Abstract
Background:
The present investigation was aimed to improve the inflammatory factors and lipoproteins concentration in patients with myocardial infarction (MI) by supplementation with coenzyme Q10 (CoQ10).
Methods:
In a double-blind, placebo-controlled study, we measured serum concentrations of one soluble cell adhesion molecules (intercellular adhesion molecule-1 [ICAM-1]), serum concentration of intereukin-6 (IL-6) and lipid profiles (high-density lipoprotein-cholesterol [HDL-C], low-density lipoprotein-cholesterol [LDL-C], total cholesterol and triglyceride [TG]) in CoQ10 supplementation group (n = 26) compared with placebo group (n = 26) in hyperlipidemic patients with MI. Fifty-two patients were randomized to receive 200 mg/day of CoQ10 or placebo for 12 weeks.
Results:
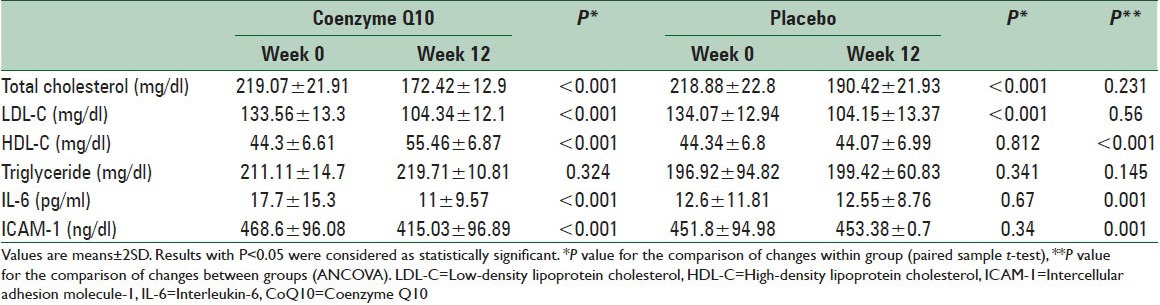
There were no significant differences for serum LDL-C, total cholesterol, and TG between two mentioned groups after the intervention. A significant enhancement in serum HDL-C level was observed between groups after the intervention (55.46 ± 6.87 and 44.07 ± 6.99 mg/dl in CoQ10 and placebo groups, respectively P < 0.001). Concentrations of ICAM-1 (415.03 ± 96.89 and 453.38 ± 0.7 ng/dl CoQ10 and placebo groups, respectively, P = 0.001) and IL-6 (11 ± 9.57 and 12.55 ± 8.76 pg/ml CoQ10 and placebo groups, respectively P = 0.001) in serum were significantly decreased in CoQ10 group.
Conclusions:
Supplementation with CoQ10 in hyperlipidemic patients with MI that have statin therapy has beneficial effects on their aspects of health.
Keywords: Coenzyme Q10, intercellular adhesion molecule-1, intereukin-6, lipoprotein, myocardial Infarction
INTRODUCTION
Myocardial infarction (MI) is a major cause of death and disability worldwide. An MI may be the first indicator of coronary artery disease (CAD), or it may occur, recurrently, in patients with established disease.[1]
Cardiovascular disease is the leading cause of death universal.[2] Hyperlipidemia is a major risk factor for CAD. A higher level of low-density lipoprotein-cholesterol (LDL-C) can increase the incidence of CAD.[3,4]
Coenzyme Q10 (CoQ10) (also called ubiquinone) is a lipid-soluble benzoquinone that has 10 isoprenyl units in its side chain and is a key component of the mitochondrial respiratory chain for adenosine triphosphate synthesis.[5,6] Statins can decrease the synthesis of cholesterol and other molecules downstream of mevalonate. Mevalonate is a precursor of CoQ10. Statins not only lower the blood cholesterol but also lower the level of CoQ10.[7] Higher levels of oxidative stress and inflammation play a role in the development of CAD.[8,9] CoQ10 is an intracellular antioxidant that protects the membrane phospholipids, mitochondrial membrane protein, and LDL-C from free radical-induced oxidative damage.[10]
Coenzyme Q10 is commonly used for the treatment of cardiomyopathy, and there is substantial evidence that heart function is developed upon administration of the lipid.[11]
Furthermore, CoQ10 decreases the production of proinflammatory cytokines, as well as blood viscosity,[12] demonstrated to be helpful in patients with heart failure and CAD. Different studies have highlighted the beneficial effects of CoQ10 supplementation in a variety of clinical conditions with emphasis on cardiovascular disease.[12,13]
Intereukin-6 (IL-6) is a powerful inducer of the hepatic acute phase response. Elevated concentrations of acute phase reactants, such as C-reactive protein (CRP), are found in patients with acute coronary syndromes, and predict future risk in apparently healthy subjects. The acute phase reaction is associated with elevated levels of fibrinogen, a strong risk factor for coronary heart disease, with autocrine and paracrine activation of monocytes by IL-6 in the vessel wall contributing to the deposition of fibrinogen. The acute phase response is accompanying to increased blood viscosity, platelet number, and activity.[14]
Intercellular adhesion molecules (ICAMs) are structurally related transmembrane glycol proteins of the immunoglobulin supergene family and are ligands for the α2 integrin molecules present on leukocytes.[15,16] Of the five ICAMs identified, ICAM-1 is the most extensively studied.[17,18] ICAM-1 specifically contributes in trafficking of inflammatory cells, in leukocyte effect or functions, in adhesion of antigen-presenting cells to T lymphocytes, in microbial pathogenesis, and in signal transduction pathways via outside-in signaling proceedings.[19] This adhesion molecule is localized to both the apical and basolateral surface of endothelial cells, making it ideally positioned to facilitate transendothelial migration of leukocytes.[15]
The purpose of this study was to investigate the effect of CoQ10 supplementation (200 mg/day) on serum concentration of ICAM-1, IL-6, and lipid profiles in hyperlipidemic patients with MI during statins therapy.
METHODS
Patients
All 52 patients (39 men, 13 women) were Iranian hyperlipidemic (cholesterol >200 mg/dl, triglyceride [TG] >150 mg/dl) individuals who were referred to the Hazrat Rasool Hospital, Tehran, Iran. All participates had MI with the age of 35–70 years. exclusive criteria included: Smoking and alcohol consumption, diabetes mellitus, kidney and liver disorders, clinical signs of acute inflammation, infectious disease during the study time, consumption of antioxidant such as ascorbic acid and α-tocopherol and omega3 supplement and nonsteroidal anti-inflammatory drugs for 3 months before the study initiation and any changes in kind of their drugs and dose of them during the study.
Participants were instructed not to change their dietary habits throughout the study. All patients were advised to consume beta blockers, thrombolysis, and statin drugs by their physician. Written informed consent was received from all patients for participating in the study before hands. The study protocol was approved by the Iran University of Medical Sciences.
Study design
This was a randomized, double-blind, placebo-controlled study to examine the effects of CoQ10 and placebo in hyperlipidemic patients with MI.
We considered two-sided significance levels of 5% and with 80% power; a sample size of 22 subjects per group was provided, and it was inflated to accommodate the anticipated dropout rate of 10%. For predicting the missing of our samples in each group, we consider 27 subjects in both groups, but two patients were excluded from our study due to diabetes and liver disease [Flow Diagram], so the 52 enrolled patients underwent a block randomization, using a computer-generated sequence: 26 patients were allocated in the intervention group and 26 patients in the placebo group. The sample size was calculated using the following formula:
Flow Diagram.
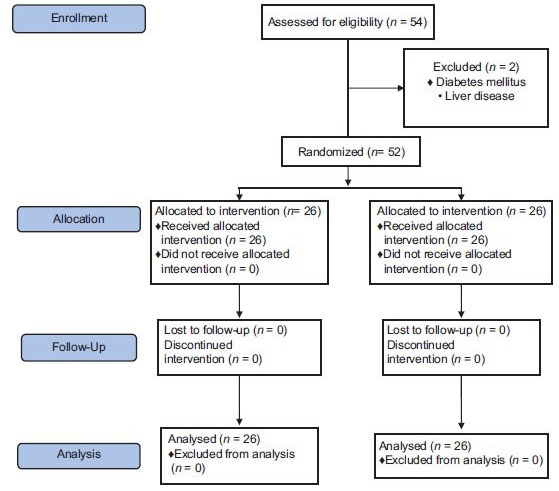
Flow diagram of patient recruitment and randomization process
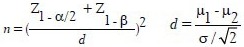
Subjects in intervention group were administered either two capsules of CoQ10 (Health Burst Co.) twice daily (200 mg of CoQ10/day) (CoQ10 group) and placebo group were received two capsules of placebo twice daily (each capsule containing lactose powder [Health Burst Co.]).
Duration of study was 12 weeks. Laboratory data before and after the intervention were determined. Serum total cholesterol, LDL-C, high-density lipoprotein-cholesterol (HDL-C), TG levels, IL-6 and ICAM-1 concentration in serum were determined before and 12 weeks after CoQ10 or placebo consumption.
Physical activity was assessed by the validated International Physical Activity Questionnaire.[10] Participants, nutrition specialists, and external assessors were blinded to the interventions into which the individuals were allocated.
Laboratory methods
A blood sample after at least 12 h of fasting was drawn at entry and end of the study in the morning. Serum was also separated by centrifugation and stored at −70°C. Serum total cholesterol, HDL-C, LDL-C, and TG were estimated enzymatically (Pars Azmoon. Co, Iran). IL-6 and ICAM-1 were also measured by ELISA method (Bender Med. Co, USA) according to the manufacturer's instructions. Technician was blind to groups.
Statistical analysis
Data are presented as mean ± standard deviation. ANCOVA method was used to compare the impact of CoQ10 treatment on the changes of studied factors. The significant differences between the groups at various time points were also assessed by paired t-test. All numeric variables were tested for normality of distribution by the Kolmogorov–Smirnov test and if necessary, subjected to logarithmic transformation before applying parametric tests. Results were considered significant if the P < 0.05. The Statistical Package for Social Sciences (version 18.0; SPSS Inc., Chicago, IL, USA) was also used for all analyses.
RESULTS
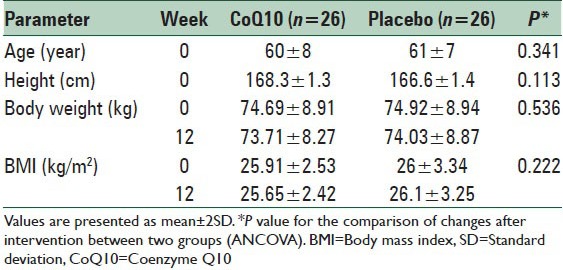
The study sample included 75% and 15% men and women, respectively. The mean of age and body mass index of subjects were 60 ± 8 years and 26 ± 3.2 kg/m2, respectively [Table 1]. A total of 52 subjects (39 men and 13 women) were enrolled in the study and completed the trial. The subjects who received CoQ10 supplement (CoQ10 group, n = 26) and those who received the placebo (placebo group, n = 26) were similar in age and sex distribution and levels of total cholesterol, LDL-C, HDL-C, TG, IL-6, and ICAM-1 in baseline.
Table 1.
The anthropometric measurements in two studies groups
Mean levels of total cholesterol, LDL-C and TG were not statistically different between the two groups after the intervention. A significant enhancement in serum HDL-C level was observed between groups after the intervention (55.46 ± 6.87 and 44.07 ± 6.99 mg/dl in CoQ10 and placebo groups, respectively P < 0.001). Concentrations of ICAM-1 (415.03 ± 96.89 and 453.38 ± 0.7 ng/dl CoQ10 and placebo groups, respectively, P = 0.001) and IL-6 (11 ± 9.57 and 12.55 ± 8.76 pg/ml CoQ10 and placebo groups, respectively P = 0.001) in serum were significantly decreased in CoQ10 group [Table 2].
Table 2.
Effect of CoQ10 on serum lipoproteins, ICAM-1 and IL-6 concentration in the study groups
For other variables, no statistically significant difference was observed between two groups in the end of the study. The increase in HDL-C level (P < 0.001) and decrease in all variables (P < 0.001) except TG (P = 0.21), showed statistical significance difference in the CoQ10 group after the intervention compared to the baseline [Figure 1].
Figure 1.
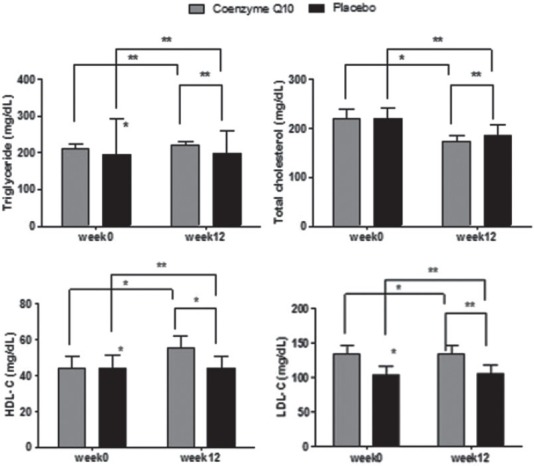
Changes in total serum cholesterol, triglyceride, low-density lipoprotein-cholesterol, and high-density lipoprotein-cholesterol concentration after 12 weeks intervention in two studies groups. P value for the comparison of changes within group (paired sample t-test) and P value for the comparison of changes between groups (ANCOVA) (*P < 0.05, **P ≥ 0.05)
Figure 2 indicates that supplementation with CoQ10 decreased serum concentration of IL-6 (P = 0.001) and ICAM-1 (P = 0.001) in CoQ10 group compared with placebo group.
Figure 2.
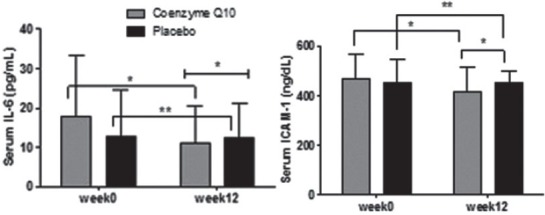
Changes in intereukin-6 and intercellular adhesion molecule-1 concentration in serum of patients after 12 weeks intervention in two studies groups. P value for the comparison of changes within group (paired sample t-test) and P value for the comparison of changes between groups (ANCOVA) (*P < 0.05, **P ≥ 0.05)
DISCUSSION
In this clinical trial, we have demonstrated that coenzymeQ10 at the dose of 200 mg/day for 12 weeks increases the HDL-C and decreases inflammation in patients with MI during statins therapy. In the present study, the levels of ICAM-1 and IL-6 in serum were significantly decreased after CoQ10 supplementation. Schmelzer et al.[20,21] demonstrated that CoQ10 could exert anti-inflammation effects via the reduction of nuclear factor-KB (NF-KB) dependent gene expression. NF-KB can be activated by the reactive oxygen species and can then up-regulate pro-inflammatory cytokines expression. However, this NF-KB - activating cascade could be inhibited by antioxidants like CoQ10.[22] Lee et al. showed that CoQ10 supplementation at 300 mg/day decreased the levels of tumor necrosis factor-α by 0.30 pg/ml and IL-6 by 0.52 pg/ml. However, CoQ10 supplementation had no effect on the level of CRP.[23]
In MI patients, who are thrombolysed, severe endothelial dysfunction in the infarct-related arteries is observed[24] with an increase in inflammatory cytokines like IL-6 and also its signaling product CRP. Circulating IL-6 levels constitute a significant pro-atherogenic cytokine, and serum IL-6 concentration was an independent predictor of cardiovascular mortality.[25]
Almost there is not any study about effects of CoQ10 on ICAM-1, however; one study has examined this relationship with other antioxidants such as vitamin E and probucol. This study showed that oxidized LDL can induce the expression of different adhesion molecules; this induction can be prevented by pretreating either the LDL or the cells with radical-scavenging antioxidant.[26]
According to Maldonado A findings, consumption of vitamin C-rich apple juice can reduce ICAM-1 and total cholesterol in healthy young adults.[27]
We have shown that the consumption of CoQ10, for 12 weeks had beneficial effects on the immune system among patients with acute MI. Our findings clearly demonstrated that CoQ10 supplementation can reduce the ICAM-1 and IL-6 serum concentration in MI patients. Inflammation plays a critical role in atherogenesis. The initial step in atherosclerosis is the adhesion of leukocytes to activated endothelial cells mediated by ICAM-1, an inflammatory protein.
The results of this double-blind, randomized clinical trial demonstrate that intake of CoQ10 (200 mg/day) for 12 weeks, leads to significant increase in serum HDL-C in MI patients. Our results also indicate a slight but not significant decrease in serum LDL-C, total cholesterol, and TG. The rise in the serum HDL-C initiates cholesterol efflux and facilitates the removal of excess cholesterol from the arteries and delivers it to the liver for being removed through reverse cholesterol transport pathway.[28]
Patients receiving statin show lower levels of plasma CoQ10, Therefore, statin treatment may cause a CoQ10 deficiency.[29] Studies have consistently demonstrated that statin therapy decreases circulating CoQ10 concentrations.[30] According to Chapidze et al.,[31] treatment with CoQ10 in a patient with ischemic heart disease, is associated with its potential independent role in lowering the markers of oxidative stress and decreasing the total cholesterol/HDL-C ratio.
Two limitations of the present study should be mentioned. First, the number of participants was small; although we did recruit more subjects than expected. Second, lack of measurement of apolipoproteins was the major limitation of our study. Markers such as fibrinogen, vitamin D, and CRP have a strong association with cardiovascular disease.
CONCLUSIONS
We have demonstrated that CoQ10 supplementation at a dose of 200 mg/day significantly increases serum HDL-C, IL-6, and ICAM-1 serum concentration in hyperlipidemic patients with MI. These patients might benefit from using CoQ10 supplements to increase their antioxidation and anti-inflammation capacity during statins therapy.
ACKNOWLEDGEMENTS
Dr. Farzad Shidfar and Dr. Mohammad Reza Vafa were as scientific advisors and critically reviewed the study proposal, Mona Mohseni collected data, provided and cared for study patients, Dr. Seyed Javad Hajimiresmail as an endocrinologist, Dr. Abbas Rahimi Forushani participated in the Statistical Analysis and Dr. Mitra Zarrati participated in writing or technical editing of the manuscript. We would like to express our sincere thanks to Iran University of Medical Sciences funding for the project with IRCT138811192709N4 number and all patients and their medical teams.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Shattuck lectur – Cardiovascular medicine at the turn of the millennium: Triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–9. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 1986;256:2823–8. [PubMed] [Google Scholar]
- 4.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 5.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 6.Bhagavan HN, Chopra RK. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–53. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 7.Chu CS, Kou HS, Lee CJ, Lee KT, Chen SH, Voon WC, et al. Effect of atorvastatin withdrawal on circulating coenzyme Q10 concentration in patients with hypercholesterolemia. Biofactors. 2006;28:177–84. doi: 10.1002/biof.5520280304. [DOI] [PubMed] [Google Scholar]
- 8.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 9.Siegel D, Devaraj S, Mitra A, Raychaudhuri SP, Raychaudhuri SK, Jialal I. Inflammation, atherosclerosis, and psoriasis. Clin Rev Allergy Immunol. 2013;44:194–204. doi: 10.1007/s12016-012-8308-0. [DOI] [PubMed] [Google Scholar]
- 10.Singh U, Devaraj S, Jialal I. Coenzyme Q10 supplementation and heart failure. Nutr Rev. 2007;65:286–93. doi: 10.1301/nr.2007.jun.286-293. [DOI] [PubMed] [Google Scholar]
- 11.Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9:273–84. doi: 10.1002/biof.5520090224. [DOI] [PubMed] [Google Scholar]
- 12.Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: An update. Nutrition. 2010;26:250–4. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig Drugs. 2010;19:535–54. doi: 10.1517/13543781003727495. [DOI] [PubMed] [Google Scholar]
- 14.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 15.Almenar-Queralt A, Duperray A, Miles LA, Felez J, Altieri DC. Apical topography and modulation of ICAM-1 expression on activated endothelium. Am J Pathol. 1995;147:1278–88. [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–86. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 17.Koning GA, Schiffelers RM, Storm G. Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium. 2002;9:161–71. doi: 10.1080/10623320213631. [DOI] [PubMed] [Google Scholar]
- 18.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: A novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105:650–8. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 19.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des. 2005;11:2383–401. doi: 10.2174/1381612054367274. [DOI] [PubMed] [Google Scholar]
- 20.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Döring F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–83. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 21.Schmelzer C, Lorenz G, Rimbach G, Döring F. In vitro effects of the reduced form of coenzyme Q (10) on secretion levels of TNF-alpha and chemokines in response to LPS in the human monocytic cell line THP-1. J Clin Biochem Nutr. 2009;44:62–6. doi: 10.3164/jcbn.08-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YP, Eber A, Yuan Y, Yang Z, Rodriguez Y, Levitt RC, et al. Prophylactic and antinociceptive effects of coenzyme Q10 on diabetic neuropathic pain in a mouse model of type 1 diabetes. Anesthesiology. 2013;118:945–54. doi: 10.1097/ALN.0b013e3182829b7b. [DOI] [PubMed] [Google Scholar]
- 23.Lee BJ, Tseng YF, Yen CH, Lin PT. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr J. 2013;12:142. doi: 10.1186/1475-2891-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iràculis E, Cequier A, Gómez-Hospital JA, Sabaté M, Mauri J, Fernández-Nofrerias E, et al. Early dysfunction and long-term improvement in endothelium-dependent vasodilation in the infarct-related artery after thrombolysis. J Am Coll Cardiol. 2002;40:257–65. doi: 10.1016/s0735-1097(02)01953-8. [DOI] [PubMed] [Google Scholar]
- 25.Fan ZX, Hua Q, Tan J, Gao J, Liu RK, Yang Z. Interleukin-6 but not soluble adhesion molecules has short-term prognostic value on mortality in patients with acute ST-segment elevation myocardial infarction. Afr J Biotechnol. 2011;10:1454–9. [Google Scholar]
- 26.Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Contessi GB, et al. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic Biol Med. 1997;22:117–27. doi: 10.1016/s0891-5849(96)00271-7. [DOI] [PubMed] [Google Scholar]
- 27.Soriano-Maldonado A, Hidalgo M, Arteaga P, de Pascual-Teresa S, Nova E. Effects of regular consumption of vitamin C-rich or polyphenol-rich apple juice on cardiometabolic markers in healthy adults: A randomized crossover trial. Eur J Nutr. 2014;53:1645–57. doi: 10.1007/s00394-014-0670-7. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap ML. Mechanistic studies of high-density lipoproteins. Am J Cardiol. 1998;82:42U–48. doi: 10.1016/s0002-9149(98)00813-3. [DOI] [PubMed] [Google Scholar]
- 29.Langsjoen PH, Langsjoen AM. The clinical use of HMG CoA-reductase inhibitors and the associated depletion of coenzyme Q10. A review of animal and human publications. Biofactors. 2003;18:101–11. doi: 10.1002/biof.5520180212. [DOI] [PubMed] [Google Scholar]
- 30.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: A systematic review. J Am Coll Cardiol. 2007;49:2231–7. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 31.Chapidze G, Kapanadze S, Dolidze N, Bachutashvili Z, Latsabidze N. Prevention of coronary atherosclerosis by the use of combination therapy with antioxidant coenzyme Q10 and statins. Georgian Med News. 2005;118:20–5. [PubMed] [Google Scholar]


