Abstract
OBJECTIVE
Sodium–glucose cotransporter 2 (SGLT2) inhibitors cause substantially less weight loss than expected from the energy excreted via glycosuria. Our aim was to analyze this phenomenon quantitatively.
RESEARCH DESIGN AND METHODS
Eighty-six patients with type 2 diabetes (HbA1c 7.8 ± 0.8% [62 ± 9 mmol/mol], estimated glomerular filtration rate [eGFR] 89 ± 19 mL ⋅ min−1 ⋅ 1.73 m−2) received empagliflozin (25 mg/day) for 90 weeks with frequent (n = 11) assessments of body weight, eGFR, and fasting plasma glucose (FPG). Time-dependent glucose filtration was calculated as the product of eGFR and FPG; time-dependent glycosuria was estimated from previous direct measurements. The relation of calorie-to-weight changes was estimated using a mathematical model of human energy metabolism that simulates the time course of weight change for a given change in calorie balance and calculates the corresponding energy intake changes.
RESULTS
At week 90, weight loss averaged −3.2 ± 4.2 kg (corresponding to a median calorie deficit of 51 kcal/day [interquartile range (IQR) 112]). However, the observed calorie loss through glycosuria (206 kcal/day [IQR 90]) was predicted to result in a weight loss of –11.3 ± 3.1 kg, assuming no compensatory changes in energy intake. Thus, patients lost only 29 ± 41% of the weight loss predicted by their glycosuria; the model indicated that this difference was accounted for by a 13% (IQR 12) increase in calorie intake (269 kcal/day [IQR 258]) coupled with a 2% (IQR 5) increase in daily energy expenditure (due to diet-induced thermogenesis). This increased calorie intake was inversely related to baseline BMI (partial r = −0.34, P < 0.01) and positively to baseline eGFR (partial r = 0.29, P < 0.01).
CONCLUSIONS
Chronic glycosuria elicits an adaptive increase in energy intake. Combining SGLT2 inhibition with caloric restriction is expected to be associated with major weight loss.
Introduction
Glycemic control is still the mainstay of type 2 diabetes (T2DM) management, as a level of HbA1c of 7% or below has been associated with a reduction in both microvascular and macrovascular complications (1). Despite the wide range of antihyperglycemic drugs available, current therapeutic strategies fall short of optimal glycemic control, especially in patients with long-standing T2DM with complications and/or multiple cardiovascular disease risk factors (2,3). In addition, current therapies have limiting side effects, such as weight gain, hypoglycemia, fluid retention, and gastrointestinal side effects (1). Thus, the search for therapeutic agents with a new mechanism of action continues, and several novel drugs are on the horizon.
The role of the kidney in glucose homeostasis has recently gained renewed attention. In severely hyperglycemic patients, the kidneys play a protective role by excreting the excess filtered glucose load. Sodium–glucose cotransporter 2 (SGLT2), a low-affinity, high-capacity transporter located on the brush-border of cells in the S1-S2 segment of the proximal convoluted tubule, is the most important mediator of glucose reabsorption from the glomerular filtrate (4). Several compounds acting as SGLT2 inhibitors have been developed, and some of them are already available for clinical use as oral agents (5–10). A number of clinical trials (11–25) have shown that blocking renal glucose reabsorption leads to a reduction in fasting plasma glucose (FPG) and HbA1c through a generalized relief of glucose toxicity that does not depend on insulin action or secretion. Furthermore, SGLT2 inhibitors have proven to be generally safe and well tolerated, and they have additional clinical benefits, such as blood pressure reduction, due to their mild diuretic effect, and a decrease in body weight, caused by the attendant chronic calorie loss (26).
However, in virtually all clinical trials, the observed weight loss has been consistently less than expected from the amount of urinary glucose excretion (25,26). Therefore, our aim was to describe and quantify the effects of SGLT2 inhibition on body weight, energy balance, and body composition in T2DM patients on stable therapy with empagliflozin, a highly selective SGLT2 inhibitor.
Research Design and Methods
Patients
We analyzed data from a per-protocol completer cohort of 86 patients with T2DM (39 women and 47 men; age 58 ± 9 years, BMI 29.8 ± 4.5 kg/m2, HbA1c 7.8 ± 0.8% [62 ± 9 mmol/mol], mean ± SD) who received empagliflozin (25 mg/day) for 90 weeks; these patients were one arm of a phase IIb 78-week extension study of two 12-week phase II trials (monotherapy and add-on to metformin) (27). Forty-six of the patients (20 women and 26 men) were drug-naïve and the other 40 patients (19 women and 21 men) were on >1.5 g/day or maximally tolerated doses of metformin. In each patient, body weight, FPG, and serum creatinine concentration were measured at timed intervals between baseline and 90 weeks (week 4, 8, 12, 18, 30, 42, 54, 66, 78, and 90; body weight not recorded at weeks 4 and 8).
Data Analysis
Estimated glomerular filtration rate (eGFR) was calculated by the MDRD formula at each time point. Filtered glucose was calculated as the product of eGFR and FPG. The time course of glycosuria was calculated as the product of glucose filtration rate and fractional glucose excretion. The latter was estimated from the relationship between plasma glucose level and fractional glucose excretion derived from previous studies in which glycosuria was measured directly in T2DM patients treated with empagliflozin (25 mg/day) (28).
A validated mathematical model (http://bwsimulator.niddk.nih.gov) simulating the weight loss time course for a given change in calorie balance was used to estimate the relation of calorie to weight changes (29). For each patient, the simulator used sex, age, height, estimated physical activity level, and weight at baseline as model inputs. Model outputs include total daily energy expenditure (TDEE) and body composition (fat mass and lean body mass) at baseline and 90 weeks and the time course of energy balance (intake/expenditure). The original model was modified to incorporate the effect of glycosuria. The simulator predicted the weight changes expected for a given calorie loss caused by glycosuria assuming no compensatory changes in calorie intake. The same mathematical model was then used to calculate the energy intake changes that must have taken place to result in the observed weight change (30). This model-based method has recently been validated to provide accurate calculations of energy intake changes in free-living people during a 2-year lifestyle intervention as compared with energy intake changes measured using repeated assessments of body composition and energy expenditure by doubly labeled water (31).
Statistical Analysis
Data are given as mean ± SD (or median [interquartile range (IQR)] for data with nonnormal distribution). Group values were compared by Mann-Whitney U test, and paired group values were compared by Wilcoxon signed rank test. Treatment-related changes of continuous variables were tested by two-way ANOVA for repeated measures with patient group (drug naïve or metformin treated) as a fixed effect. Multivariate regression analysis was carried out by standard methods. P values ≤0.05 were considered statistically significant.
Results
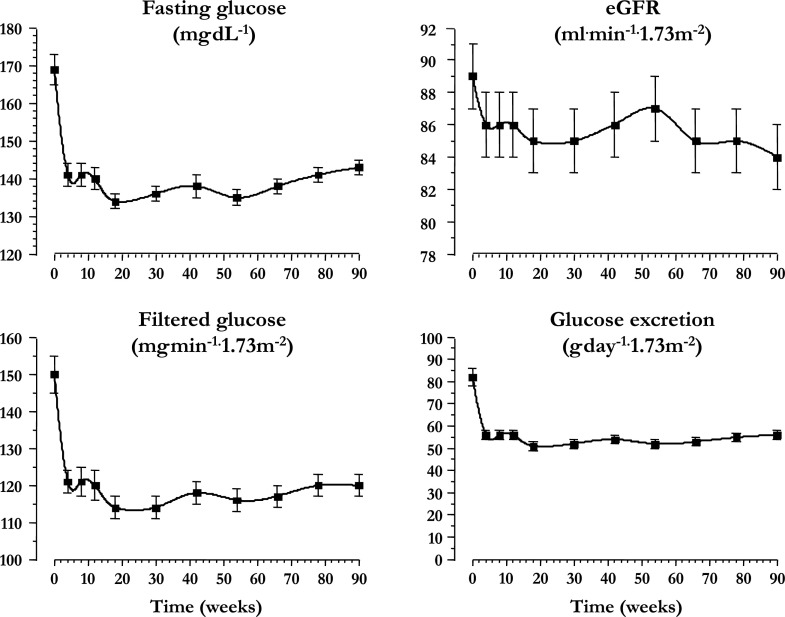
The clinical data at baseline and week 90 are shown in Table 1. As expected, HbA1c improved with treatment (by 0.56 ± 0.86 and 0.81 ± 0.93% in women and men, respectively, P = 0.19 for the sex difference). FPG and eGFR both decreased significantly during the 90 weeks of treatment. Such decreases were sharper during the first 4 weeks and remained essentially stable thereafter; both calculated filtered glucose and glucose excretion followed the same time course (Fig. 1). Over the 90 weeks of drug administration, glycosuria was calculated to be 51 g/day (IQR 26) in women and 52 g/day (IQR 18) in men (P = 0.57). Thus, in the whole cohort, urinary energy loss averaged 206 kcal/day (IQR 90). Neither LDL-cholesterol nor serum triglycerides changed significantly, whereas HDL-cholesterol levels were significantly higher at week 90 compared with baseline (Table 1).
Table 1.
Clinical characteristics at baseline and end of study
| Baseline | 90th week | P# | |
|---|---|---|---|
| n (women/men) | 86 (39/47) | — | — |
| Age (years) | 58 ± 9 | — | — |
| Height (cm) | 169 ± 11 | — | — |
| Body weight (kg) | 85.9 ± 18.2 | 82.7 ± 17.3 | <0.0001 |
| BMI (kg/m2) | 29.8 ± 4.5 | 28.7 ± 4.1 | <0.0001 |
| HbA1c (%) [mmol/mol] | 7.8 ± 0.8 [62 ± 9] | 7.1 ± 0.7 [54 ± 8] | <0.0001 |
| LDL-cholesterol (mg/dL) | 111 ± 32 | 117 ± 43 | NS |
| HDL-cholesterol (mg/dL) | 48 ± 11 | 55 ± 14 | <0.001 |
| Triglycerides (mg/dL) | 194 ± 175 | 181 ± 89 | NS |
| eGFR (mL · min−1 · 1.73 m−2) | 89 ± 19 | 84 ± 18 | 0.0003 |
| FPG (mg/dL) | 169 ± 41 | 143 ± 22 | <0.0001 |
| Fat-free mass (kg) | 55.3 ± 12.5 | 54.4 ± 12.4 | <0.0001 |
| Fat mass (kg) | 30.6 ± 10.2 | 28.4 ± 9.2 | <0.0001 |
| TDEE (kcal/day) | 2,139 [693] | 2,192 [652] | 0.0037 |
Data are mean ± SD or median [IQR].
#Wilcoxon signed rank test.
Figure 1.
Time course of FPG concentrations, eGFR, renal glucose filtration rate, and urinary glucose excretion rate in 86 patients with T2DM treated with empagliflozin (25 mg/day) for 90 weeks. Plots are mean ± SEM.
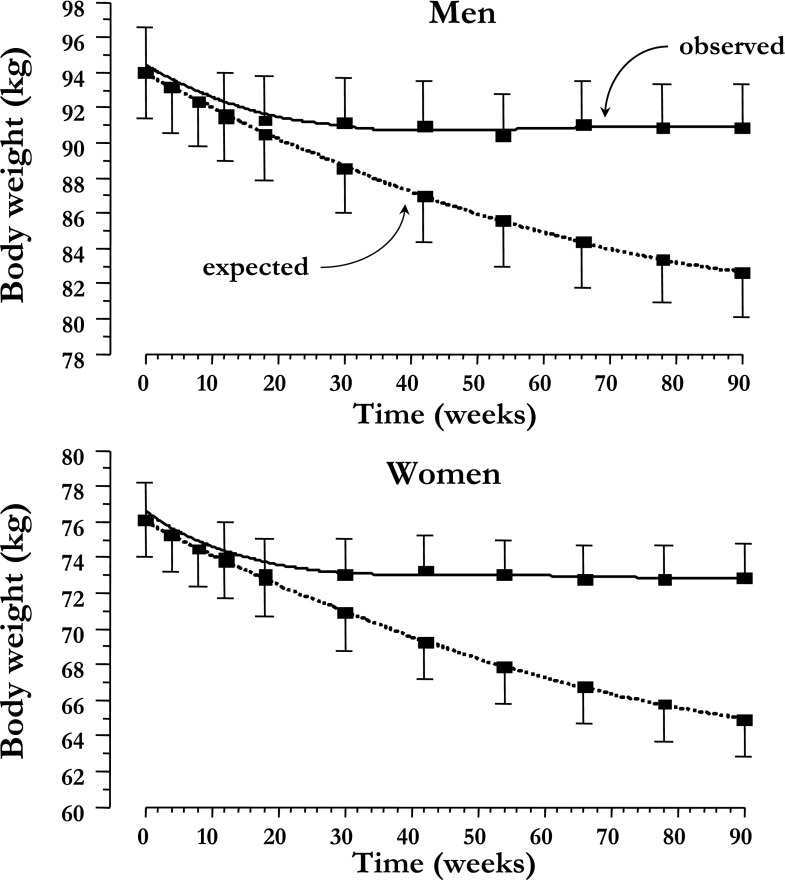
At week 90, observed weight loss averaged −3.2 ± 3.6 kg (range −17.0 to +1.3) in women and −3.1 ± 4.6 kg (range −16 to +5.5) in men (P = 0.99 for the sex difference); in both groups, body weight was stable after the initial ∼24 weeks of treatment (Fig. 2). This weight change was estimated to consist of one-third fat-free mass loss and two-thirds fat mass loss (Table 1) and was positively related to the change in HbA1c (Supplementary Fig. 1).
Figure 2.
Time course of observed and predicted (by glycosuria) weight loss in men and women with T2DM treated with empagliflozin (25 mg/day) for 90 weeks. Plots are mean ± SEM.
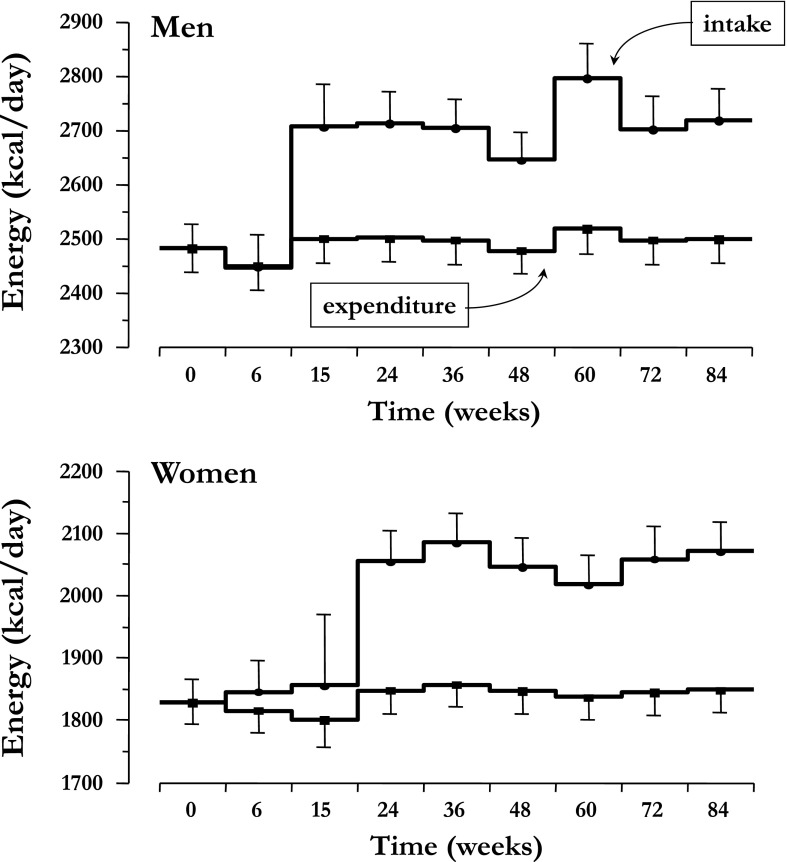
When accounting for glycosuria but assuming no changes in energy intake, the model-predicted weight loss was much larger than was observed, averaging −11.1 ± 3.2 kg in women and −11.4 ± 3.0 kg in men (P = 0.74 for the sex difference). As depicted in Fig. 2, observed and predicted weight loss began to diverge at ∼24 weeks, when observed body weight stabilized while expected weight continued to fall. Therefore, energy intake must have increased to explain the attenuation of weight loss. The model-calculated time course of energy intake and expenditure corresponding to the observed weight changes are shown in Fig. 3: whereas TDEE was slightly, by 31 kcal/day (IQR 91; P = 0.03), but significantly increased over the final 6-week period, energy intake increased markedly, especially after the first ∼10–20 weeks of treatment. Over the last 6 weeks, energy intake exceeded baseline energy intake by 269 kcal/day (IQR 258). On the other hand, if the same calorie loss had been achieved by reduced calorie intake instead of glycosuria, TDEE would have decreased steadily over the 90 weeks, to reach a level ∼100 kcal/day below baseline (Supplementary Fig. 2).
Figure 3.
Calculated rates of energy intake and TDEE in men and women with T2DM treated with empagliflozin (25 mg/day) for 90 weeks. Plots are mean ± SEM averaged over the indicated time intervals.
When searching for individual determinants of the difference between observed and expected energy expenditure (or weight loss), we found that both baseline BMI and eGFR had an independent influence. Thus, in multivariate analysis, the difference between expected and observed weight loss was higher in leaner patients and in individuals with a higher eGFR (Supplementary Fig. 3).
Finally, we analyzed the difference between patients on metformin and drug-naïve patients. Baseline clinical characteristics (including body weight) and treatment-induced changes in HbA1c and FPG levels were not significantly different between these groups (data not shown). In contrast, observed weight loss was significantly greater in metformin-treated (−4.4 ± 4.4 kg) than drug-naïve patients (−2.0 ± 3.6 kg), both in men and women (P = 0.024 after adjusting for sex and baseline body weight).
Conclusions
The current results with empagliflozin confirm the benefits of SGLT2 inhibition in patients with T2DM shown in previous studies (meta-analyzed in Vasilakou et al. [26]). In our patient cohort (rather typical for age, BMI, and glycemic control), 90 weeks of therapy led to a significant and sustained decrease in HbA1c and FPG. The initial decrease in eGFR, which averaged 4 ± 10 mL ⋅ min−1 ⋅ 1.73 m−2 and did not differ by sex or previous treatment, is likely a hemodynamic effect that has been observed with other members of this class of agents (26) and has been shown to be fully reversible upon interrupting the treatment (32,33).
With regard to body weight changes, the reduction seen in these patients is in line with the findings of virtually all trials using SGLT2 inhibitors: a rapid decrement, typically averaging 2–3 kg and made up by roughly two-thirds of fat tissue, which is maintained as long as treatment is continued (Fig. 1). Using the mathematical model, one can calculate that the observed weight loss corresponds to a daily energy deficit of 51 kcal (IQR 112), which is much less than the estimated daily calorie loss through glycosuria (206 kcal [IQR 90]). Even a conservative estimate of glycosuria such as used here (based on fasting rather than day-long plasma glucose concentrations) predicts a 2.7-fold larger weight loss (∼11 kg). As previous studies have shown that acute or chronic (4 weeks) empagliflozin treatment does not alter resting or postprandial energy expenditure as measured by indirect calorimetry (28), the discrepancy between observed and expected weight loss implies that energy intake is increased. In fact, after about 10 weeks of treatment, the model predicts a 13% (IQR 12) increase in energy intake, which is maintained throughout (Fig. 3). Because an increase in water (34) and metabolizable calorie consumption leads to an increase in energy usage (i.e., diet-induced thermogenesis, associated with nutrient processing and absorption [29]), TDEE is slightly augmented (by 2% [IQR 5]) relative to baseline. It is interesting to reflect that if the same amount of energy deficit had been achieved with dietary restriction, energy expenditure would have decreased substantially (Supplementary Fig. 2). A similar discrepancy between expected and observed weight loss has been reported in preliminary form by Polidori et al. (35) using the same model as in the current study in patients with T2DM on treatment with canagliflozin. An increase in calorie consumption has also been found in patients with malabsorption due to Crohn disease (36).
This adaptive response to glycosuria was accentuated in association with lower BMI and higher eGFR (Supplementary Fig. 3). Conceivably, leaner patients were less concerned about their weight and tended to overcompensate, and in patients with preserved renal function, SGLT2 inhibition led to greater urinary glucose excretion. In addition, in patients on chronic metformin therapy, who lost significantly more weight than drug-naïve patients, the increase in energy intake tended to be lower (228 kcal/day [IQR 267] vs. 291 kcal/day [IQR 226], P = 0.07). These findings indicate that marked, consistent energy loss through the urine triggers an anabolic response by which enhanced appetite and, possibly, carbohydrate craving partially offset glycosuria and defend body weight (a basic phenomenon in humans [37]). In support of this interpretation, a study in rats treated with dapagliflozin showed that weight loss was four times larger when animals were pair-fed as compared with ad libitum diet (38). Furthermore, a recent study in patients with type 1 diabetes treated with empagliflozin for 8 weeks (39) documented an increased consumption of carbohydrates, averaging ∼50 g/day, a figure very close to the estimated increase in energy intake of the present cohort. Finally, the intrinsic restraining effect of metformin on appetite (40) likely accounts for the observed differences in weight loss and energy intake from our drug-naïve patients.
Further investigation is needed to better understand the mechanisms whereby glycosuria signals to the central nervous system to alter appetite regulation. From the clinical standpoint, patients with T2DM could benefit more from SGLT2 treatment in terms of weight loss and glycemic control if more stringent dietary recommendations were implemented to curb appetite and overeating.
Supplementary Material
Article Information
Funding. This work was supported by a grant from the Italian Ministry of Education, University and Research (MIUR 2010329EKE). A.S. and K.D.H. were supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. The clinical trial that provided the data for this study was funded by Boehringer Ingelheim and Eli Lilly and Company. T.H. and S.C. are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. E.F. has received research grant support from Boehringer Ingelheim and Eli Lilly and Company and does consulting work for Boehringer Ingelheim, Eli Lilly and Company, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Takeda, Medtronic, and Intarcia. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.F. was involved in the acquisition and analysis of data and wrote the first draft of the manuscript. T.H. designed the study. S.C. was involved in the statistical analysis. A.S. and K.D.H. performed the model analysis. E.F. designed the study. All authors reviewed and edited the manuscript. E.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00881530, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0355/-/DC1.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 4.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794 [DOI] [PubMed] [Google Scholar]
- 5.Plosker GL. Dapagliflozin: a review of its use in type 2 diabetes mellitus. Drugs 2012;72:2289–2312 [DOI] [PubMed] [Google Scholar]
- 6.Paisley AN, Yadav R, Younis N, Rao-Balakrishna P, Soran H. Dapagliflozin: a review on efficacy, clinical effectiveness and safety [published correction appears in Expert Opin Investig Drugs 2013;22:939]. Expert Opin Investig Drugs 2013;22:131–140 [DOI] [PubMed] [Google Scholar]
- 7.Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol 2013;53:601–610 [DOI] [PubMed] [Google Scholar]
- 8.Nagata T, Fukazawa M, Honda K, et al. Selective SGLT2 inhibition by tofogliflozin reduces renal glucose reabsorption under hyperglycemic but not under hypo- or euglycemic conditions in rats. Am J Physiol Endocrinol Metab 2013;304:E414–E423 [DOI] [PubMed] [Google Scholar]
- 9.Tahara A, Kurosaki E, Yokono M, et al. Antidiabetic effects of SGLT2-selective inhibitor ipragliflozin in streptozotocin-nicotinamide-induced mildly diabetic mice. J Pharmacol Sci 2012;120:36–44 [DOI] [PubMed] [Google Scholar]
- 10.Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:613–621 [DOI] [PubMed] [Google Scholar]
- 11.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:2223–2233 [DOI] [PubMed] [Google Scholar]
- 13.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 2011;13:928–938 [DOI] [PubMed] [Google Scholar]
- 14.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012;35:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilding JP, Woo V, Soler NG, et al.; Dapagliflozin 006 Study Group . Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 2012;156:405–415 [DOI] [PubMed] [Google Scholar]
- 16.Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract 2012;66:446–456 [DOI] [PubMed] [Google Scholar]
- 17.Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 2011;34:2015–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract 2013;67:1267–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941–950 [DOI] [PubMed] [Google Scholar]
- 21.Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 2014;16:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med 2013;125:181–189 [DOI] [PubMed] [Google Scholar]
- 24.Rosenstock J, Aggarwal N, Polidori D, et al.; Canagliflozin DIA 2001 Study Group . Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–1031 [DOI] [PubMed] [Google Scholar]
- 26.Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–274 [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 2013;36:4015–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall KD, Sacks G, Chandramohan D, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall KD, Chow CC. Estimating changes in free-living energy intake and its confidence interval. Am J Clin Nutr 2011;94:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanghvi A, Redman LM, Martin CK, Ravussin E, Hall KD. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am J Clin Nutr. 3 June 2015 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012;8:495–502 [DOI] [PubMed] [Google Scholar]
- 33.Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 2014;63:1228–1237 [DOI] [PubMed] [Google Scholar]
- 34.Boschmann M, Steiniger J, Hille U, et al. Water-induced thermogenesis. J Clin Endocrinol Metab 2003;88:6015–6019 [DOI] [PubMed] [Google Scholar]
- 35.Polidori D, Sanghvi A, Seeley R, Hall K. Predicted temporal changes in energy intake and energy expenditure in subjects with type 2 diabetes treated with canagliflozin. Diabetologia 2014;57(Suppl. 1):S331 [Google Scholar]
- 36.Mayberry JF, Rhodes J, Allan R, et al. Diet in Crohn’s disease two studies of current and previous habits in newly diagnosed patients. Dig Dis Sci 1981;26:444–448 [DOI] [PubMed] [Google Scholar]
- 37.Ferrannini E, Rosenbaum M, Leibel RL. The threshold shift paradigm of obesity: evidence from surgically induced weight loss. Am J Clin Nutr 2014;100:996–1002 [DOI] [PubMed] [Google Scholar]
- 38.Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring) 2012;20:1645–1652 [DOI] [PubMed] [Google Scholar]
- 39.Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480–1483 [DOI] [PubMed] [Google Scholar]
- 40.Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res 1998;6:47–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.