Abstract
Without pigments, we are nothing. Life presents us with a kaleidoscope of colors. From the green grass of home to a forest's ruddy autumn hues, we are surrounded by living colors. Living things obtain their colors, with few exceptions, from natural pigments. In addition to their role in coloration, natural pigments carry out a variety of important biological functions. Of the various classes of pigments in nature, the carotenoids are among the most widespread and important ones, especially due to their varied functions. Lycopene is a red plant pigment found in tomatoes, apricots, guavas, watermelons, papayas, and pink grapefruits, with tomatoes being the largest contributor to the dietary intake of humans. Lycopene exhibits higher singlet oxygen quenching ability. Due to its strong color and nontoxicity, it is a useful food coloring agent. Moreover, it plays a multifunctional role as a nonsurgical aid in the treatment of oral diseases like leukoplakia, oral submucous fibrosis, lichen planus, oral squamous cell carcinoma, and also prevents the destruction of periodontal tissues. This review article focuses mainly on the role of lycopene in the prevention of various oral diseases.
Keywords: Carotenoids, leukoplakia, lichen planus, lycopene, oral cancer, oral health, oral submucous fibrosis
INTRODUCTION
Without pigments, we are nothing. Life presents us with a kaleidoscope of colors. From the green grass of home to a forest's ruddy autumn hues, we are surrounded by living colors. Living things obtain their colors, with few exceptions, from natural pigments. In addition to their role in coloration, natural pigments carry out a variety of important biological functions.[1] Of the various classes of pigments in nature, the carotenoids are among the most widespread and important ones, especially due to their varied functions.[2]
Carotenoids are a family of compounds of over 600 fat-soluble plant pigments that provide much of the color we see in nature. They are important nutritious substances for the human body owing to their pro-vitamin A and antioxidant (AO) activities.[3]
Carotenoids are classified according to the structure as follows:[4]
Carotenes: The hydrocarbon carotenoids. For example: β-carotene and lycopene
Xanthophylls: The oxygenated carotenoids which are derivatives of these hydrocarbons. For example: Zeaxanthin and lutein (hydroxy), spirilloxanthin (methoxy), echinenone (oxo), and antheraxanthin (epoxy).
Lycopene is a fat-soluble carotenoid discovered by Ernest et al. in 1959.[5] It is a natural constituent of red fruits and vegetables and of certain algae and fungi. Tomatoes and tomato-based products[6] are the major sources of lycopene in the human diet [Table 1]. Other sources of lycopene are apricot, Cranberry, grapes, pink grapefruit, guava, papaya, peaches, and watermelon.[7] Tomato sauce and ketchup are concentrated sources of lycopene as compared to unprocessed tomatoes.[8]
Table 1.
Dietary sources of lycopene

STRUCTURE
It is one of the most potent AOs among dietary carotenoids. The chemical name of lycopene is 2,6,10,14,19,23,27,31-octamethyl 2,6,8,10,12,14,16,18,20,22,24,26,30 -dotriacontatridecaene. Common names include Ψ,Ψ-carotene, all-trans-carotene, and (all-E)-lycopene.[3] Lycopene is a carotenoid with the chemical formula C40H56. It has a molecular weight of 536.85 and Chemical Abstract Service Registry Number is 502-65-8. Its structural formula is [Figure 1]:
Figure 1.
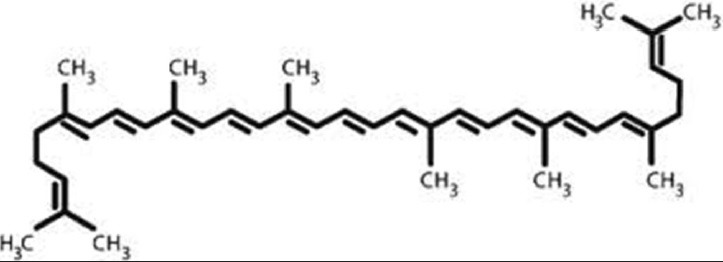
Structural formula of lycopene
Like all carotenoids, lycopene is a polyunsaturated hydrocarbon (an unsubstituted alkene). Structurally, it is a tetraterpene assembled from eight isoprene units, composed entirely of 40 carbon atoms and 56 hydrogen atoms and is insoluble in water. Lycopene's 11 conjugated double bonds give it a deep red color and are responsible for its AO activity.[9]
Lycopene from natural plant sources exists predominantly in trans configuration, the most thermodynamically stable form. In human plasma, lycopene is an isomeric mixture containing 50% of the total lycopene as cis isomers. All trans, 5-cis, 9-cis, 13-cis, and 15-cis are most commonly identified isomeric forms of lycopene. Lycopene, ingested in its natural transform found in tomatoes, is poorly absorbed. Recent studies have shown that heat processing of tomatoes and tomato products induces isomerization of lycopene to the cis form which in turn increases its bioavailability. The mean plasma level of lycopene ranges from 0.22 to 1.06 nmol/ml, and it contributes to about 21–43% of the total carotenoids.[10]
SYNTHESIS ROUTE OF LYCOPENE
A complex mechanism persists in the biosynthesis of lycopene that starts when chlorophyll degrades to yield white colored leucoplast thus yielding specialized red color pigmented organelles, that is, chromoplast. A stepwise addition of isopentenyl diphosphate (IPP) takes place with dimethylallyl diphosphate giving rise 20-C precursor, geranylgeranyl diphosphate (GGPP). On desaturation of GGPP, 11 conjugated double bonds are produced that exist as lycopene in nature. From this point, cyclic conversion takes place converting it to α- and β-carotene that on oxidation produce xanthophylls.[11]
BENEFITS ON HUMAN HEALTH
Lycopene has some beneficial effects in the treatment of certain diseases of oral cavity including oral cancer and precancerous lesions;[7] lycopene does not have the pro-vitamin A activity[12] and its various benefits on human health can be explained based on its properties of:
AO activity
Inhibition of cancer cell proliferation
Interference with growth factor stimulation
Inducing phase II enzymes
Regulation of transcription and
Restoration of gap junctions.
Lycopene exerts its AO activity by physical and chemical quenching of free radicals and is the most efficient singlet oxygen quenching carotenoid.[13] Because lycopene is not converted to vitamin A, it may be entirely available for other properties (e.g., antioxidation). The lack of the b-ionone ring structure for lycopene may increase its AO activity. The stereochemical properties of lycopene are quite different from those of other commonly consumed carotenoids, making it uniquely present in specific subcellular environments. Lycopene appears to be the most efficient quencher of singlet oxygen and free radicals among the common carotenoids in vitro. In some population, lycopene is the predominant carotenoid in plasma and various tissues. The unique biochemical properties of lycopene may render it able to protect cellular components against specific types of damage from highly reactive oxygen species (ROS).[14] Inhibitory effect of lycopene on cancer cells has been accompanied by inhibition of cell cycle progression from the Go/G1 to the S phase.[15]
ROLE OF LYCOPENE IN PREVENTION OF ORAL DISEASES
Oral cancer
Neoplasm is a multistage disease process, where a single cell can develop from an otherwise normal tissue into malignancy that can eventually destroy the very base. The series of cellular and molecular changes that occur through the development of cancers can be mediated by a diversity of endogenous and other free radicals which have long been known to be mutagenic. Further, these free radicals have more recently emerged as the mediators of the other phenotypic and genotypic changes that can lead from mutation to neoplasia. It has therefore been felt that free radicals may have a major contribution to the cancer development in the human.[16]
Oral cancer is one of the most common malignancies worldwide and ranks 12th among all cancers. Oral squamous cell carcinoma (OSCC) develops through a multi-step process of genetic, epigenetic, and metabolic changes resulting from exposure to carcinogens. Among these factors, the role of diet and nutrition in the prevention of oral malignancies has drawn a great interest.[17]
Although neoplasia is treatable with surgery or radiotherapy in its early stages, most patients are diagnosed only at advanced stages of the disease. At these late stages, therapy outcomes have not dramatically improved in recent years. Reduced incidence of this disease may be attainable through preventive measures. Preventive strategies are designed to suppress, reverse or prevent the formation of premalignant lesions and their subsequent development through the multistep process of initiation, promotion, and progression into OSCC.[18]
Lycopene has been hypothesized to prevent carcinogenesis and atherogenesis by protecting critical cellular biomolecules, including lipids, lipoproteins, proteins, and DNA.[19]
The anticancer activity of lycopene has been demonstrated both in vitro and in vivo tumor models. The mechanisms underlying the inhibitory effects of lycopene on carcinogenesis could involve ROS scavenging, up-regulation of detoxification systems, interference with cell proliferation, induction of gap-junctional communication, inhibition of cell cycle progression. Lycopene has been reported to increase p53 protein levels in cancer cells.[15]
Cell–cell gap junctions are considered to be important in the maintenance of tissue homeostasis. Any alteration in this can give rise to the neoplastic phenotype. Studies have shown that the lycopene in various doses results in a decrease in the proliferation of oral tumor cells, Killer B1 (KB1) cells. These cells originating from a human oral cavity tumor were incubated with different concentrations of lycopene delivered via the cell culture media from stock solutions in tetrahydrofuran. Lycopene strongly and dose dependently inhibited the proliferation of KB1 human oral tumor cells.[20]
Lycopene also increases the expression of a gene encoding connexin-43, a gap junction protein, effect being independent of pro-vitamin A or AO properties. Administration of lycopene suppresses DMBA-induced oral carcinogenesis.[21]
Oral leukoplakia
Oral leukoplakia (OL) is a premalignant lesion described as “a predominant white lesion of the oral mucosa which cannot be defined as any other known lesion.”[22] Association between tobacco usage and OL has been determined for a long period and beyond doubt. Tobacco usage also has definite roles in the etiopathogenesis of oral cancers by generating increased reactive free radicals and active oxygen species, which mediate phenotypic and genotypic alterations and lead mutations to carcinogenesis.[16] In order to conduct treatment for OL, the degree of epithelial dysplasia may be assessed. In the presence of moderate or severe epithelial dysplasia, surgical treatment is recommended.[23] However, OL presenting low to moderate malignant risk may be either completely removed or not, and the decision should consider other factors such as location, size, and in the case of smokers, the patient's engagement in smoking cessation.[24] OL surgical treatment may be performed either through conventional surgery,[25] electro cauterization, laser ablation[26] or cryosurgery.[27]
Lycopene appears to be a very promising AO as a treatment modality in OL and can protect cells against cell damage and play a protective role against progression of dysplasia by inhibiting tumor cell proliferation and the first report of efficacy of lycopene against human oral cancer cell was published describing the significant therapeutic effect.[28] Nagao et al. tried to investigate the association between serum micronutrient levels and OL. The serum levels of lycopene among men with OL were significantly lower than those of controls.[29]
Gupta et al. tried to estimate the relation between nutrient intake and prevalence of OL. They observed that tomato consumption-the main source of lycopene has the most protective effect on OL among all dietary factors. Up to today, only one study evaluated the efficacy of lycopene in the clinical resolution of OL.[30]
A study conducted at Belgaum, Karnataka showed lycopene to be efficacious in the treatment of OL. They also reported that a daily dose of 8 mg of lycopene was more efficacious than 4 mg a day. This efficacy of lycopene was associated to its AO properties.[31]
Zakrzewska in their study on 58 patients concluded that lycopene brings about histological changes of a significant degree in patients with OL.[32]
Oral submucous fibrosis
Oral submucous fibrosis (OSMF) is a chronic, progressive, scarring, disease that predominantly affects the people of South-East Asian origin.[33] OSMF is found to be of multifactorial etiology including excessive chilli consumption, genetic susceptibility, autoimmunity, iron and vitamin deficiency,[34] but it is strongly associated with areca nut chewing and pan masala.[35] Experiments have shown that ethanolic extracts of areca nut stimulate collagen synthesis in human dermal fibroblasts and also stabilize collagen fibrils and render them resistant to degradation by collagenase leading to fibrosis.[36] It has been found that some amount of copper is also present in areca nut which up-regulates collagen production by increasing lysyl oxidase, involved in collagen synthesis and cross-linking.[37]
Oral submucous fibrosis has a similarity in behavior and malignant changes to other premalignant lesions of the oral cavity. Hence, it has been felt that the disease process in OSMF also could be reversed and inhibited by the use of AOs, as is observed in other pre malignant lesion therapies of the oral cavity. Many authors are of the opinion that conservative treatment is preferable than the conventional ones.[38]
Hazardous treatments like the submucosal injections of steroids, hyaluranidase, and placental extracts should be avoided. Several studies in humans have confirmed the cancer preventive nature of AOs. The oral intake of retinoids has a significant toxic effect on the normal tissue. A less toxic group of micronutrients are the carotenoids, which include lycopene. Its mode of action may involve stimulation of the immune system or a direct action on the tumor cells. Lycopene has been shown to inhibit hepatic fibrogenesis in liver endothelial cell rats by Kitade et al., and it may also exert a similar inhibition on the abnormal fibroblasts in OSMF.[16] Lycopene also up-regulates the lymphocyte resistance to stress and suppresses the inflammatory response.[39]
Oral lichen planus
LP is a chronic inflammatory mucocutaneous disease that occurs in about 0.2–4% of the general population, affecting skin and/or mucosa.[40] Although the exact etiology of the disease is unknown, the role of free radicals and oxidative stress has been implicated in its pathogenesis.[41]
Further, an affirmative treatment remains elusive, and a vast array of empirical treatments reported in the literature indicates the continuing search for the solution.[42] The role of lycopene, a potent AO being used in the management of various systemic and few oral diseases including cancer and precancerous lesions, suggested to be caused by the oxidative stress has not been assessed in the prevention or treatment of oral lichen planus (OLP). However, one study has reported significantly decreased levels of lycopene in patients with atrophic and erosive OLP,[43] and its role in the disease pathology needs further investigation. Further, by virtue of its AO and anticancer properties,[44] it may be useful in the prevention of malignant transformation in the OLP.
PERIODONTAL DISEASES
The periodontal tissues also provide an ideal medium to study the mechanisms of ROS-mediated tissue damage and AO defense in response to bacterial colonization, through the noninvasive collection of gingival crevicular fluid.[45] ROS cause tissue damage by a variety of different mechanisms, which include DNA damage, lipid peroxidation (through activation of cyclooxygenases and lipooxygenases), protein damage, including gingival hyaluronic acid and proteoglycans, oxidation of important enzymes, e.g. anti-proteases, and stimulation of pro-inflammatory cytokine release by monocytes and macrophages. While most ROS have extremely short half-lives, they can cause substantial tissue damage by initiating free radical chain reactions. It is, therefore, not surprising that the body contains a number of protective AO mechanisms whose specific role is to remove harmful oxidants or ROS as soon as they form or to repair the damage caused by ROS in vivo.[46]
Carotenoids are powerful AO agents which are important in the maintenance of overall health of an individual and have a protective role against cancer, heart diseases, and oral malignancies and diseases. Among the carotenoids, lycopene is the most potent AO. It also enhances the effect of other carotenoids. It also possesses antibacterial and antifungal properties.[47] It is an effective adjuvant in the treatment of gingivitis along with oral prophylaxis. Lycopene exerts potent antifungal activity against Candida albicans by causing significant damage to the cell membrane.[48] A randomized, placebo-controlled, split-mouth study of gingivitis was performed by Chandra et al. (2007) in 20 healthy subjects with clinical signs of gingivitis. The treatment group (n = 10) was supplemented with 8 mg/day lycopene (LycoRed®), whereas the control group (n = 10) received a placebo daily for 2 weeks. In this study, patients receiving the lycopene treatment showed statistically significant reductions in gingivitis and bleeding index.[49] Lycopene has been found to be more effective with other AOs like vitamin C.[50]
A relationship exists between periodontitis and risk of congestive heart failure, and high monthly total consumption of lycopene appears to affect this relationship in a positive direction in periodontitis subjects.[51] Lycopene has also been associated with a decrease in oxidative stress as it is inversely related to malondialdehde which is a marker oxidative stress[9]
SAFETY OF LYCOPENE
Lycopene has been recognized as a safe product for daily dietary intake. Large amounts of dietary intake also do not show any adverse effects on the health of an individual. Based on various safety studies reviewed, no adverse effects were observed at the highest intake level provided, that is, 3 g/kg/day of dietary or formulated lycopene.[52] Excessive carotenoid intake have been reported in a middle-aged woman who had prolonged and excessive consumption of tomato juice, her skin and liver were colored orange-yellow and she had elevated levels of lycopene in her blood. After 3 weeks on a lycopene-free diet, her skin color returned to normal.[53] However, since lycopene is a lipidsoluble AO, it has been reported that concomitant intake of some cholesterollowering drugs such as probucol and cholestyramine significantly decreases the serum concentration of lycopene due to impairment of gastrointestinal absorption.[54]
CONCLUSIONS
Lycopene is a promising candidate in reducing cancer and oral diseases in human beings; however, further research is needed to clarify its potential function in human oral health according to the following criteria:[55]
Factors influencing the uptake of lycopene in the diet, including the way it interacts with other carotenoids
Human metabolism and the possible function of the metabolites and cis-trans isomers
Mechanisms of the direct or indirect modulation of cancer
Studies based on evidences of treatment in human beings
Mechanisms of lycopene deposition in human tissues
Lycopene effects in the immunological system.
ACKNOWLEDGEMENT
This work has been put together by all the authors. Dr. Sonia Gupta contributed to the conception or design of the work; Dr. Vikram, Dr. Nishant, and Dr. Vipul worked on or the acquisition, analysis, or interpretation of data. Dr. Manveen Kaur edited the manuscript and gave the final revision.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ong AS, Tee ES. Tee. Natural sources of carotenoids from plants and oils. Methods Enzymol. 1992;213:142–67. [Google Scholar]
- 2.Eldahshan OA, Singab AN. Carotenoids. J Pharmacogn Phytochem. 2013;2:225–34. [Google Scholar]
- 3.Aghel N, Ramezani Z, Amirfakhrian S. Isolation and quantification of lycopene from tomato cultivated in Dezfoul, Iran. Jundisha J Nat Pharm Prod. 2011;6:9–15. [Google Scholar]
- 4.Elumalai M, Karthika B, Usha V. Lycopene-role in cancer prevention. Int J Pharma Bio Sci. 2013;4:371–8. [Google Scholar]
- 5.Saawarn N, Shashikanth MC, Saawarn S, Jirge V, Chaitanya NC, Pinakapani R. Lycopene in the management of oral lichen planus: A placebo-controlled study. Indian J Dent Res. 2011;22:639–43. doi: 10.4103/0970-9290.93448. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen ML, Schwartz SJ. Lycopene: Chemical and biological properties. Food Technol. 1999;53:38–45. [Google Scholar]
- 7.Mehta DN. Lycopene: Structure, pharmacokinetics and role in oral cancer precancerous lesions. J Res Adv Dent. 2012;1:44–9. [Google Scholar]
- 8.Heber D, Lu QY. Overview of mechanisms of action of lycopene. Exp Biol Med (Maywood) 2002;227:920–3. doi: 10.1177/153537020222701013. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj R, Chaudhary K, Kaur S, Gupta R, Kamal R, Kumar M. Lycopene in oral health. Indian J Oral Sci. 2013;4:125–9. [Google Scholar]
- 10.Schierle J, Bretzel, W, Bühler I, Faccin N, Hess D, Steiner K, et al. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997;59:459–65. [Google Scholar]
- 11.Naz A, Butt MS, Sultan MT, Qayyum MM, Niaz RS. Watermelon lycopene and allied health claims. EXCLI J. 2014;13:650–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Bhuvaneswari V, Nagini S. Lycopene: A review of its potential as an anticancer agent. Curr Med Chem Anticancer Agents. 2005;5:627–35. doi: 10.2174/156801105774574667. [DOI] [PubMed] [Google Scholar]
- 13.Stahl W, Sies H. Lycopene: A biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E. RESPONSE: Re: Tomatoes, tomato-based products, lycopene, and prostate cancer: Review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:1331A. doi: 10.1093/jnci/91.15.1331a. [DOI] [PubMed] [Google Scholar]
- 15.Palozza P, Simone RE, Catalano A, Mele MC. Tomato lycopene and lung cancer prevention: From experimental to human studies. Cancers (Basel) 2011;3:2333–57. doi: 10.3390/cancers3022333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gowda BB, Yathish TR, Sankappa PS, Naik HK, Somayaji P, Anand D. The response of oral submucous fibrosis to lycopene – A carotenoid antioxidant: A clinicopathological study. J Clin Diagn Res. 2011;5:882–6. [Google Scholar]
- 17.Reddy ES. Role of antioxidants in precancerous lesions. J Indian Dent Assoc. 2011;5:99–101. [Google Scholar]
- 18.D’Ambriosio SM, D’Ambriosio RG, Milo GE, Casto B, Kelloff GJ, Steele VE. Differential response of normal, premalignant human oral epithelia cells to growth inhibition by chemopreventive agents. Anticancer Res. 2000;20:2273–80. [PubMed] [Google Scholar]
- 19.Bansal M, Vashisth S, Gupta N, Singh S. Antioxidants – Its preventive role in oral cancer. Indian J Dent Sci. 2012;4:103–5. [Google Scholar]
- 20.Livny O, Kaplan L, Reifen R, Polak-Charcon S, Madarand Z, Schwartz B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J Nutr. 2002;132:3754–9. doi: 10.1093/jn/132.12.3754. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Krishanappa R, Bagewadi A, Keluskar V. Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral Oncol. 2004;40:591–6. doi: 10.1016/j.oraloncology.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro AS, Salles PR, da Silva TA, Mesquita RA. A review of the nonsurgical treatment of oral leukoplakia. Int J Dent. 2010;2010:186018. doi: 10.1155/2010/186018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reibel J. Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14:47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 24.van der Waal I, Schepman KP, van der Meij EH, Smeele LE. Oral leukoplakia: A clinicopathological review. Oral Oncol. 1997;33:291–301. doi: 10.1016/s1368-8375(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 25.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 26.Ishii J, Fujita K, Komori T. Laser surgery as a treatment for oral leukoplakia. Oral Oncol. 2003;39:759–69. doi: 10.1016/s1368-8375(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes G. Beta-carotene supplementation: Friend or foe? J Lab Clin Med. 1997;129:285–7. doi: 10.1016/s0022-2143(97)90175-x. [DOI] [PubMed] [Google Scholar]
- 28.Uma TN. Treatment of oral leukoplakia with antioxidants – A systematic review. Int J Pharm Bio Sci. 2013;4:33–4. [Google Scholar]
- 29.Nagao T, Ikeda N, Warnakulasuriya S, Fukano H, Yuasa H, Yano M, et al. Serum antioxidant micronutrients and the risk of oral leukoplakia among Japanese. Oral Oncol. 2000;36:466–70. doi: 10.1016/s1368-8375(00)00037-3. [DOI] [PubMed] [Google Scholar]
- 30.Gupta PC, Hebert JR, Bhonsle RB, Sinor PN, Mehta H, Mehta FS. Dietary factors in oral leukoplakia and submucous fibrosis in a population-based case control study in Gujarat, India. Oral Dis. 1998;4:200–6. doi: 10.1111/j.1601-0825.1998.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 31.Aung WP. The use of lycopene in oral potentially malignant disorders. Myan Dent J. 2013;20:58–63. [Google Scholar]
- 32.Zakrzewska JM. Oral lycopene – An efficacious treatment for oral leukoplakia? Evid Based Dent. 2005;6:17–8. doi: 10.1038/sj.ebd.6400285. [DOI] [PubMed] [Google Scholar]
- 33.Rajendran R. Oral submucous fibrosis. J Oral Maxillofac Pathol. 2003;7:1–4. doi: 10.4103/0973-029X.86678. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Koshti SS, Barpande S. Quantification of plasma fibrinogen degradation products in oral submucous fibrosis: A clinicopathological study. J Oral Maxillofac Pathol. 2007;11:48–50. [Google Scholar]
- 35.Shah N, Sharma PP. Role of chewing and smoking habits in the etiology of oral submucous fibrosis (OSF): A case-control study. J Oral Pathol Med. 1998;27:475–9. doi: 10.1111/j.1600-0714.1998.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 36.Kuo MY, Chen HM, Hahn LJ, Hsieh CC, Chiang CP. Collagen biosynthesis in human oral submucous fibrosis fibroblast cultures. J Dent Res. 1995;74:1783–8. doi: 10.1177/00220345950740111101. [DOI] [PubMed] [Google Scholar]
- 37.Trivedy C, Meghji S, Warnakulasuriya KA, Johnson NW, Harris M. Copper stimulates human oral fibroblasts in vitro: A role in the pathogenesis of oral submucous fibrosis. J Oral Pathol Med. 2001;30:465–70. doi: 10.1034/j.1600-0714.2001.030008465.x. [DOI] [PubMed] [Google Scholar]
- 38.Borle RM, Borle SR. Management of oral submucous fibrosis: A conservative approach. J Oral Maxillofac Surg. 1991;49:788–91. doi: 10.1016/0278-2391(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 39.Kumar A, Bagewadi A, Keluskar V, Singh M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:207–13. doi: 10.1016/j.tripleo.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Gorsky M, Raviv M. Efficacy of etretinate (Tigason) in symptomatic oral lichen planus. Oral Surg Oral Med Oral Pathol. 1992;73:52–5. doi: 10.1016/0030-4220(92)90153-h. [DOI] [PubMed] [Google Scholar]
- 41.Sander CS, Ali I, Dean D, Thiele JJ, Wojnarowska F. Oxidative stress is implicated in the pathogenesis of lichen sclerosus. Br J Dermatol. 2004;151:627–35. doi: 10.1111/j.1365-2133.2004.06142.x. [DOI] [PubMed] [Google Scholar]
- 42.Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: Etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9:86–122. doi: 10.1177/10454411980090010501. [DOI] [PubMed] [Google Scholar]
- 43.Nagao T, Warnakulasuriya S, Ikeda N, Fukano H, Yamamoto S, Yano M, et al. Serum antioxidant micronutrient levels in oral lichen planus. J Oral Pathol Med. 2001;30:264–7. doi: 10.1034/j.1600-0714.2001.300502.x. [DOI] [PubMed] [Google Scholar]
- 44.Levy J, Sharoni Y. The functions of tomato lycopene and its role in human health. Herbalgram. 2004;62:49–56. [Google Scholar]
- 45.Bhardwaj A, Bhardwaj SV. Role of antioxidants in periodontal disease and its therapy. Asian J Med Res. 2012;1:62–7. [Google Scholar]
- 46.Chapple IL. Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Clin Mol Pathol. 1996;49:M247–55. doi: 10.1136/mp.49.5.m247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahan K, Fennal M, Kumar NB. Lycopene in the prevention of prostate cancer. J Soc Integr Oncol. 2008;6:29–36. [PubMed] [Google Scholar]
- 48.Sung WS, Lee IS, Lee DG. Damage to the cytoplasmic membrane and cell death caused by lycopene in Candida albicans. J Microbiol Biotechnol. 2007;17:1797–804. [PubMed] [Google Scholar]
- 49.Chandra RV, Prabhuji ML, Roopa DA, Ravirajan S, Kishore HC. Efficacy of lycopene in the treatment of gingivitis: A randomised, placebo-controlled clinical trial. Oral Health Prev Dent. 2007;5:327–36. [PubMed] [Google Scholar]
- 50.Kanwar L. The role of parry tomato lycopene complex in human health. Parry Neutraceuticals. 2011;7:1–13. [Google Scholar]
- 51.Wood N, Johnson RB. The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. J Clin Periodontol. 2004;31:574–80. doi: 10.1111/j.1600-051X.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 52.Singh D, Aggarwal S. Lycopene in oral diseases. Guident. 2012;5:73–4. [Google Scholar]
- 53.Michael McClain R, Bausch J. Summary of safety studies conducted with synthetic lycopene. Regul Toxicol Pharmacol. 2003;37:274–85. doi: 10.1016/s0273-2300(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 54.Elinder LS, Hådell K, Johansson J, Mølgaard J, Holme I, Olsson AG, et al. Probucol treatment decreases serum concentrations of diet-derived antioxidants. Arterioscler Thromb Vasc Biol. 1995;15:1057–63. doi: 10.1161/01.atv.15.8.1057. [DOI] [PubMed] [Google Scholar]
- 55.Bramley PM. Is lycopene beneficial to human health? Phytochemistry. 2000;54:233–6. doi: 10.1016/s0031-9422(00)00103-5. [DOI] [PubMed] [Google Scholar]


