in ordinal logistic regression models with level of cognitive impairment as the outcome and T1D status as the main covariate (Table 3).
Table 3.
Ordinal logistic (proportional odds) regression models showing the independent effects of T1D on the odds of cognitive impairment level
| Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| T1D | 5.13 (2.97–8.85) | <0.0001* | 4.56 (2.62–7.93) | <0.0001* | 4.82 (2.42–9.60) | <0.0001* | 4.31 (2.39–7.76) | <0.0001* | 3.85 (2.18–6.81) | <0.0001* |
| Years of education | 0.87 (0.78–0.97) | 0.001* | 0.87 (0.78–0.96) | 0.001* | 0.87 (0.78–0.97) | 0.01* | 0.88 (0.79–0.98) | 0.03 | ||
| Diastolic blood pressure | 1.00 (0.97–1.03) | 0.94 | ||||||||
| History of high blood pressure or using antihypertensive medication | 1.18 (0.61–2.31) | 0.63 | ||||||||
| Weekly physical activity estimate (kcal/100) | 0.99 (0.97–1.01) | 0.25 | ||||||||
ORs here should be interpreted proportionately as the odds of any cognitive impairment (one or more vs. no tests impaired at ≥1.5 SD compared with published norms); also two or more vs. no or one test impaired. Different variables were included in each model: model 1, T1D; model 2, T1D + years of education; model 3, T1D + years of education + diastolic blood pressure; model 4, T1D + years of education + history of high blood pressure; model 5, T1D + years of education + estimated weekly physical activity. *Statistically significant using Bonferroni correction (model 1: P < 0.05; model 2: P < 0.03; models 3–5, P < 0.02).
Sensitivity analysis, excluding participants with a history of high blood pressure (≥140/90 mmHg or using antihypertensive medication), was conducted to ensure that the imbalance in high blood pressure was not confounding results.
In analyses restricted to participants with T1D, characteristics were compared first across level of cognitive impairment using the Fisher exact test for categorical variables and ANOVA for continuous variables (Table 1). Characteristics that differed at FDR = 0.20 then were entered into separate ordinal logistic regression models with level of cognitive impairment as the outcome, controlling for years of education (Supplementary Table 1). All models met the proportional odds assumption (P > 0.1).
To adjust for multiple comparisons, we applied an FDR of 0.20 per the Benjamini-Hochberg correction method. Education-adjusted P values were sorted from lowest to highest, then the Benjamini-Hochberg correction was applied to identify statistically significant variables, accepting that 20% may be false positives. This corresponded to a P value of 0.03 (Supplementary Table 2) when comparing factors between participants by T1D status (Table 1, “No T1D” and “T1D” columns), of 0.06 (Supplementary Table 3) when comparing factors among participants with T1D by cognitive status (Table 1, “Degree of Cognitive Impairment” columns), and of 0.10 (Supplementary Table 4) when comparing cognitive test scores by T1D status (Table 2). We chose this method over family-wise correction methods because of the paucity of prior studies; we would rather falsely identify factors as deserving further investigation than reject factors that actually warrant additional study.
In the ordinal logistic regression models (Table 3, Supplementary Table 1), the more stringent Bonferroni method was used to control for multiple comparisons.
Results
Both cohorts were comparable in age, male-to-female distribution, systolic blood pressure, BMI, and apolipoprotein E4 (ApoE4) status (Table 1). Compared with participants without T1D, participants with T1D were significantly more likely to have a history of high blood pressure (38% vs. 8%; P < 0.0001), higher serum glucose (174.5 vs. 91.3 mg/dL; P < 0.0001), lower diastolic blood pressure (66 vs. 80 mmHg; P < 0.0001), and lower estimated weekly physical activity (1092 vs. 1628 kcal; P = 0.03) (Table 1).
Compared with participants without T1D, those with T1D had lower raw cognitive test scores on measures of psychomotor speed, executive function, visuoconstruction ability, and verbal intelligence (Table 2). Large effect sizes (d ≥ 0.60) were found for NAART, DSST, GP, and ROCF-copy. Moderate effect sizes (d 0.3–0.6) were found for Stroop Color-Word, TMTB, VF, LN Sequence, and 4WSTM 5-s list scores (Table 2, Supplementary Fig. 2). The effect size for the 4WSTM 15-s list fell within the “moderate” range, but between-group differences on this and other memory test scores were not statistically significant using an FDR of 0.20.
A similar pattern was observed when test scores were classified as “normal” versus “impaired” (i.e., the raw test score was ≥1.5 SD worse than the published norm). Compared with participants without T1D, those with T1D had significantly higher rates of impairment on tests of psychomotor speed (DSST: 25% vs. 8%; GP: 45% vs. 10%), executive function (TMTB: 12% vs. 5%; VF: 10% vs. 2%; VF Animals: 8% vs. 1%; Stroop Color-Word: 8% vs. <1%), and visuoconstruction ability (ROCF-copy: 20% vs. 4%) (Fig. 1B). Participants with T1D were also more likely to have two or more tests impaired, hence meeting our definition of clinically relevant cognitive impairment (28% vs. 5%; P < 0.0001; Fig. 1A).
In ordinal logistic regression models with level of cognitive impairment as the outcome, having T1D was related to fivefold increased odds of having cognitive impairment (Table 3). This relationship remained stable when controlling for education. Importantly, no other factors significantly modified this between-group difference. In sensitivity analyses excluding participants with a history of high blood pressure, having T1D remained significantly associated with higher odds of cognitive impairment (odds ratio [OR] 4.35; P = 0.001), and no other factors significantly modified this relationship.
When comparing participants with T1D by level of cognitive impairment, we found no statistically significant differences by sex, age, T1D duration, age at T1D diagnosis, serum glucose at time of cognitive testing, estimated glomerular filtration rate, ApoE4 status, physical activity, blood pressure measures, or prevalence of coronary artery disease, cardiac autonomic neuropathy, or overt neuropathy measured 5 years earlier (Table 1). Compared with cognitively normal participants with T1D, those with mild or clinically relevant cognitive impairment had significantly fewer years of education, higher BMI, and worse long-term glycemic control; were more likely to have a 20-year prior average ABI >1.3; and were more likely to have prevalent distal symmetric polyneuropathy or proliferative retinopathy measured 5 years earlier (Table 1). Relationships remained similar when controlling for serum glucose or A1c at the time of neurocognitive testing.
Among participants with T1D, ordinal logistic regression models with level of cognitive impairment as the outcome showed that having a 14-year (1996–2013) average HbA1c ≥ 7.5% (58 mmol/mol) tripled the odds of cognitive impairment (Supplementary Table 1, model 1). Results were similar for prevalent proliferative retinopathy or distal symmetric polyneuropathy (Supplementary Table 1, models 2 and 3). Each unit increase in BMI was associated with a 10% increased odds of cognitive impairment (Supplementary Table 1, model 4). An average ABI >1.3/noncompressible quadrupled the odds of cognitive impairment (Supplementary Table 1, model 5). These relationships remained statistically significant after adjusting for years of education and the interval of time from the most recent EDC exam (2004–2006) to the time of neurocognitive testing after Bonferroni correction.
Conclusions
Unlike previous reports of mild/modest cognitive dysfunction in young adults with T1D (1,2), we detected clinically relevant cognitive impairment in 28% of our middle-aged participants with T1D. This prevalence rate in our T1D cohort is comparable to the prevalence of mild cognitive impairment typically reported among community-dwelling adults aged 85 years and older (29%) (20).
We know of only one prior study that investigated clinically relevant cognitive impairment in T1D, and this was done in a pediatric population (4). Overall, 12.8% of the pediatric participants with T1D met this early study’s definition of clinically relevant cognitive impairment, a rate less than half that observed in our middle-aged population with T1D (28%). Interestingly, rates observed in the participants who did not have T1D were similar (5–6%) in both studies. This suggests that, for people without T1D, the rate of cognitive impairment remains basically unchanged from childhood into middle age, whereas the rate more than doubles over the same time among people with childhood-onset T1D. Unlike the pediatric study, we found no effect of early versus late age at T1D diagnosis (age <6 or >6 years) on cognitive impairment. It is possible that during youth an earlier T1D onset contributes to worse brain function, but this effect becomes secondary to other factors as people with T1D age, prolonging their exposure to the deleterious effects of this metabolic disorder.
Our findings contradict results from two previous studies of older participants with T1D, which reported “mild disturbances in cognitive function” (21) and no between-group differences in the rate of cognitive decline (22). It is important to note differences between our study and these prior studies that may contribute to the disparate findings. Only 33% of the participants in the earlier studies were diagnosed during childhood, whereas 100% of our participants with T1D were diagnosed during childhood. Consequently, although younger, with a mean age of 49 years compared with 61 (21) and 65 years (22), our participants with T1D had a longer T1D duration (mean 41, 34, and 38 years, respectively). We postulate that the developing brain (i.e., during childhood) may be especially vulnerable to insults of glycemic dysregulation and fluctuating insulin concentrations compared with the adult brain. While mild cognitive differences can be detected early, more severe effects may become especially apparent later in life as these individuals grow older and experience the effects of normal age-related changes in brain function and structure in combination with the effects of long-term T1D and its comorbidities (e.g., hypertension, microvascular complications).
Our results motivate further study of longitudinal changes over time. While the DCCT/EDIC study found “no evidence of substantial long-term declines in cognitive function” over 18 years (23), many of the DCCT participants were diagnosed during adulthood (at DCCT baseline, mean age 27.0 years and mean duration of diabetes 5.7 years), unlike our cohort that includes only individuals diagnosed during childhood. In addition, the DCCT participants were young adults at their baseline neurocognitive exam and displayed relatively good metabolic control.
Having T1D was the only factor significantly associated with the between-group difference in clinically relevant cognitive impairment in our sample. Traditional risk factors for age-related cognitive impairment, in particular older age and high blood pressure (24), were not related to the between-group difference we observed. The design of our study does not allow us to determine whether these traditional risk factors contributed to the development and/or progression of cognitive impairment in these participants, only that these factors were not related to the degree of cognitive impairment already present in these participants at the time of cognitive testing.
Among participants with T1D, a 14-year average HbA1c ≥7.5% (58 mmol/mol), prevalent distal symmetric polyneuropathy, and proliferative retinopathy assessed 5 years earlier were related to cognitive impairment, with effect sizes larger than those reported in prior studies (21,25). Two other measures of glycemic control, HbA1c >7.5% (58 mmol/mol) at the time of cognitive testing and “25-year A1c months,” also were related to higher odds of having cognitive impairment. We chose to study the relationship between cognitive impairment and 14-year average A1c rather than “A1c months” because the latter is not widely understood; we chose 14-year average A1c rather than A1c at a single assessment concurrent with cognitive testing to assess the chronicity of hyperglycemia. All analyses were repeated, controlling separately for serum glucose then for A1c at the time of cognitive testing, to ensure glucose concentrations were not confounding study results.
Similar to previous studies of younger adults with T1D (14,26), we found no relationship between the number of severe hypoglycemic episodes and cognitive impairment. Rather, we found that chronic hyperglycemia, via its associated vascular and metabolic changes, may have triggered structural changes in the brain that disrupt normal cognitive function. Like others (14), we found no statistically significant relationships between cognitive impairment and ApoE4 status. Whereas earlier studies found relationships between cognitive dysfunction and age at T1D diagnosis (4,27–29), diabetes duration (29,30), and high blood pressure (21,29,30), these factors were not significantly correlated with cognitive impairment in our sample. This could be a result of our outcome of cognitive impairment compared with previous studies’ outcome of cognitive dysfunction. In addition, participants in those earlier studies were younger, on average, than our participants and may therefore have not yet developed cognitive impairment.
We also identified two less-studied factors related to cognitive impairment in our sample. First, we found that higher BMI was related with higher odds of cognitive impairment (OR 1.10; Table 3), similar to results reported by Brismar et al. (29) showing relationships between higher BMI and deficits in psychomotor speed as well as general intelligence in adults with T1D. While the exact mechanism relating BMI and cognitive function remains unclear, one plausible route is via an inflammatory pathway (31). In animal models of T1D, higher levels of circulating glucocorticoids and higher susceptibility to stress were associated with high BMI, increased brain inflammation, and reduced neurogenesis (32). Higher BMI could also indicate insulin resistance, which also is related to worse cognitive outcomes in older participants who are healthy or have type 2 diabetes (33). Future studies should further investigate the influence of BMI on cognitive function in young participants with T1D to determine whether this could be a risk factor suitable for early intervention.
Second, we found that participants with an average ABI >1.3/noncompressible had quadrupled odds of clinically relevant cognitive impairment. High ABI is a marker of subclinical vascular conditions including atherosclerosis, vessel stiffness, and calcification, all of which are known risk factors for cognitive impairment in older adults without T1D (34,35). Peripheral vascular stiffness, as a result of endothelial dysfunction, may serve as an indicator of early changes to brain vasculature and structure (36). As an example, hepatic hypoperfusion resulting from endothelial dysfunction leads to “increased adhesion of circulating inflammatory cells to the endothelium, activation of the coagulation cascade and constriction of the microvasculature” (37). Cerebral hypoperfusion likely exerts similar effects on the brain’s vasculature; diffuse subclinical ischemia and resultant damage to oligodendrocytes and focal necrosis in gray and white matter offer a mechanistic explanation for the relationship with cognitive impairment. While the applicability of this finding is limited because of the small sample size, this novel potential risk factor deserves further investigation in participants with T1D.
Performance on several neurocognitive tests used in this study (e.g., DSST, GP, ROCF-copy) depend on visual acuity and motor capabilities, skills that are compromised in participants with T1D with proliferative retinopathy and distal symmetric polyneuropathy. While these complications may contribute to poor performance on these tasks, psychomotor speed and executive function appear to be affected even in pediatric populations with T1D, well before such microvascular complications occur (3,4). Furthermore, performance on several tasks requiring the same degree of visual acuity and fine motor skills (e.g., the ROCF delayed task) did not differ by cognitive status among participants with T1D, suggesting that prevalent retinopathy and/or polyneuropathy alone do not explain the cognitive impairment observed in these individuals. Rather, it suggests that long-term T1D causes microvascular damage in the brain similar to that known to affect the eyes and nerves, thereby explaining why participants with proliferative retinopathy and distal symmetric polyneuropathy were more likely to meet the study definition of clinically relevant cognitive impairment.
The number of participants with T1D completing each neurocognitive task varied (see Supplementary Fig. 2), and this is a limitation of this study. However, the completers did not significantly differ from the noncompleters by retinopathy, polyneuropathy, 14-year average A1c > 7.5%, ABI > 1.3, BMI, age at cognitive testing, or T1D duration. Overall, incompleteness was due to time constraints, that is, insufficient time to complete all tests within the 90 min allotted: 12 participants arrived at the cognitive testing center ≥10 min late, and another 6 were hypoglycemic, requiring at least 15–30 min for serum glucose concentrations to normalize before initiating cognitive testing. Lack of time affected completion of the final tasks (FAS, VF Animals, Stroop, GP); we do not believe this was dependent on cognitive status.
Our study results may not be entirely generalizable to all adults with T1D. First, we included only participants with childhood-onset diabetes; the cognitive effects of T1D on participants diagnosed during adulthood may differ from our participants who were exposed to chronic hyperglycemia in childhood, during crucial stages of brain and cognitive functioning development (38). In addition, 99% of our participants are Caucasian, whereas 93% of people diagnosed with T1D in Allegheny County, Pennsylvania, from 1965–1979 were Caucasian (39). A survivor bias may be present in that these participants have survived with T1D for a substantial period of time. A selection bias is also possible; those participating in this cognitive study are, in general, healthier than the parent EDC cohort, especially since the eligibility criteria for participation in the cognitive study included being able to undergo brain imaging. Many causes of mortality and morbidity that prevented participation in this study are related to cognitive impairment, potentially underestimating its true prevalence in T1D. Because of the study design, we cannot determine direction of the relationships between cognitive impairment and factors assessed concurrently, such as A1c on the day of cognitive testing; it could be that those with worse cognitive impairment are less able to manage their diabetes, leading to higher A1c concentrations. The study design also prohibits determination of the life stage at which clinically relevant cognitive impairment first developed in our participants.
Strengths of this study include the use of a well-defined cohort with over 20 years of data, thus allowing computation of long-term trajectories of risk factor profiles, and the use of an extensive neuropsychological test battery to assess multiple cognitive domains. The cognitive impairment algorithm has been used in previous studies (13), is easily replicable, and could be adapted by others to improve comparability across studies.
In summation, results of this study show that a large percentage of middle-aged adults with childhood-onset T1D are currently living with clinically relevant cognitive impairment. The effects of cognitive impairment on these individuals—diabetes self-management, health service utilization, disability, and quality-of-life issues—deserve further investigation. Future studies with larger sample sizes and a wider range of ages at diagnosis are needed to improve our understanding of the development and progression of T1D-related cognitive impairment. Such studies should use repeated neurocognitive testing, beginning shortly after the time of diagnosis. Furthermore, incorporating repeated neuroimaging with extensive measures of metabolic control and vascular health would help to clarify the mechanisms contributing to the development of cognitive impairment in these individuals.
Supplementary Material
Article Information
Funding. C.R. is the principal investigator of the neurocognitive study of participants with T1D, National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK089028. T.J.O. is the principal investigator of the T1D cohort/EDC Study, National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK034818-25, which provided data on population characteristics for the participants with T1D. J.R.J. is the principal investigator of the MR Hyper Study, National Heart, Lung, and Blood Institute grant RO1 101959-05, which provided neurocognitive and other data on participants without T1D.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.A.N. and C.R. analyzed the data, interpreted the results, and wrote the manuscript. C.M.R. provided neurocognitive test batteries to both cohorts. C.M.R. and J.A.S. oversaw cognitive impairment scoring, interpretation of cognitive test results, and algorithms to determine degree of cognitive impairment and developed the manuscript. C.M.R., J.R.J., H.J.A., J.C.Z., T.C., T.J.O., and J.A.S. reviewed and edited the manuscript. R.M.B. and R.M. provided guidance regarding statistical analyses. C.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Prior Presentation. Portions of the cognitive test findings were presented as an abstract and oral presentation at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0041/-/DC1.
References
- 1.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol 2008;7:184–190 [DOI] [PubMed] [Google Scholar]
- 2.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 2005;28:726–735 [DOI] [PubMed] [Google Scholar]
- 3.Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care 2008;31:1892–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 1985;75:921–927 [PubMed] [Google Scholar]
- 5.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007;64:1734–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004;15:1904–1911 [DOI] [PubMed] [Google Scholar]
- 7.Arlt S, Lindner R, Rösler A, von Renteln-Kruse W. Adherence to medication in patients with dementia: predictors and strategies for improvement. Drugs Aging 2008;25:1033–1047 [DOI] [PubMed] [Google Scholar]
- 8.Zhu CW, Sano M, Ferris SH, Whitehouse PJ, Patterson MB, Aisen PS. Health-related resource use and costs in elderly adults with and without mild cognitive impairment. J Am Geriatr Soc 2013;61:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royall DR, Palmer R, Chiodo LK, Polk MJ. Executive control mediates memory’s association with change in instrumental activities of daily living: the Freedom House Study. J Am Geriatr Soc 2005;53:11–17 [DOI] [PubMed] [Google Scholar]
- 10.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, Oxford University Press, 2006 [Google Scholar]
- 11.Ardila A. Normal aging increases cognitive heterogeneity: analysis of dispersion in WAIS-III scores across age. Arch Clin Neuropsychol 2007;22:1003–1011 [DOI] [PubMed] [Google Scholar]
- 12.Dore GA, Elias MF, Robbins MA, Elias PK, Brennan SL. Cognitive performance and age: norms from the Maine-Syracuse Study. Exp Aging Res 2007;33:205–271 [DOI] [PubMed] [Google Scholar]
- 13.Saxton J, Snitz BE, Lopez OL, et al.; GEM Study Investigators . Functional and cognitive criteria produce different rates of mild cognitive impairment and conversion to dementia. J Neurol Neurosurg Psychiatry 2009;80:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson AM, Ryan CM, Cleary PA, et al.; Diabetes Control and Complications Trial/EDIC Research Group . Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia 2011;54:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 16.Orchard TJ, Forrest KY, Ellis D, Becker DJ. Cumulative glycemic exposure and microvascular complications in insulin-dependent diabetes mellitus. The glycemic threshold revisited. Arch Intern Med 1997;157:1851–1856 [PubMed] [Google Scholar]
- 17.Ix JH, Miller RG, Criqui MH, Orchard TJ. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J Vasc Surg 2012;56:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekikawa A, Shin C, Curb JD, et al. Aortic stiffness and calcification in men in a population-based international study. Atherosclerosis 2012;222:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ, Lawrence Earlbaum Associates, 1988 [Google Scholar]
- 20.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003;60:1385–1389 [DOI] [PubMed] [Google Scholar]
- 21.Brands AMA, Kessels RPC, Hoogma RPLM, et al. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes 2006;55:1800–1806 [DOI] [PubMed] [Google Scholar]
- 22.Duinkerken Ev, Brands AMA, van den Berg E, Henselmans JM, Hoogma RP, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group . Cognition in older patients with type 1 diabetes mellitus: a longitudinal study. J Am Geriatr Soc 2011;59:563–565 [DOI] [PubMed] [Google Scholar]
- 23.Jacobson AM, Musen G, Ryan CM, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension 2014;63:894–903 [DOI] [PubMed] [Google Scholar]
- 25.Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156 [DOI] [PubMed] [Google Scholar]
- 26.Ryan CM, Williams TM, Finegold DN, Orchard TJ. Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: effects of recurrent hypoglycaemia and other chronic complications. Diabetologia 1993;36:329–334 [DOI] [PubMed] [Google Scholar]
- 27.Ferguson SC, Blane A, Wardlaw J, et al. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 2005;28:1431–1437 [DOI] [PubMed] [Google Scholar]
- 28.Northam EA, Rankins D, Lin A, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care 2009;32:445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brismar T, Maurex L, Cooray G, et al. Predictors of cognitive impairment in type 1 diabetes. Psychoneuroendocrinology 2007;32:1041–1051 [DOI] [PubMed] [Google Scholar]
- 30.Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia 2003;46:940–948 [DOI] [PubMed] [Google Scholar]
- 31.Das UN. Is obesity an inflammatory condition? Nutrition 2001;17:953–966 [DOI] [PubMed] [Google Scholar]
- 32.Beauquis J, Homo-Delarche F, Revsin Y, De Nicola AF, Saravia F. Brain alterations in autoimmune and pharmacological models of diabetes mellitus: focus on hypothalamic-pituitary-adrenocortical axis disturbances. Neuroimmunomodulation 2008;15:61–67 [DOI] [PubMed] [Google Scholar]
- 33.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol 2012;32:2060–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelick PB, Scuteri A, Black SE, et al.; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011;42:2672–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J Alzheimers Dis 2012;32:541–549 [DOI] [PubMed] [Google Scholar]
- 36.Rosano C, Watson N, Chang Y, et al. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension 2013;61:160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Golen RF, van Gulik TM, Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med 2012;52:1382–1402 [DOI] [PubMed] [Google Scholar]
- 38.Ryan CM. Searching for the origin of brain dysfunction in diabetic children: going back to the beginning. Pediatr Diabetes 2008;9:527–530 [DOI] [PubMed] [Google Scholar]
- 39.Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, Orchard TJ. Mortality trends in type 1 diabetes. The Allegheny County (Pennsylvania) Registry 1965-1999. Diabetes Care 2001;24:823–827 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.