ABSTRACT
Herpes simplex virus 1 (HSV-1) and HSV-2 infect many humans and establish a latent infection in sensory ganglia. Although some infected people suffer periodic recurrences, others do not. Infected people mount both cell-mediated and humoral responses, including the production of virus-neutralizing antibodies (Abs) directed at viral entry glycoproteins. Previously, we examined IgGs from 10 HSV-seropositive individuals; all neutralized virus and were directed primarily against gD or gD+gB. Here, we expand our studies and examine 32 additional sera from HSV-infected individuals, 23 of whom had no recurrent disease. Using an Octet RED96 system, we screened all 32 serum samples directly for both glycoprotein binding and competition with known neutralizing anti-gD and -gB monoclonal Abs (MAbs). On average, the recurrent cohort exhibited higher binding to gD and gB and had higher neutralization titers. There were similar trends in the blocking of MAbs to critical gD and gB epitopes. When we depleted six sera of Abs to specific glycoproteins, we found different types of responses, but always directed primarily at gD and/or gB. Interestingly, in one dual-infected person, the neutralizing response to HSV-2 was due to gD2 and gB2, whereas HSV-1 neutralization was due to gD1 and gB1. In another case, virus neutralization was HSV-1 specific, with the Ab response directed entirely at gB1, despite this serum blocking type-common anti-gD and -gB neutralizing MAbs. These data are pertinent in the design of future HSV vaccines since they demonstrate the importance of both serotypes of gD and gB as immunogens.
IMPORTANCE We previously showed that people infected with HSV produce neutralizing Abs directed against gD or a combination of gD+gB (and in one case, gD+gB+gC, which was HSV-1 specific). In this more extensive study, we again found that gD or gD+gB can account for the virus neutralizing response and critical epitopes of one or both of these proteins are represented in sera of naturally infected humans. However, we also found that some individuals produced a strong response against gB alone. In addition, we identified type-specific contributions to HSV neutralization from both gD and gB. Contributions from the other entry glycoproteins, gC and gH/gL, were minimal and limited to HSV-1 neutralization. Knowing the variations in how humans see and mount a response to HSV will be important to vaccine development.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) and HSV-2 cause human diseases, ranging from benign cold sores to life-threatening infections such as encephalitis and neonatal HSV disease (1, 2). The hallmark of HSV infection is the ability of each serotype to establish a lifelong latent infection in sensory neurons. Reactivation can lead to clinical symptoms such as oral or genital lesions. Currently, 47% of the U.S. population is seropositive for HSV-1 and 16% are seropositive for HSV-2 (3). Many seropositive individuals are asymptomatic, although a number of people shed virus regardless of clinical signs of disease (4). However, the frequency of shedding is greater in those with recurrent clinical outbreaks (5). Control of virus shedding has been ascribed to cellular immunity (6), whereas protection correlates well with virus neutralization (7, 8). Thus, knowing the neutralizing antibody (Ab) response to infection is important for understanding how vaccination may elicit these protective Abs.
Viral glycoproteins that are critical for entry can stimulate production of neutralizing antibodies (9–14). These proteins include the cell receptor-binding glycoprotein D (gD), along with the heterodimer gH/gL and the fusion protein gB (9, 15, 16). Both gH/gL and gB are the “core fusion machinery” of all herpesviruses, and both crystallographic data and electron microscopy show that these proteins are highly homologous across herpesviruses in structure, implying a common mechanism by which they function (16–21). Our current hypothesis is that HSV entry involves a cascade of events (15, 22) beginning with the binding of gD to a cellular receptor, necessitating a conformational change to gD that allows it to interact with gH/gL. Once gH/gL is activated by gD, it upregulates the fusion activity of gB. In addition to these four proteins, gC has been shown to play a critical role in virus attachment via heparan sulfate (23, 24). In animal models, gC, gD, gH/gL, and gB stimulate a neutralizing Ab response (13, 25–29). gC also plays a critical role in immune evasion, acting as a complement receptor; immunization of animals with gC blocks this function (25, 27, 30, 31). A recent vaccine trial using gD as the sole immunogen failed to protect doubly seronegative women against genital HSV-2 disease but did show some efficacy against HSV-1 (7, 32, 33). In 10 samples with the most potent neutralizing activity, we found that the strongest correlates of neutralization were the overall gD-specific IgG content, and the combined response to the four major gD epitopes (34). These data suggest that optimal virus neutralization, and possibly protection, would be achieved by strong and balanced responses to the major neutralizing epitopes of gD.
Having established that in a vaccine setting, gD stimulates neutralizing Ab responses, we next examined the relationship between viral shedding and the properties of Abs to the viral entry glycoproteins in a small cohort of HSV-infected people, 5 of whom shed large amounts of virus and 5 of whom shed lower amounts (5, 35). We purified IgGs from the sera and assayed (i) the neutralization of HSV-1 and HSV-2, (ii) the ability to bind the HSV entry glycoproteins, (iii) the recognition of gD and gB epitopes (10, 12, 29, 36, 37) using a Biacore-based Ab competition assay, and (iv) the contribution of anti-gD and anti-gB Abs to overall neutralization, using affinity chromatography to isolate glycoprotein-specific IgGs. We concluded that the dominant immune response that accounted for neutralization was due to gD or a combination of gD+gB, irrespective of whether the sample was from an individual demonstrating low or high virus shedding. Moreover, both high- and low-virus-shedding individuals had similar ranges of neutralizing titers and similar repertoires of gD and gB epitopes. Nonetheless, Abs to three epitopes of gD, two involved in receptor binding (DL11 and MC23) and the third involved in gD-gH/gL interaction (MC5), correlated quite well with total IgG neutralization. For gB, the Ab response was directed almost entirely to a region of gB close to its fusion loops, as well as a region associated with gB-gH/gL interaction (38).
Because our initial study was of a small cohort, we decided to extend our analysis and focus on a comparison between sera from 9 people who were infected long-term and suffered recurrent episodes with sera from 23 people who remained asymptomatic following a primary genital HSV infection. These 32 samples, plus the original 10 samples from our previous study, came from a much larger set of human sera from people enrolled in a sexually transmitted disease surveillance study of HSV infection (5, 35). Our major goal was to determine whether a lack of recurrent symptoms correlates with differences in virus neutralization and glycoprotein binding and/or epitope recognition. A second goal was to be able to establish assays that could be expanded to examine the larger numbers of patient specimens that we have on hand. Octet RED96, a label-free system based upon biolayer interferometry, enables us to carry out binding and blocking studies using small volumes of patient sera. Furthermore, we now use streptavidin-coated magnetic beads to capture biotinylated proteins as a means to deplete serum specimens of specific Abs in order to determine the contribution of the missing Ab to overall neutralization.
We focused our binding and monoclonal Ab (MAb) blocking studies on gD and gB, since IgG against these two glycoproteins accounted for virtually all of the HSV-2 neutralization activity in our previous study (35). Although the average neutralization titers, glycoprotein binding, and epitope blocking activity were all higher in sera from the symptomatic subjects than those from the asymptomatic group, most of the differences were below the threshold of significance. This prompted us to delve more deeply and dissect the sera for what comprised the response to HSV infection.
Using the magnetic bead-based Ab depletion assay, we focused on six samples (two from the recurrent group and four from the nonrecurrent group). As in the previous study, we found samples where the dominant immune response was due to either gD or gD+gB. However, we also identified samples where the virus neutralization activity was due to solely gB. Furthermore, we found several examples where type-specific responses to gD and/or gB accounted for neutralization of either HSV-1 or HSV-2. In the end, the six serum samples that we dissected fell into six different categorical responses of neutralizing Abs against HSV glycoproteins, emphasizing the complexity of the human response to HSV infection.
MATERIALS AND METHODS
Cells and soluble proteins.
African green monkey kidney (Vero) cells were grown in Dulbecco modified Eagle medium with 5% fetal bovine serum. All soluble HSV glycoproteins were produced from baculovirus-infected insect (Sf9) cells. HSV-1 and HSV-2 gD(285t) and gD(306t) were purified using a DL6 immunosorbent column (36). gC1(457t) was purified using a heparin column as previously described (39). gH1(803t)/gL1 was purified via a C-terminal His6 tag by use of Ni-nitrilotriacetic acid resin and elution with imidazole (40). gB1(730t) and gB2(727t) were purified by using a DL16 immunosorbent column (41, 42).
Antibodies.
The gD-specific MAbs 1D3, DL11, MC2, MC5, MC14, and MC23 (29, 36, 43, 44) and the gB-specific MAbs SS144, C226, and SS10 (10) were characterized previously. Epitope mapping of these MAbs onto the gD and gB crystal structures, along with an abbreviated MAb tree for each glycoprotein, was published by Cairns et al. (35).
Human samples.
Serum samples were obtained from HSV-2-infected persons enrolled in natural history studies at the University of Washington Virology Research Clinic in Seattle, WA. The samples were purposefully chosen for the present study according to recurrence of genital lesions and time after primary infection. All samples were also known to be HIV negative. IRB 815813 (Human sera with HSV2 antibodies [approval date, April 2014]).
Biosensor experiments.
Experiments were performed at room temperature using either purified sample IgG on a Biacore 3000 biosensor at 25°C as described previously (34, 35) or serum samples on an Octet RED96 system (Pall ForteBio) at 30°C (45). For the Octet analysis, we used SA (streptavidin) biosensors and kinetics buffer (phosphate-buffered saline, 0.1% bovine serum albumin, 0.02% Tween) in all experiments. One milligram of each glycoprotein was biotinylated at a 1:1 ratio with EZ-Link Sulfo-NHS-Biotin (Pierce Biotechnology, Rockford, IL) so that it would bind to the SA biosensor. The concentrations of protein, human serum, and MAb IgG were optimized prior to the experiments. To determine how much protein to bind to the biosensor, we used a serum sample from our previous study (35) which we knew had high concentrations of anti-gD and anti-gB Abs. Using a concentration of 2% serum of this sample, we determined the titers of gD2(285t) and gB2(730t) to choose a concentration of each that would generate both a saturating signal and a good signal against the competing MAb: 0.15 μg/ml for gD2 and 0.625 μg/ml for gB2. However, when we did competition with the anti-gD MAb MC2, we used a gD2 concentration of 1.5 μg/ml to get a signal comparable to that when other anti-gD MAbs were used. We believe that the need for a 10-fold-higher concentration of MC2 is due to partial blocking of the MC2 gD epitope by biotin. A human serum that was negative for HSV-1 and HSV-2 was diluted to 2% in kinetics buffer and used to block the biosensors prior to the addition of the test serum. Each test serum was also diluted to 2% in kinetics buffer prior to use. MAbs were used at 20 to 50 μg of IgG/ml to achieve a signal suitable for competition studies. The Octet assay protocol was as follows: baseline (kinetic buffer) for 60 s, loading (soluble bt-gD2 or bt-gB2) for 60 s, blocking (negative serum) for 360 s, association of test sera for 600 s, MAb baseline (kinetic buffer) for 210 s, MAb IgG (diluted in kinetics buffer) for 180 s, and finally kinetics buffer for 120 s.
Virus neutralization assays.
Serial dilutions of IgG were mixed with HSV-1(KOS) or HSV-2(333), and the mixture was incubated at 37°C for 1 h. Monolayers of Vero cells grown in 48-well plates were then incubated with the IgG-virus mixture for 24 h. Cells were fixed with methanol-acetone (1:1), and plaques were visualized by the black plaque assay (39).
Statistical analysis of serum samples.
Neutralizing Ab titers were normally distributed after log2 transformation; we therefore used a two-tailed Student t test for unpaired samples (46) to compare recurrent and nonrecurrent individuals. Other data, including total Ab binding to gD and gB, and serum competition with MAb binding were not normally distributed. Therefore, we used a two-tailed Mann-Whitney U test for unpaired samples of unequal variance (47) to compare recurrent and nonrecurrent individuals. The data were judged to be normally distributed using a Shapiro test (48), as well as visual inspection of a quantile-quantile plot against normal distribution. Statistical analysis and plotting was performed using the R Project for Statistical Computing (49). A Student t test was performed as implemented in the function “t.test,” and a Mann-Whitney U test was performed as implemented in the function “wilcox.test.”
Depletion of glycoprotein-specific Abs from human sera via streptavidin-conjugated magnetic beads.
According to the manufacture's protocol, 1 mg of soluble glycoprotein [gD2(306t), gD1(306t), gB2(727)t, gB1(730)t, gC1t, or gH1(803t)t/gL1] was biotin-labeled using EZ-Link Sulfo-NHS-Biotin (Pierce Biotechnology, Rockford, IL). Excess nonreacted biotin was removed with Ultracel-30 Amicon ultracentrifugal filters (Millipore). Then, 5 mg of Pierce streptavidin magnetic beads (Thermo Scientific, catalog no. 88816) were prewashed three times with washing and binding buffer (50 mM Tris, 0.15 M NaCl, 0.1% Tween 20) using a magnetic stand (DynaMag-Spin; Life Technology, Grand Island, NY) to separate the beds from the buffer. Each biotinylated glycoprotein (1 ml at a concentration of 0.5 mg/ml) was incubated with the magnetic beads at 4°C overnight. Glycoprotein-conjugated magnetic beads were then washed three times in buffer, followed by incubation with 1 ml of each serum sample (5% serum diluted in buffer) at 4°C overnight. Supernatants (now depleted of Abs against specific HSV glycoproteins) were collected using the magnetic stand and then tested in an enzyme-linked immunosorbent assay (ELISA) and neutralization assays. The glycoprotein-conjugated magnetic beads were regenerated with 0.1 M ethanolamine, suspended in buffer, and stored at 4°C.
RESULTS
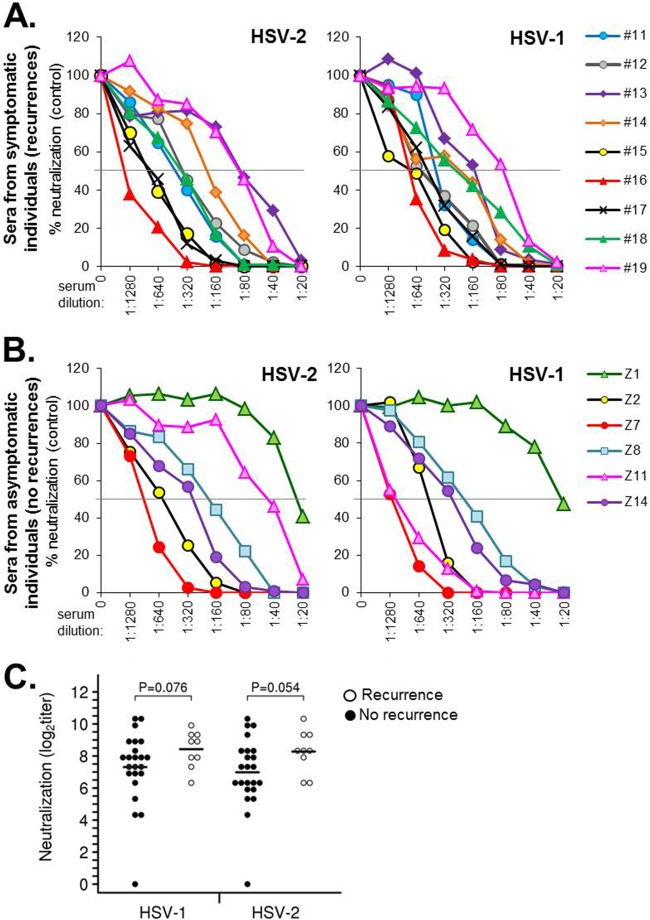
We obtained a number of serum samples from people enrolled in natural history studies at the University of Washington Virology Research Clinic (35). These samples included 9 people who had suffered multiple recurrences of genital HSV infections over a long period of time (>20 years; Table 1) and 23 people who were asymptomatic for disease following a primary infection (5). The samples contained a mix of individuals who were either infected with HSV-2 only or dually infected with both HSV-2 and HSV-1 (Tables 1 and 2). We first tested each serum sample for neutralization against HSV-2(333) and HSV-1(KOS) using a standard 50% plaque reduction assay (Fig. 1). The same color scheme used to display neutralization data (Fig. 1) is maintained in the binding and blocking data (see Fig. 3 and 4). For the symptomatic cohort, the plots show the results for all 9 sera (Fig. 1A). For the larger cohort of asymptomatic specimens, we show plots of the data for 6 representative samples (Fig. 1B) typical of the range of titers seen in the full cohort of 23 (Table 2). The average titers for the nonrecurrent group were somewhat lower against both HSV-2 (P = 0.054) and HSV-1 (P = 0.076) than those of the recurrent group, but the differences were just above the level of statistical significance (Fig. 1C). Two samples in the asymptomatic group, Z1 and Z21, had little to no neutralization activity against either virus. In all cases except one (sample Z11), the neutralizing titers against each virus were within a 0 to 1 dilution of each other. For Z11, the titer against HSV-1 was 1:1,280 but only 1:40 against HSV-2, indicating a type 1-specific response. We concluded that there was a difference in the total neutralizing response by the two groups but that it did not strongly correlate with recurrence versus nonrecurrence with this sample size.
TABLE 1.
Characterization of sera from symptomatic individuals
| Sample | Time (yr) after primary infection | No. of recurrences/yr | HSV statusa | % blockingb |
HSV neutralization activityc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| αgD MAb |
αgB MAb |
|||||||||||||
| 1D3 | MC23 | DL11 | MC2 | MC5 | MC14 | SS144 | C226 | SS10 | HSV-1 | HSV-2 | ||||
| 11 | 28 | 4 | 1,2 | 29 | 94 | 78 | 65 | 84 | 10 | 88 | 27 | 27 | 480 | 320 |
| 12 | 27 | 18 | 1,2 | 16 | 81 | 67 | 28 | 71 | 7 | 99 | 27 | 26 | 640 | 320 |
| 13 | 27 | 4 | 1,2 | 15 | 44 | 36 | 28 | 52 | 6 | 52 | 27 | 5 | 160 | 80 |
| 14 | 22 | 10 | 2 | 26 | 91 | 48 | 51 | 71 | 5 | 94 | 27 | 34 | 240 | 240 |
| 15 | 22 | 3 | 1,2 | 48 | 100 | 87 | 88 | 99 | 12 | 81 | 61 | 34 | 640 | 640 |
| 16 | 23 | 13 | 2 | 37 | 100 | 88 | 88 | 96 | 4 | 92 | 52 | 48 | 960 | 2,560 |
| 17 | 34 | 2 | 2 | 34 | 88 | 64 | 62 | 100 | 4 | 42 | 24 | 49 | 480 | 640 |
| 18 | 27 | 4 | 2 | 19 | 64 | 53 | 36 | 61 | 4 | 97 | 48 | 40 | 240 | 320 |
| 19 | 29 | 4 | 1,2 | 14 | 53 | 34 | 15 | 49 | 0 | 37 | 17 | 20 | 80 | 80 |
TABLE 2.
Characterization of sera from individuals with zero recurrences (asymptomatic)
| Sample | Time (yr) after primary infectiona | HSV status | % blockingb |
HSV neutralization activityc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| αgD MAb |
αgB MAb |
||||||||||||
| 1D3 | MC23 | DL11 | MC2 | MC5 | MC14 | SS144 | C226 | SS10 | HSV-1 | HSV-2 | |||
| Z1 | ? | 2 | 4 | 22 | 14 | 15 | 40 | 3 | NB | NB | NB | 20 | 20 |
| Z2 | 4 | 2 | 35 | 98 | 83 | 82 | 95 | 5 | 87 | 52 | 39 | 480 | 640 |
| Z3 | ? | 1,2 | 19 | 80 | 73 | 73 | 91 | 0 | 87 | 58 | 64 | 480 | 960 |
| Z4 | 4 | 2 | 14 | 26 | 11 | 17 | 29 | 9 | 25 | 13 | 31 | 20 | 60 |
| Z5 | ? | 1,2 | 6 | 45 | 40 | 26 | 61 | 0 | NB | NB | NB | 120 | 80 |
| Z6 | ? | 1,2 | 14 | 62 | 46 | 61 | 61 | 2 | 64 | 25 | 52 | 240 | 80 |
| Z7 | ? | 2 | 44 | 93 | 79 | 96 | 100 | 0 | NB | NB | NB | 1,280 | 960 |
| Z8 | 0.7 | 1,2 | 11 | 71 | 48 | 6 | 46 | 0 | 81 | 15 | 16 | 240 | 240 |
| Z9 | ? | 1,2 | 24 | 67 | 58 | 45 | 79 | 5 | 100 | 18 | 1 | 240 | 240 |
| Z10 | ? | 1,2 | 11 | 33 | 28 | 19 | 49 | 5 | NB | NB | NB | 40 | 40 |
| Z11 | 10 | 1 | 28 | 50 | 39 | 24 | 70 | 7 | 81 | 27 | 22 | 1,280 | 40 |
| Z12 | ? | 1,2 | 28 | 50 | 33 | 26 | 70 | 2 | 85 | 18 | 35 | 80 | 160 |
| Z13 | 16 | 2 | 24 | 77 | 73 | 39 | 83 | 5 | 0 | 27 | 12 | 160 | 320 |
| Z14 | ? | 2 | 16 | 61 | 50 | 38 | 81 | 1 | 100 | 48 | 85 | 320 | 320 |
| Z15 | ? | 2 | 31 | 81 | 52 | 42 | 85 | 7 | 72 | 7 | 0 | 160 | 160 |
| Z16 | ? | 1,2 | 53 | 45 | 17 | 19 | 46 | 7 | 78 | 7 | 1 | 240 | 60 |
| Z17 | ? | 2 | 19 | 64 | 30 | 0 | 34 | 7 | 32 | 0 | 0 | 120 | 160 |
| Z18 | ? | 1,2 | 23 | 100 | 71 | 63 | 50 | 0 | 21 | 0 | 16 | 960 | >1,280 |
| Z19 | ? | 1,2 | 25 | 52 | 52 | 34 | 70 | 0 | 73 | 26 | 19 | 480 | 80 |
| Z20 | 5 | 1,2 | 1 | 50 | 38 | 14 | 41 | 0 | NB | NB | NB | 160 | 80 |
| Z21 | 4 | 2 | 7 | 13 | 13 | 18 | 14 | 6 | 0 | 5 | 24 | – | – |
| Z22 | ? | 2 | 17 | 67 | 71 | 43 | 75 | 0 | 90 | 8 | 23 | 240 | >320 |
| Z23 | ? | 2 | 16 | 56 | 37 | 15 | 50 | 8 | NB | NB | NB | 120 | 80 |
A question mark (?) indicates that the number of years when the sample was taken after the primary infection is not known.
That is, the percent activity compared to the no-antibody control. NB, no binding to gB.
Values represent the dilution of serum required for a 50% reduction in plaques. Example, 1:20 is listed as 20. –, no neutralization.
FIG 1.
Virus neutralization. Sample sera were tested for their ability to neutralize HSV (types 1 and 2). Plaque numbers were determined for each sample and plotted as a percentage of plaques obtained in the absence of human serum (y axis). Serum dilutions are indicated on the x axis. Sera were obtained from either long-term-infected individuals with recurrent disease (symptomatic) (A) or individuals with no (zero) recurrence of disease (asymptomatic) (B). For samples from nonrecurrent individuals, a select set of sera are graphed in panel B. HSV neutralization data for all of the samples are provided in Table 2. (C) Statistical analysis of the neutralizing titer of patient sera from individuals with recurrent or nonrecurrent disease. The data from individuals with no clinical HSV recurrence are represented with black circles, individuals with HSV recurrence are represented with white circles, and horizontal black bars indicate the mean value. Symptomatic individuals produced a higher titer of neutralizing antibodies on average, but the variance between individuals resulted in a P value above 0.05.
FIG 3.
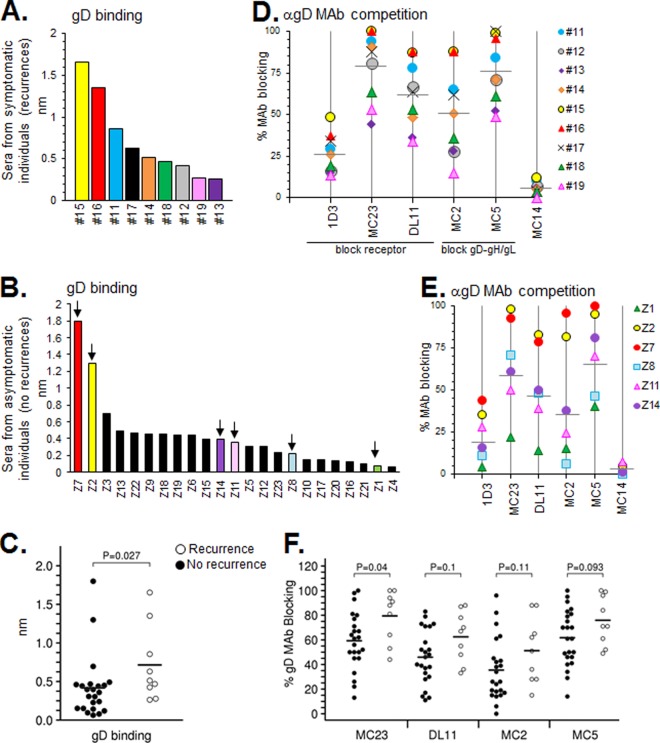
(A and B) Antibody binding to gD was tested using human sera on an Octet RED96 system as described in Materials and Methods. Samples are ranked from high to low for gD binding. (D and E) Blocking of neutralizing anti-gD MAbs via human subject serum. Each point indicates the percent blocking activity (compared to a no-Ab control) against the MAbs tested. Horizontal gray bars denote average blocking of that particular MAb among the samples. Sera from individuals with recurrent infection are shown in panels A and D, while those without are shown in panels B and E. For those with no recurrences, only selected samples from panel B (arrows) were graphed in panel E. Samples are color-coded the same between gD binding and MAb competition graphs. Anti-gD MAb competition data for all of the nonrecurrent samples are provided in Table 2. (C and F) Statistical analysis of the gD binding and MAb blocking capability of patient sera from individuals with recurrent or nonrecurrent disease. The data from individuals with no clinical HSV recurrence are represented with black circles, individuals with HSV recurrence are represented with white circles, and horizontal black bars indicate the mean value. Using Octet data from panels A and B, we found significantly more Abs targeted gD in recurring individuals than nonrecurring individuals (C). Likewise, individuals with recurrent disease produced more Abs directed toward all of the gD MAb epitopes we studied, and yet only the MC23 epitope reached statistical significance (F).
FIG 4.
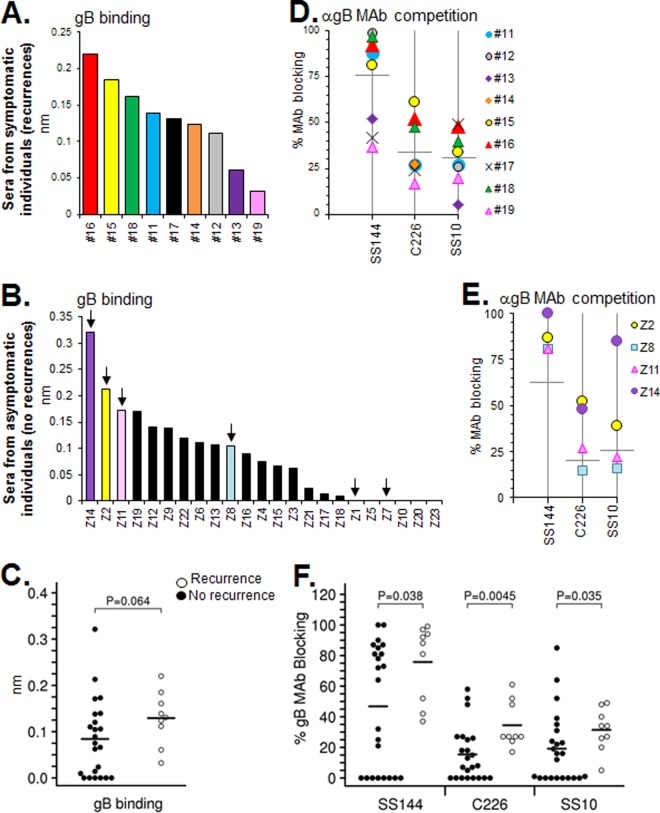
(A to F) Human sera binding to gB, competition with neutralizing anti-gB MAbs, and statistical analysis of these data was set up in the same manner as described for gD in Fig. 2. For sera from individuals with no recurrent disease, only selected samples from panel B (arrows) were graphed in panel E. Anti-gB MAb competition data for all of the nonrecurrent samples is provided in Table 2. Samples Z1 and Z7, which did not bind gB in panel B, were not tested for MAb competition in panel E. Those with recurrent infections produced Abs that exhibited greater gB binding and anti-gB MAb competition; blocking of all three anti-gB epitopes tested was statistically different between recurrent and nonrecurrent individuals.
The neutralization results in Fig. 1 represent potential contributions from all of the glycoproteins of both serotypes that can individually stimulate neutralizing Abs. However, we previously found that Abs against gD or gD+gB accounted for most, if not all, of the neutralizing activity (35). Still, we remained open to the possibility that gH/gL and/or gC might also play a role in some cases. The neutralization results from our current study raised the following questions. (i) How well did the sera bind to gD and gB? (ii) Which anti-gD and anti-gB epitopes were recognized by the sera? (iii) To what extent did each individual entry glycoprotein (including gC and gH/gL) contribute to HSV-1 and HSV-2 neutralization, and would gD and gB account for all virus neutralization as seen in our previous study?
Use of the Octet RED96 system to analyze binding and epitope profiles of human sera.
We previously used the BIAcore 3000 to assay IgG purified from 10 serum human samples for glycoprotein binding and competition with MAbs to key epitopes (35). Aside from the need to purify IgG, the study required a relatively large sample volume. In order to more efficiently examine multiple samples, we have now used the ForteBio Octet RED96 system (45). This system uses an eight-channel, dip-and-read format that has several advantages: (i) use of serum rather than IgG, (ii) smaller sample volume, and (iii) rapid screening.
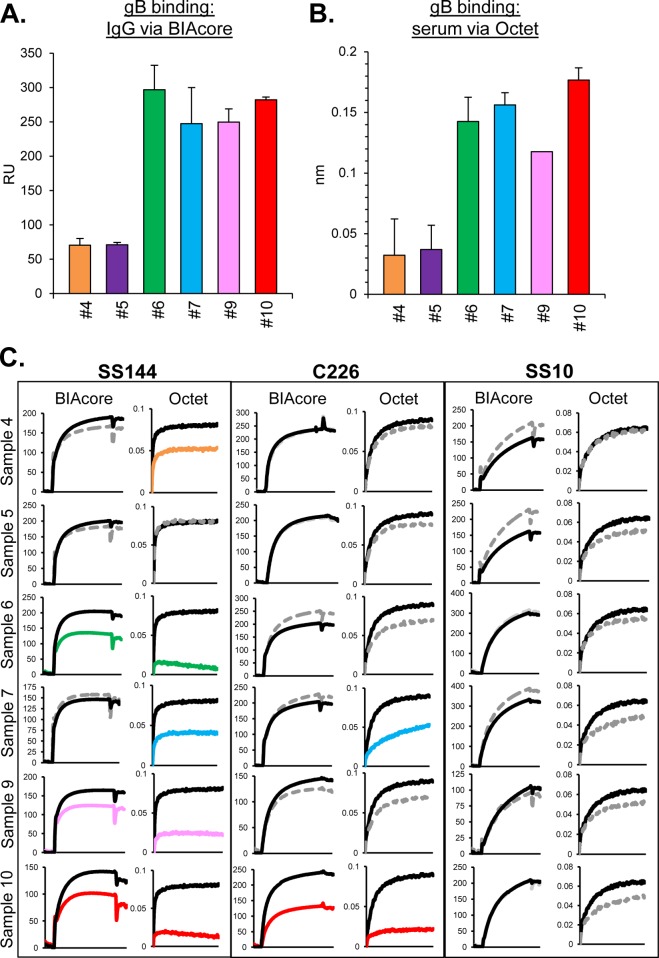
To be certain that we could compare the results from the present study with our previous one, we selected six samples from our prior study that were analyzed IgG/BIAcore and now analyzed sera from each sample on the Octet RED96 system. To compare the two methods, we tested them for gB2 binding (Fig. 2A and B) and blocking of anti-gB MAbs (Fig. 2C). Octet data were generated by dipping a streptavidin biosensor [coated with biotinylated gB2(727t)] into 2% serum (see Materials and Methods). Binding data using the BIAcore were generated by capturing His-tagged gB onto a CM5 chip (presenting amine-coupled anti-His MAb), followed by flowing the test IgGs across the chip. Comparable levels of gB binding were noted using either IgG/BIAcore (Fig. 2A) or serum/Octet (Fig. 2B).
FIG 2.
Samples from HSV-infected humans, serum versus IgG. Antibody binding to gB was tested using purified IgG on a BIAcore 3000 biosensor (A) or using serum on an Octet RED96 system (B) as described in Materials and Methods. Relative binding units (RU) are indicated on the y axis. The averages of at least two experiments are shown; error bars indicate the standard errors. (C) Blocking of neutralizing anti-gB MAbs via human subject IgG (BIAcore) or sera (Octet). Binding curves for the association of test MAbs (SS144, C226, and SS10) to gB are shown. Black lines indicate MAb binding to gB that was not exposed to human subject samples (positive control). Gray dotted lines denote samples that block MAb binding less than 25% that of control. The curves reflect the extent to which Abs within the serum blocked MAb binding (steep curve, close to the black control curve = no MAb blocking; shallow curve = MAb blocking). The data using human IgG on the BIAcore for these six samples was first reported in Cairns et al. (35).
Next, we tested the six sera in a MAb competition assay. Using the Octet RED96 system, the gB-coated biosensors were first loaded with one serum (Fig. 2B) and then dipped into the indicated anti-gB MAb (Fig. 2C). A biosensor with gB (but not loaded with serum) was used as a positive control for the binding of each MAb (Fig. 2C, black line). Regardless of which method we used, none of the samples (IgG or serum) blocked the binding of SS10 (Fig. 2C). In addition, all samples that were positive for the blocking of SS144 and C226 via IgG BIAcore were also positive when measured on the Octet, although the extent of blocking was greater on the Octet. Moreover, by Octet (but not by BIAcore) we detected blocking of MAb SS144 by samples 4 and 7 and blocking of MAb C226 by sample 7. The greater sensitivity of the Octet may be due to the use of serum (which could include other Ab subtypes and/or lower affinity Abs) rather than purified IgG. Overall, our results using the Octet gave us confidence to use this method for the results presented here.
Comparison of gD binding and anti-gD MAb blocking by sera from subjects with recurrent versus nonrecurrent HSV infection.
All of the sera from the recurrent group bound to gD2 but to various extents (Fig. 3A). Although two samples from the nonrecurrent group showed high levels of gD binding, the majority of the 23 sera exhibited lower levels of binding (Fig. 3B). Of the nine sera in the recurrent group, binding to gD was greater in seven cases than the mean value of the sera from nonrecurring individuals (Fig. 3C). Correspondingly, sera from the recurrent cohort exhibited a statistically greater level of gD binding than those in the nonrecurrent cohort (P = 0.03; Fig. 3C). We conclude that recurrent infection results in higher levels of gD binding by total serum.
Six anti-gD MAbs were used to determine the epitope composition of the sera from the two cohorts. Each affects gD function, but in different ways. Three MAbs block gD-receptor binding (1D3, MC23, and DL11), while two (MC2 and MC5) block another function, presumably the interaction between gD and gH/gL (29, 36, 37). All five of these MAbs neutralize HSV. We also included the non-neutralizing MAb MC14, which significantly enhances neutralization by MAb MC2 (36). In two previous studies of either gD-vaccinated subjects (34) or naturally infected people (35), IgGs from all samples blocked key conformational anti-gD epitopes (MC23, DL11, MC2, and MC5), and yet none blocked binding to linear epitopes (1D3 and MC14). In the present study, all nine sera in the recurrent group blocked the binding of MC23, DL11, and MC5 above a 20% threshold (Fig. 3D). All but one (sample 19) blocked the binding of MC2. Thus, Abs that affect both functions of gD were generated by the human immune response in this cohort, similar to what we found previously. Also as before, none of the sera blocked MC14. However, we were now able to detect appreciable competition with MAb 1D3 in several of the sera (Fig. 3D), which had not been seen in any of the IgGs previously evaluated. The extent to which each serum sample blocked the MAb panel correlated well with gD binding, i.e., the highest levels of gD binding (samples 11, 15, and 16; Fig. 3A) also showed the highest levels (>60%) of MAb blocking for MC2, MC5, MC23, and DL11 (Fig. 3D and Table 1). Those that showed the lowest levels of gD binding (samples 13 and 19) showed much less blocking of all of the anti-gD MAbs, particularly MC2. Thus, the degree to which the sera blocked any one MAb correlated with the level of gD binding.
In the nonrecurrent group, there were also variations in how well each serum blocked anti-gD MAb binding. Due to the larger number of samples tested, Fig. 3E only shows representative data for 6 sera; data for the complete cohort are presented in Table 2. As with the recurrent group, these sera targeted epitopes analogous to those that interfere with both functions of gD, and did so in accord with their level of gD binding. Competition (>20%) with the MAb 1D3 was also noted in 10 of 23 nonrecurrent samples (Table 2). MAb MC14 failed yet again to compete with any of the sera. It was difficult to ascribe the binding and blocking data for the sera to the level of neutralization. However, we observed that some sera with high neutralization titers (samples Z2 and Z7) also had had gD binding and MAb blocking levels (Tables 1 and 2). Likewise, sample Z1 had a low virus titer and was also a poor gD binder.
A comparison of MAb blocking by sera from symptomatic versus asymptomatic subjects (Fig. 3F) showed that the mean value for blocking of MAb MC23 was statistically higher (P = 0.04) in the recurrent cohort. Mean values for blocking of other neutralizing MAbs (DL11, MC2, and MC5) were all higher for the recurrent cohort, but the variant in blocking was not statistically significant within this cohort size. Thus, although many of the sera in both cohorts recognized key gD epitopes, there was only one (MC23, which blocks receptor binding) that correlated significantly with recurrent infection.
Comparison of gB binding and anti-gB MAb blocking by sera from subjects with recurrent versus nonrecurrent HSV infection.
Similar to our observations with gD-binding Abs (Fig. 3A and B), the concentration of gB binding Abs varied among individuals with recurrent and nonrecurrent HSV infection (Fig. 4A and B). For the recurrent group, all of the sera bound to gB at a detectable level (Fig. 4A). In contrast, 6 of the 23 sera from the nonrecurrent cohort (26%) did not bind to gB at the given concentration (Fig. 4B). Serum binding to gB was greater for the recurrent group, but the difference was just above the threshold of statistical significance (Fig. 4C). Interestingly, for the recurrent group, the gB binding pattern generally followed that of gD. For example, two sera from this cohort that bound very well to gD also bound well to gB (samples 15 [yellow] and 16 [red]), whereas the two poorest gB binders also bound poorly to gD (samples 13 [purple] and 19 [pink]; compare Fig. 4A to Fig. 3A). We plotted gD binding values (RU) against the binding of gB and calculated R2 values for each plot (data not shown). As predicted, the level of gD-gB binding correlated in the recurrent group (R2 = 0.66). However, in the asymptomatic group of sera, binding of gD (Fig. 3B) did not correlate with gB binding (R2 = 0.02) (Fig. 4B). For example, sample Z7, which had the highest level of gD binding of any in the nonrecurrent group (Fig. 3B, red), had no detectable binding to gB (Fig. 4B, arrow).
To analyze the epitopes of gB seen by the sera, we used three MAbs that block gB function in different ways and map to three distinct functional regions (FR) (10). MAb SS144, in FR1, contains the gB fusion loops; MAb C226, in FR2, blocks the interaction between gB and gH/gL; and MAb SS10, in FR3, blocks the association of gB with target cells (10, 42, 50–52). In our previous study (35), response to these gB epitopes was limited: 3 of the 10 human IgGs tested blocked SS144, 1 blocked C226, and none blocked SS10. However, reexamination of these samples on the Octet RED96 system revealed an additional two samples that competed with SS144, one of which also competed with C226 (Fig. 2C). In contrast to what we found in our earlier study, we detected blocking of SS144 in all serum samples from the recurrent cohort and blocking of C226 in all except sample 19 (Fig. 4D). We were also able to detect for the first time competition with FR3 MAb SS10 (in 7 of the 9 samples). In part, this difference is explained by the greater sensitivity of the Octet system (Fig. 2C). However, it may also reflect the selection of only long-term infected individuals in this cohort (Table 1); sera from our previous study that blocked SS144 and C226 were also from those infected long term (data not shown).
For the nonrecurrent cohort (Fig. 4E and Table 2), sera that showed high levels of binding to gB (e.g., samples Z2, Z11, and Z14) also exhibited high levels of blocking of SS144 and blocking of C226. A statistical analysis showed that blocking of SS144, C226, and SS10 was significantly greater in samples from the recurrent group versus the nonrecurrent group (Fig. 4F). The most significant difference was seen with MAb C226 (P = 0.0045). As we saw with gD, in several cases high binding and blocking of gB MAbs was associated with the overall titer of virus neutralizing activity (e.g., samples 16 and Z1 both had poor neutralization titers and also poor binding to gB). However, it was clear that the best way to relate neutralization to binding and/or blocking was to dissect the sera according to glycoprotein specificity and then reassess the neutralizing activity.
Dissection of the neutralizing Abs in sera from naturally infected people.
Previously (35), we used antigen-sorbent columns to capture glycoprotein-specific IgGs from sera of naturally infected people and showed that the total neutralizing activity of each IgG was due almost exclusively to Abs against gD or a combination of gD and gB. For the present study, we decided to use a different methodology that used antigen-coated magnetic bead to capture Abs to specific glycoproteins directly from sera. This technique has several advantages: (i) it uses small amounts of serum and eliminates the need to purify IgG for each sample, (ii) it can be adapted to screen large numbers of samples, and (iii) it is easier and faster to sequentially remove Abs to several different glycoproteins. In this assay, each biotinylated glycoprotein was immobilized on streptavidin-coated magnetic beads and then incubated with human serum. The beads selectively bound specific Abs against the glycoprotein, while the unbound fraction (supernatant) contained the remaining Abs. We used ELISA to confirm that each supernatant had been depleted of Abs against the particular protein. Finally, the depleted serum was assayed for HSV-1 and HSV-2 neutralization using a 50% plaque reduction assay. Based on what we had found in our prior study, we focused initially on gD and gB for capture.
We selected six human serum samples: samples 16 and 18 from the recurrent cohort and samples Z2, Z7, Z8, and Z11 from the nonrecurrent cohort (Tables 1 and 2). Selection of these six samples was based on neutralization titer, as well as MAb competition assays, which suggested that each sample had different mixes and quantities of Abs directed against gD and gB epitopes. For clarity, we report our analysis of each sample separately.
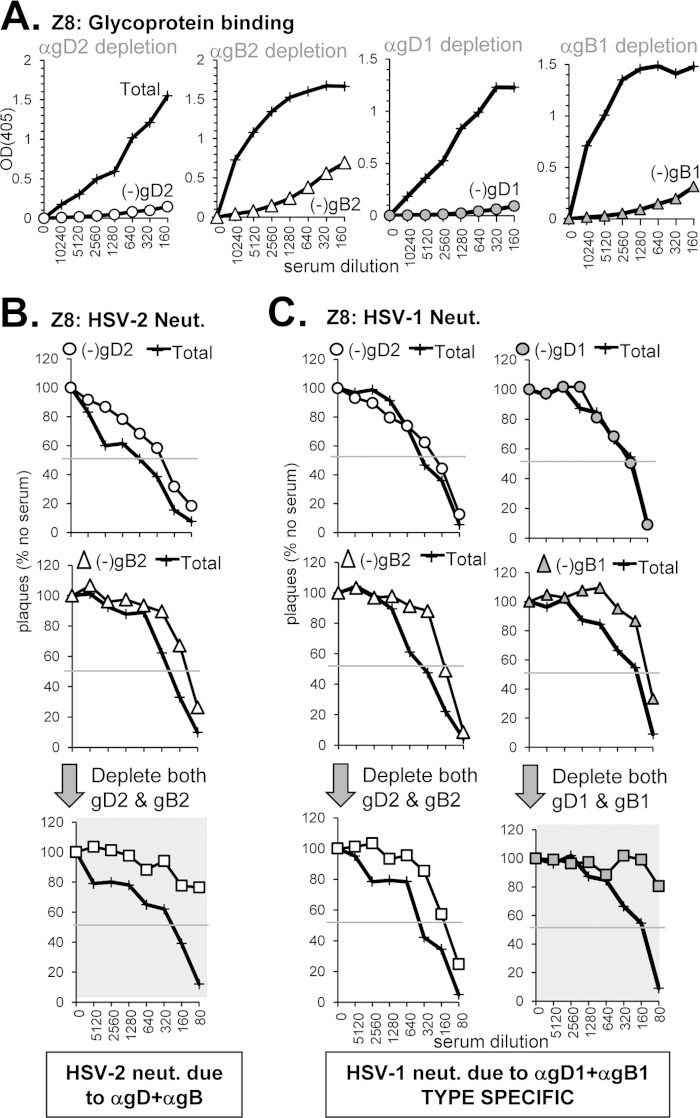
(i) Anti-gD neutralizing response without anti-gB (sample Z7).
Sample Z7 (from an HSV-2-infected subject) was chosen because it bound well to gD but not to gB (Fig. 3B and 4B), blocked five neutralizing anti-gD MAbs (Fig. 3E), and had the highest titers of type-common neutralizing activity (Fig. 1B and Table 2). Therefore, we predicted that the overall neutralization activity of Z7 should be due to type-common anti-gD Abs. Indeed, when we used gD2 to deplete Z7 of anti-gD Abs (Fig. 5A), all neutralization activity was lost against both HSV-2 and HSV-1 (Fig. 5B and C, highlighted in gray). As expected, when Z7 was depleted of anti-gB Abs (Fig. 5A) there was no change in its ability to neutralize either serotype of HSV (Fig. 5B and C). We conclude that all neutralization activity of Z7 serum is due to anti-gD Abs that recognize both serotypes of gD, and this correlated precisely with the binding and blocking data.
FIG 5.
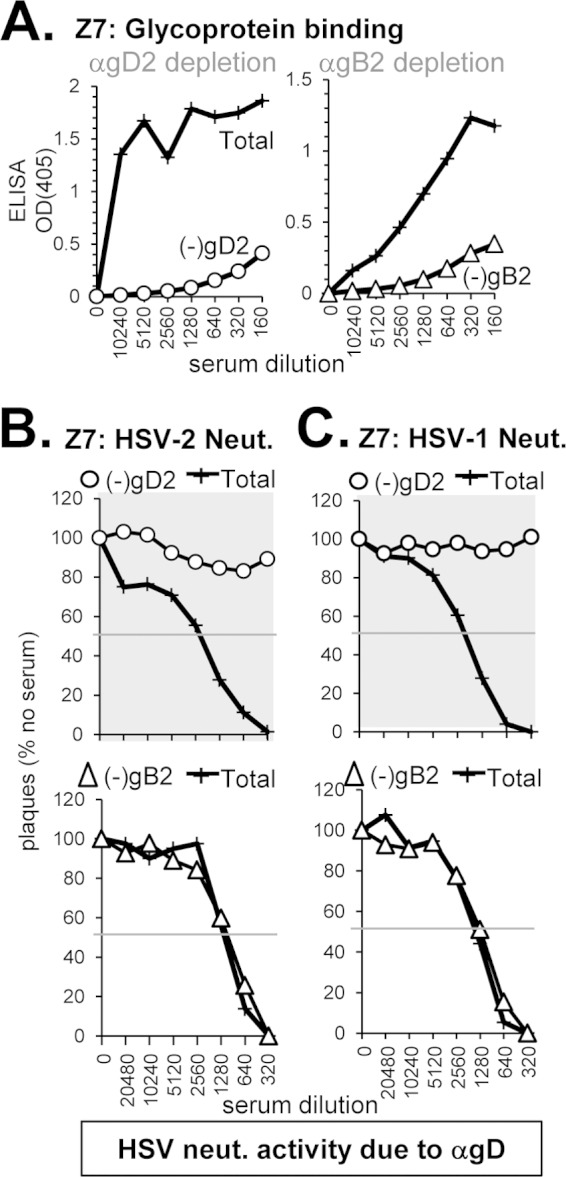
Depletion of gD- and gB-specific Abs from human serum sample Z7. To deplete human serum of glycoprotein-specific Abs, serum was incubated with biotinylated gD2 or gB2 that was bound to streptavidin-coated magnetic beads. (A) After depletion, the sample supernatant was tested by ELISA for binding to soluble gD2 (○) or gB2 (△); the optical density at 405 nm (OD405) is shown on the y axis, and the serum dilution is indicated on the x axis of each ELISA graph. Next, the depleted sample was tested for the ability to neutralize either HSV-2 (B) or HSV-1 (C). Plaque numbers were determined for each sample and plotted as a percentage of plaques obtained in the absence of human serum (y axis). Serum dilutions are indicated on the x axis. Curves where the neutralization activity was successfully depleted are highlighted with a gray background.
(ii) Type-specific neutralizing responses to gD plus gB (sample Z8).
Serum sample Z8 (from a dually infected subject) had a moderate type-common neutralization titer (Fig. 1B), exhibited fairly low levels of gD binding (Fig. 3B), and moderate gB binding (Fig. 4B). Although Z8 did not compete with the neutralizing anti-gD MAb MC2, it did compete with other key anti-gD MAbs (MC23, DL11, and MC5) (Fig. 3E). It also blocked anti-gB MAb SS144 very well but not C226 or SS10 (Fig. 4E). We questioned whether this narrow response to Abs against selected epitopes was sufficient to generate significant neutralization activity that could be attributed to anti-gD and/or anti-gB Abs. Depletion of Abs against either gD2 or gB2 (Fig. 6A) had only a minor effect on HSV-2 neutralization (Fig. 6B). We found that abrogation of HSV-2 neutralization could only be achieved when both anti-gD and anti-gB Abs were depleted from the serum (Fig. 6B, highlighted in gray), suggesting that neutralization activity is due to the additive effect of Abs to each glycoprotein. However, we were surprised to find that depletion of Abs to gD2 and gB2 had only a minimal effect on HSV-1 neutralization (Fig. 6C). We speculated that HSV-1 neutralization might be more type-specific, i.e., due to either gD1, gB1, or a combination of both. To test this idea, we first depleted the serum with gD1 (Fig. 6A) and found no change in HSV-1 neutralizing activity (Fig. 6C, upper right panel). When we depleted the Z8 serum using gB1 (Fig. 6A), there was a slight shift in the neutralization curve, similar to what we saw when anti-gB2 Abs were depleted (Fig. 6C, middle panels). However, we when depleted the serum of Abs against both gD1 and gB1, all HSV-1 neutralizing activity was lost (Fig. 6C, lower right panel). Thus, HSV-1 neutralization is almost entirely due to a combination of Abs against gD1 and gB1. The overall neutralizing activity of Z8, which appears to be type common from the 50% neutralization titers (Fig. 1B), does in fact have a type-specific component. This serum was obtained from a dually infected person and the type 1 specific response to gD and gB reflects this status. Because we had to use gD1 and gB1 in addition to gD2 and gB2 to tease out the answer for sample Z8, we decided to use both serotypes of glycoproteins for the remaining samples that we tested.
FIG 6.
Depletion of glycoprotein-specific Abs from human serum sample Z8. (A) Antibody depletion of serum was carried out as described in Fig. 4A. Depletion with gD1 is indicated with gray circles, and that of gB1 is indicated with gray triangles. The depleted serum was tested for neutralization activity against either HSV-2 (B) or HSV-1 (C) as in Fig. 4B and C, respectively. The bottom row in panel B and panel C depict samples where both Ab against gD and gB have been depleted (squares, white for type 2 and gray for type 1).
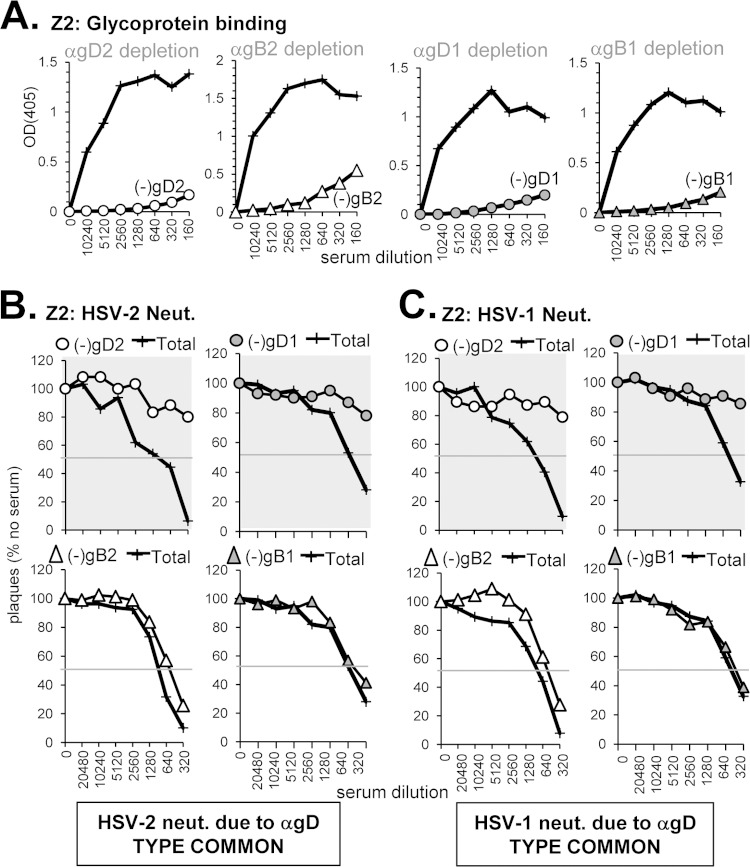
(iii) Type-common anti-gD neutralizing response in conjunction with a non-neutralizing anti-gB response (sample Z2).
Sample Z2 (from an HSV-2-infected subject) was chosen because it contained high levels of neutralizing Abs to both HSV-1 and HSV-2 (Fig. 1B), bound well to both gD and gB (Fig. 3B and 4B), and competed against each anti-gD and anti-gB neutralizing MAb tested (Fig. 3E and 4E). Therefore, we predicted that Z2 would contain both anti-gD and anti-gB neutralizing Abs. When Z2 was depleted of Abs against gD2 or gD1 (Fig. 7A), neutralizing activity was lost against both serotypes of HSV (Fig. 7B and C, top panels). Unexpectedly, depletion of Abs to either gB2 or gB1 (Fig. 7A) had little effect on neutralization of either virus (Fig. 7B and C, bottom panels). Because serum Z2 binds gB well and blocks neutralizing anti-gB MAbs, the depletion results suggest that this serum contains non-neutralizing anti-gB Abs that bind closely to these epitopes and therefore compete with the neutralizing MAbs. Indeed, several anti-gB MAbs that we have previously described are non-neutralizing and compete with SS144 or SS10 (10). In conclusion, we determined that the neutralizing activity of serum Z2 is due to type-common anti-gD Abs.
FIG 7.
Depletion of glycoprotein-specific Abs from human serum sample Z2. (A) Antibody depletion of serum was carried out as described in Fig. 5A. The depleted serum was tested for neutralization activity against either HSV-2 (B) or HSV-1 (C) as in Fig. 5B and C, respectively.
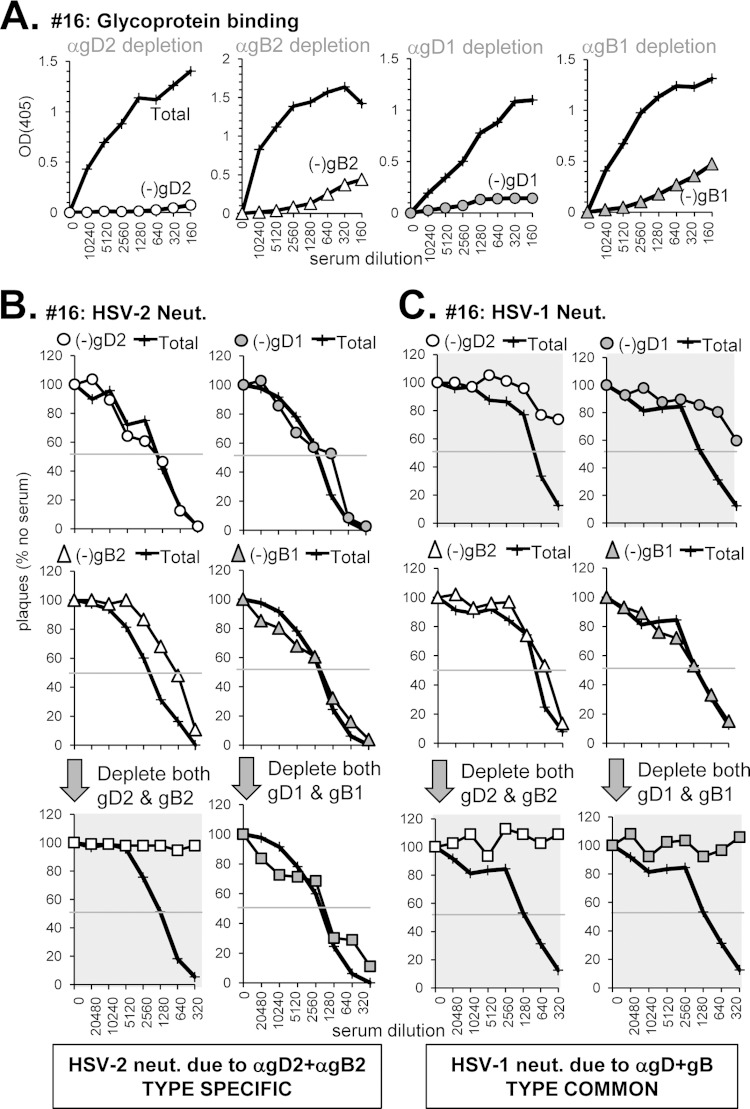
(iv) Neutralization via both type-specific and type-common anti-gD and anti-gB Abs (sample 16).
Serum sample 16 (from an HSV-2-infected subject) had the highest neutralizing titer of the 32 sera tested (1:2,560 for HSV-2, 1:960 for HSV-1) (Fig. 1, Table 1). It bound well to both gD and gB and blocked binding of MAbs to key epitopes of both (Fig. 3 and 4; Table 1). From these data, we predicted that neutralization activity would be due to type-common Abs against both gD and gB. However, analysis of this serum revealed a more complex pattern of neutralization.
For HSV-2, depletion of Abs directed at either gD2 or gB2 (Fig. 8A) did not significantly affect HSV-2 neutralization activity, whereas removal of Abs to both glycoproteins resulted in a complete loss of neutralization (Fig. 8B). This additive effect resembles what we found for HSV-2 neutralization by sample Z8 (Fig. 6B). However, when we depleted the serum of Abs to gD1 and gB1, there was no effect on HSV-2 neutralization, indicated that the combined response to HSV-2 is type specific.
FIG 8.
Depletion of glycoprotein-specific Abs from human serum sample 16. (A) Antibody depletion of serum was carried out as described in Fig. 5A. The depleted serum was tested for neutralization activity against either HSV-2 (B) or HSV-1 (C) as in Fig. 5B and C, respectively.
For HSV-1, the situation was quite different. In this case, depletion of Abs to gD2 or gD1 drastically reduced neutralizing activity (Fig. 8C, top panels), indicating a type-common response to gD comprises most of the neutralization activity in sample 16. However, type-common anti-gB Abs also appeared to contribute to some extent, as the neutralization curves further flattened upon sequential removal of gD and gB Abs (Fig. 8C, bottom panels). That sample 16 did not contain type 1 specific Abs was expected since the subject's infection status was HSV-2 only (Table 1).
Thus, our data show that type-2 specific Abs to gD and gB account for HSV-2 neutralization, but type-common Abs to gD (and a lesser extent gB) account for HSV-1 neutralization. Clearly, the overall titers against the two viruses involve different Abs.
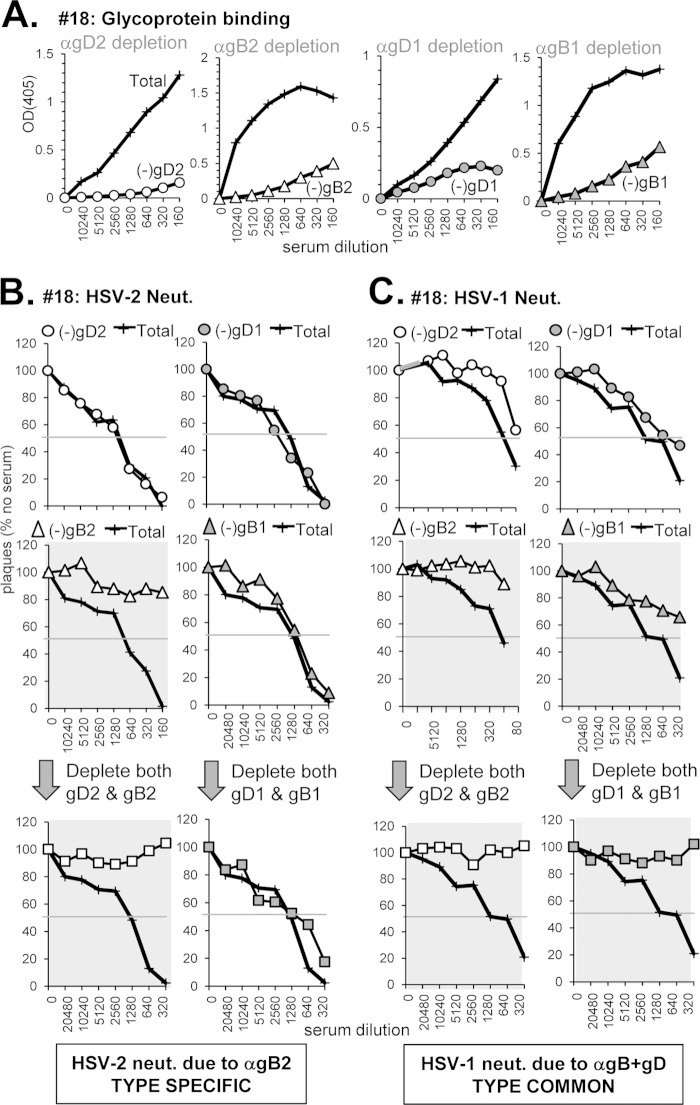
(v) Neutralization of HSV-2 by type-specific Abs to gB and neutralization of HSV-1 by type-common Abs to gD and gB (sample 18).
Sample 18 was chosen because it had the opposite profile of Z7: it bound gB well (Fig. 4A) and blocked key neutralizing anti-gB epitopes (Fig. 4D). However, compared to other sera from the long-term-infected, recurrent group it was diminished in gD binding and MAb blocking (Fig. 3A and D and Table 1). We predicted that the majority of its neutralization activity would be due to anti-gB Abs. Indeed, when anti-gD Abs of either serotype were depleted from the serum (Fig. 9A), there was no effect on HSV-2 neutralization (Fig. 9B) and only a minimal effect on HSV-1 (Fig. 9C). In contrast, depletion with gB2 resulted in a loss of neutralization activity for both HSV-2 and HSV-1 (Fig. 9B and C). However, depletion of Abs to gB1 had no effect on HSV-2 neutralization (Fig. 9B); this activity is therefore type specific and directed against gB2. Depletion of anti-gB1 Abs decreased HSV-1 activity by three dilutions (Fig. 9C). Further depletion with both gB1 and gD1 resulted in a complete loss of HSV-1 activity; the same result for HSV-1 neutralization was seen when gB2 and gD2 were used to deplete the serum (Fig. 9C, bottom panels). We conclude that for sample 18, neutralization of HSV-2 is due to type-specific anti-gB2 Abs but neutralization of HSV-1 is due to a mixture of type-common anti-gB and anti-gD Abs.
FIG 9.
Depletion of glycoprotein-specific Abs from human serum sample 18. (A) Antibody depletion of serum was carried out as described in Fig. 5A. The depleted serum was tested for neutralization activity against either HSV-2 (B) or HSV-1 (C) as in Fig. 5B and C, respectively.
(vi) Anti-gB-mediated neutralization specific for HSV-1 (sample Z11).
Sample Z11 was chosen because it neutralized HSV-1 very well (1:1280) but not HSV-2 (1:40) (Fig. 1B and Table 2). In fact, Z11 was isolated from an individual infected with HSV-1 only (Table 2). We were curious to see which Abs contributed to this HSV-1 specific neutralization. Sample Z11 bound to both gD and gB (Fig. 3B and 4B) and blocked neutralizing MAbs against both (Fig. 3E and 4E). Because of the type 1 specificity of the serum, we confined the depletion analysis to the effects on HSV-1 neutralization. We predicted that the type 1-specific neutralizing activity of Z11 could be due to a combination of Abs to both gD1 and gB1. As expected from the neutralization titer, depletion with gD2, gB2, or a combination of both (Fig. 10A) resulted in very little change to HSV-1 neutralization activity (Fig. 10B). Surprisingly, when we depleted this serum of Abs to gD1 there was also no effect (Fig. 10B). However, when we used gB1 for depletion there was a major effect on neutralization. Further depletion of anti-gD1 Abs in combination with anti-gB1 had a minor effect, as did depletion with a combination of gD1, gB1, gC1, and gH/1gL1 (Fig. 10B). We conclude that most of the neutralizing activity of Z11 is due to type 1-specific Abs to gB, with a small contribution from gD1 and either gC1 and/or gH1/gL1.
FIG 10.
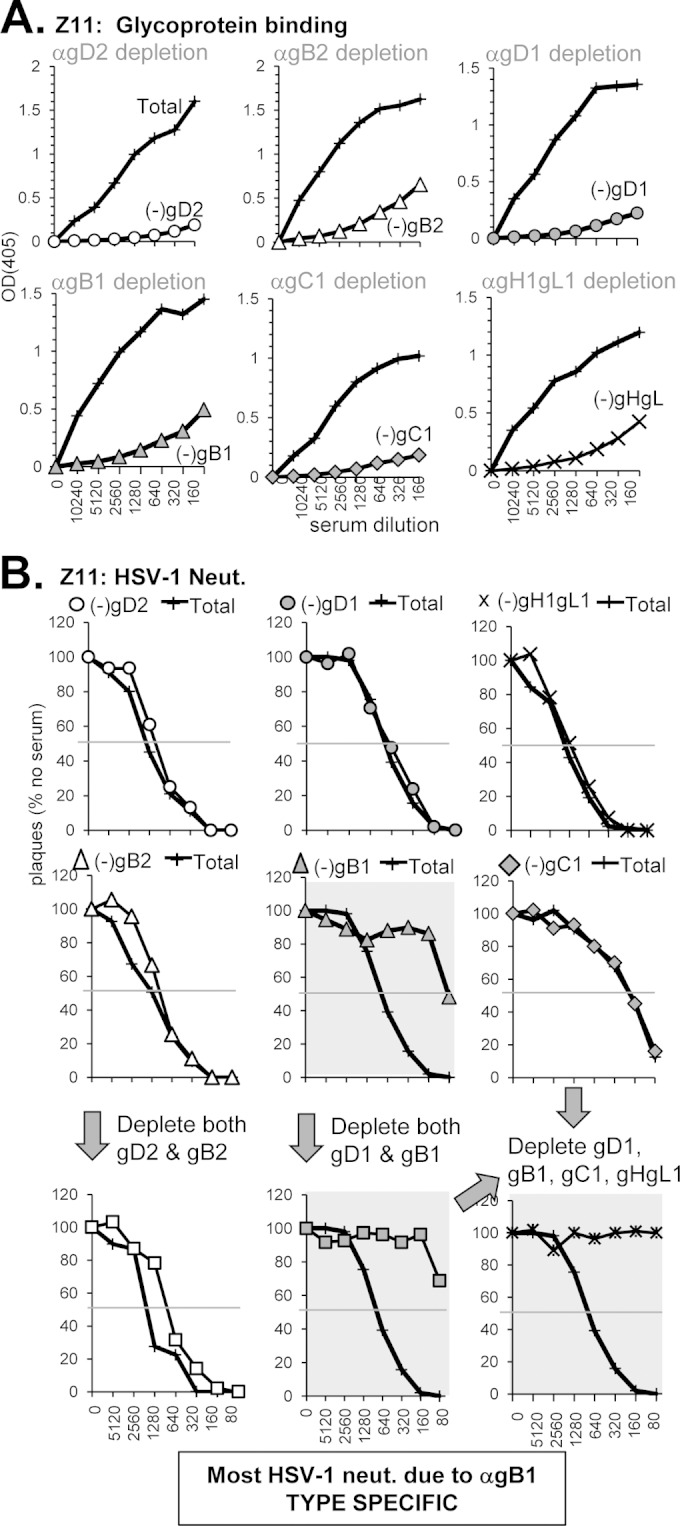
Depletion of glycoprotein-specific Abs from human serum sample Z2. (A) Antibody depletion of serum was carried out as described in Fig. 5A. Depletion with gC1 is indicated with gray diamonds, and that of gH1/gL1 is indicated by “×” symbols. The depleted serum was tested for neutralization activity against HSV-1 (B) as in Fig. 5C. The bottom right neutralization curve in panel B depicts a sample where Abs against gD1, gB1, gC1, and gH1/gL1 have all been removed.
To summarize, the neutralization profiles of the six serum samples we tested fell into six different categories (Tables 3 and 4): (i) type-common neutralization due to anti-gD Abs (Z2 and Z7); (ii) type-common HSV-1 neutralization due to anti-gD and anti-gB Abs (samples 16 and 18); (iii) type-specific HSV-2 neutralization due to anti-gD2 and anti-gB2 Abs (sample 16); (iv) type-specific HSV-2 neutralization due to anti-gB2 Abs (sample 18); (v) type-specific HSV-1 neutralization due to anti-gD1 and anti-gB1 Abs (Z8); and (vi) type-specific HSV-1 neutralization due to primarily anti-gB1 Abs (Z11). Thus, neutralization of HSV-1 and HSV-2 can be achieved in multiple ways by the contributions of different Abs directed at type-common and type-specific epitopes to glycoproteins involved in HSV entry.
TABLE 3.
Neutralization of HSV-2 before and after depletion of anti-gD and anti-gB Abs
| Sample | Total serum | Neutralization after depletiona |
Neutralization type | |||||
|---|---|---|---|---|---|---|---|---|
| Anti-gD1 | Anti-gB1 | Anti-gD1 anti-gB1 | Anti-gD2 | Anti-gB2 | Anti-gD2 anti-gB2 | |||
| Z7 | + | ND | ND | ND | – | + | ND | Type undetermined (gD) |
| Z8 | + | ND | ND | ND | + | + | – | Type undetermined (gD, gB) |
| Z2 | + | – | + | – | – | + | ND | Type common (gD) |
| 16 | + | + | + | + | + | + | – | Type specific (gD2, gB2) |
| 18 | + | + | + | + | + | – | – | Type specific (gB2) |
| Z11 | – | NA | NA | NA | NA | NA | NA | NA |
+, Neutralizes virus; –, does not neutralize virus or neutralizes poorly; ND, not determined; NA, not applicable.
TABLE 4.
Neutralization of HSV-1 before and after depletion of anti-gD, anti-gB Abs
| Sample | Total serum | Neutralization after depletiona |
Neutralization type | |||||
|---|---|---|---|---|---|---|---|---|
| Anti-gD1 | Anti-gB1 | Anti-gD1 anti-gB1 | Anti-gD2 | Anti-gB2 | Anti-gD2 anti-gB2 | |||
| Z7 | + | ND | ND | ND | – | + | ND | Type common (gD) |
| Z8 | + | + | + | – | + | + | + | Type specific (gD1, gB1) |
| Z2 | + | – | + | – | – | + | ND | Type common (gD) |
| 16 | + | – | + | – | – | + | – | Type common (gD, gB) |
| 18 | + | +/– | – | – | +/– | – | – | Type common (gD, gB) |
| Z11 | + | + | – | – | ND | ND | ND | Type specific (mostly gB1) |
+, Neutralizes virus; –, does not neutralize virus; ND, not determined.
DISCUSSION
In this report, we have continued our study of what comprises the human humoral response to the glycoproteins following HSV infection. In our prior study, we focused on a small cohort of people, five who either shed copious amounts of virus or five who shed very little (35). In that study we found a broad range in the overall neutralization titers against HSV-1 and HSV-2 in both shedding groups but no absolute correlation between the titer of neutralizing Ab and the degree of shedding. All of the IgGs purified from the sera bound to gD and gB and blocked at least one MAb directed at gD; fewer blocked MAbs to gB and those that did contained Abs directed against the base of gB (FR1) (10, 38). In that study, we found that the dominant neutralizing response to HSV was directed against gD or a combination of gD+gB. Contributions of Abs to gC were minimal and HSV-1 specific. We could detect no contribution from anti-gH/gL Abs.
Here, we compared the properties of sera from 9 subjects who had suffered multiple recurrences over a long period of time (>20 years) versus a cohort of 23 people who did not suffer any clinical recurrences. We sought to determine whether any parameters examined in our dissection of the sera correlated with the appearance of recurrent lesions. Although it is well established that CD8+ T cells play a major role in virus clearance, protection from multiple recurrences likely have both a T cell and a humoral component (6–8, 53). Interestingly, Kahn et al. (54) recently showed that asymptomatic people seropositive for HSV-1 have a significantly higher proportion of differentiated and multifunctional gB-specific effector memory CD8+ T cells compared to HSV-1-infected people who suffer frequent recurrences. The latter cohort has less differentiated and mono-functional memory CD8+ T cells. In both cases, the CD8+ T cells were directed at gB but at different sites, suggesting that asymptomatic people mount a different T cell response. The humoral response was not evaluated in that study.
Here, we modified our original approaches used to carry out glycoprotein binding, MAb competition, and serum dissection. The Octet RED96 system proved to be a powerful way to analyze multiple serum samples for both glycoprotein binding and blocking of MAbs. We validated this approach using samples previously evaluated for gB binding by BIAcore and found that relative binding of purified IgGs by BIAcore (Fig. 2A) was identical to the results obtained when we analyzed the sera directly by Octet (Fig. 2B). Notably, the Octet appeared to be more sensitive in detecting the level of competition with MAbs and revealed the presence of competing Abs to SS144 in samples 4 and 7 and to C226 in sample 7 (Fig. 2C). The complex nature of sera may account for the enhanced sensitivity of the Octet for at least two possible reasons. One, sera might have more immunoglobulin subclasses than just IgG that participate in binding or even competition. Two, some Abs in the polyclonal sera may have a lower affinity for gB and might be below the threshold of BIAcore sensitivity. Indeed, Li et al. (55) found the Octet system to be more sensitive than other methods for detecting binding of Abs with low affinity for their ligand. Moreover, purification of IgGs using affinity chromatography might have excluded Abs with low affinity.
In this report we captured biotinylated glycoproteins on streptavidin-coated magnetic beads and used them to deplete sera of Abs to both serotypes of gD and gB, as well as gC1 and gH1/gL1. This method avoids the need to use IgG and also uses small volumes of serum in a format that allowed us to readily analyze multiple samples. In fact, once the beads are coated with a specific glycoprotein, they can be reused multiple times. Heinz et al. (56, 57) used a similar magnetic bead assay to dissect the Ab response of sera from people vaccinated against yellow fever virus or tick borne encephalitis virus. These researchers depleted the sera using subdomains of the surface glycoproteins and evaluated the residual neutralization response. For gB, we also have purified several subdomains of the trimer (unpublished data) that can be used in future experiments in a similar fashion. For gD, we have many mutant forms of the protein, including deletion and point mutants that affect receptor binding (11, 58–61) and MAb-resistant mutants (37). Thus, we anticipate being able to use multiple mutant versions of the glycoproteins to deplete sera in an epitope-specific manner and generate quantitative data on the fraction of Abs directed at that epitope.
Here, our approach was to analyze which epitopes of gD and gB were recognized by the sera by using MAbs that bind to distinct sites and are associated with functions in virus entry and cell-cell fusion. For example, the anti-gD MAb 1D3 interferes with the binding of the HSV receptor HVEM, whereas MC23 and DL11 interfere with gD binding to both HVEM and a second receptor, nectin-1 (14, 29, 36, 37). Anti-gD MAbs MC2 and MC5 do not interfere with receptor binding but block a post-receptor binding step involving the interaction of gD with gH/gL (36). For gB, we used MAbs that bind to distinct epitopes on each of three functional regions (10, 17, 38). MAb SS144 binds to FR1 near the fusion loops (38), MAb C226 to FR2 (the domain associated with gB-gH/gL interaction) (50), and MAb SS10 to FR3 (the domain associated with the interaction of gB with cell surface molecules) (52). In almost every case, we have identified non-neutralizing MAbs that compete with these neutralizers (10; unpublished data). The existence of non-neutralizing competitor MAbs likely explains some of our depletion data, where the sera competed with one or more neutralizing MAbs but did not account for which glycoprotein dominated the neutralization response (e.g., samples 18 and Z2).
Which factor(s) correlate with recurrence or nonrecurrence?
Both symptomatic and asymptomatic cohorts exhibited a range of neutralization titers and variations in binding to gD and gB. The average neutralization titer against HSV-2 was higher in the recurrent cohort compared to the nonrecurrent cohort but was just above the level of significance (P = 0.054). However, we did observe a significant difference in binding of the sera to gD, i.e., the average value for binding was higher for the recurrent group than for the nonrecurrent group (P = 0.027). Binding of the sera to gB was also greater on average for the recurrent group, although it did not reach the level of statistical significance (P = 0.064).
For gD, the competition studies showed a similar statistical trend in that there was greater inhibition of the binding of each of four major neutralizing anti-gD MAbs in the recurrent cohort. Furthermore, competition with anti-gD MAb MC23 (which binds an epitope overlapping the receptor binding domains) was significantly different (P = 0.04). In addition, we detected modest competition between Abs in the human sera against MAb 1D3, which binds a linear epitope on the N terminus of gD. Strikingly, none of the 32 sera blocked the binding of non-neutralizing MAb MC14 (Tables 1 and 2). The epitope for MC14 lies in a region of gD that is important for the interaction of gD with gH/gL; this MAb also enhances the neutralization activity of anti-gD MAb MC2 and inhibits HSV glycoprotein-induced cell-cell fusion (36; unpublished data). To date, none of the sera or IgG we have isolated from either HSV-infected (35) or gD-vaccinated (34) human subjects have competed with MC14. We believe that future vaccines may benefit if designed to enhance the immunogenicity of this important region, e.g., incorporating peptides that represent this linear epitope.
In our prior study (35), only 3 of the 10 samples tested competed with MAbs against gB; each of the 3 competed with MAb SS144 in gB FR1 and one competed with MAb C226 in FR2. Here, all of the sera from the symptomatic group and all those that bound gB in the asymptomatic group competed with SS144 above a 20% threshold (Tables 1 and 2). For C226, 8 of9 symptomatic but only 7 of 23 asymptomatic individuals competed. The difference in competition for these two MAbs between recurrers and nonrecurrers was statistically significant (P = 0.038 for SS144, P = 0.0045 for C226). Unlike our previous study (35), we now detected competition with SS10 in the sera of 8 of 9 individuals from the recurrent cohort and 9 of 23 individuals from the nonrecurrent cohort, which was again a significant difference (P = 0.035). The inability of the previous set of 10 sera to block SS10 was verified on the Octet (Fig. 2C). It is not clear why none of those samples blocked SS10; it could be simply due to the small sample size. However, as was noted before, for gB the major response in both cohorts was to an epitope in FR1 (SS144), which contains the fusion loops (17, 38, 51).
There was no one epitope present in the nonrecurrent cohort that correlated to protection from recurrence. Rather, our results suggest that neutralizing Abs such as MC23, SS144, C226, and SS10 act as a readout for the severity of infection and likelihood of recurrences (more neutralizing Abs means more recurrences) but do not confer protection from recurrence.
Mixtures of human Abs directed at type-common and type-specific epitopes of gD and/or gB account for HSV neutralization.
Previously, we showed that the virus neutralizing activity against naturally acquired HSV was directed at gD or a combination of gD+gB (35). Several very interesting observations emerged from the expansion of our analysis to include both serotypes of gD and gB in this new study. We specifically selected six samples to dissect the role of these glycoproteins in contributing to HSV neutralization. Using our glycoprotein binding and MAb competition data, we were able to accurately predict which HSV glycoproteins contributed to the neutralization activity for three of these sera (samples 16, 18, and Z7). However, in several of these cases we were surprised to find that the neutralizing activity was type-specific. For example, sample 18 displayed a type-common response to gD and gB when assays for HSV-1 neutralization (Table 4), yet it was type-specific Abs against gB2 that were responsible for neutralizing HSV-2 (Table 3). Sample 16 represents another complex example of what contributes to neutralization. This sample had very high overall titers of neutralizing antibodies to both HSV-1 and HSV-2. Moreover, this serum bound very well to gD and gB and competed with key neutralizing MAbs to both glycoproteins. Taken together, the data suggested that type-common Abs to gD and gB would account for neutralization of both HSV-1 and HSV-2, which was indeed the case for HSV-1 neutralization (Fig. 8C and Table 4). However, neutralization of HSV-2 involved type-specific Abs to both gD2 and gB2 (Fig. 8B and Table 3). We have evidence of this phenomenon in our panel of mouse MAbs: anti-gB MAb SS63 binds both gB1 and gB2 and yet only neutralizes HSV-1 (data not shown). The composition of these sera emphasizes the fact that HSV-1 and HSV-2, although very similar, are different enough to provoke different responses to the envelope glycoproteins.
In the case of sample Z11, the neutralization was almost entirely directed at gB1 (Fig. 10B). Serum sample Z8 also generated a type-1 specific response against gD1 and gB1 (Fig. 6C and Table 4). Because this serum came from a dually infected person, we can speculate that this person was perhaps first infected with HSV-1 and mounted a type-specific immune response to that serotype and then was subsequently infected with HSV-2 (thereby explaining the ability of HSV to superinfect). Type-specific responses may also explain the HSV superinfection in patients 16 and 18; however, in these cases it would be infection with HSV-2 (type-specific response) and then superinfection with HSV-1 (type-common response). Type-specific responses to gD and gB that account for neutralization of either HSV-1 or HSV-2 were a consistent theme in this study.
Implications for vaccines.
It is clear from all of the data presented here, as well as in our previous study (35), that the dominant neutralizing Ab response to HSV infection is due to gD and gB, but we now know that it can be directed at one or both serotypes of these glycoproteins. HSV glycoproteins gH/gL and gC did not provoke a major neutralizing Ab response, although we did detect a small contribution from these proteins to HSV-1 neutralization (Fig. 10B) (35). Perhaps a subunit vaccine with one or both of these proteins may provide an additional protective neutralizing response. In addition, it has been argued that immunization with gC may protect against immune evasion (25, 27).
Our data suggest that first, more attention must be paid to gB as a prophylactic vaccine candidate. Second, a subunit vaccine should include both serotypes of gB and gD. It is worth noting that an ever increasing number of genital HSV infections are linked to HSV-1 (62). In the recent HSV-2 vaccine trial by GSK, this was certainly the case (7). Making the assumption that gD2 alone should suffice is not borne out by our data.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grants AI-18289 (to G.H.C.), and AI-076231 and AI-056045 (to R.J.E).
We thank Pall ForteBio LLC for use of the Octet RED96 system. We also thank our lab coworkers Doina Atanasiu and Wan-Ting Saw for helpful discussions.
REFERENCES
- 1.Grinde B. 2013. Herpesviruses: latency and reactivation—viral strategies and host response. J Oral Microbiol 2013:5. doi: 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitley RJ. 1993. Neonatal herpes simplex virus infections. J Med Virol Suppl 1:13–21. [DOI] [PubMed] [Google Scholar]
- 3.Bradley H, Markowitz LE, Gibson T, McQuillan GM. 2014. Seroprevalence of herpes simplex virus types 1 and 2: United States, 1999-2010. J Infect Dis 209:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 4.Sacks SL, Griffiths PD, Corey L, Cohen C, Cunningham A, Dusheiko GM, Self S, Spruance S, Stanberry LR, Wald A, Whitley RJ. 2004. HSV shedding. Antivir Res 63(Suppl 1):S19–S26. [DOI] [PubMed] [Google Scholar]
- 5.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffer JT. 2013. Mucosal HSV-2 specific CD8+ T cells represent containment of prior viral shedding rather than a correlate of future protection. Front Immunol 4:209. doi: 10.3389/fimmu.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. 2014. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 209:828–836. doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awasthi S, Friedman HM. 2014. A paradigm shift: vaccine-induced antibodies as an immune correlate of protection against herpes simplex virus type 1 genital herpes. J Infect Dis 209:813–815. doi: 10.1093/infdis/jit658. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpesvirus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender FC, Samanta M, Heldwein EE, de Leon MP, Bilman E, Lou H, Whitbeck JC, Eisenberg RJ, Cohen GH. 2007. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J Virol 81:3827–3841. doi: 10.1128/JVI.02710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitbeck JC, Muggeridge MI, Rux AH, Hou W, Krummenacher C, Lou H, van Geelen A, Eisenberg RJ, Cohen GH. 1999. The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain. J Virol 73:9879–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns TM, Shaner MS, Zuo Y, Ponce-de-Leon M, Baribaud I, Eisenberg RJ, Cohen GH, Whitbeck JC. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J Virol 80:2596–2608. doi: 10.1128/JVI.80.6.2596-2608.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski JM, Eisenberg RJ, Cohen GH. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol 72:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol 72:3595–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol 84:12292–12299. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol 17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 18.Backovic M, DuBois RM, Cockburn JJ, Sharff AJ, Vaney MC, Granzow H, Klupp BG, Bricogne G, Mettenleiter TC, Rey FA. 2010. Structure of a core fragment of glycoprotein H from pseudorabies virus in complex with antibody. Proc Natl Acad Sci U S A 107:22635–22640. doi: 10.1073/pnas.1011507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backovic M, Longnecker R, Jardetzky TS. 2009. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A 106:2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. 2010. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc Natl Acad Sci U S A 107:22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciferri C, Chandramouli S, Donnarumma D, Nikitin PA, Cianfrocco MA, Gerrein R, Feire AL, Barnett SW, Lilja AE, Rappuoli R, Norais N, Settembre EC, Carfi A. 2015. Structural and biochemical studies of hCMV gH/gL/gO and pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A 112:1767–1772. doi: 10.1073/pnas.1424818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci U S A 104:18718–18723. doi: 10.1073/pnas.0707452104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svennerholm B, Jeansson S, Vahlne A, Lycke E. 1991. Involvement of glycoprotein C (gC) in adsorption of herpes simplex virus type 1 (HSV-1) to the cell. Arch Virol 120:273–279. doi: 10.1007/BF01310482. [DOI] [PubMed] [Google Scholar]
- 24.Rux AH, Lou H, Lambris JD, Friedman HM, Eisenberg RJ, Cohen GH. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324–332. doi: 10.1006/viro.2001.1326. [DOI] [PubMed] [Google Scholar]
- 25.Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2011. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J Virol 85:10472–10486. doi: 10.1128/JVI.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weir JP, Bennett M, Allen EM, Elkins KL, Martin S, Rouse BT. 1989. Recombinant vaccinia virus expressing the herpes simplex virus type 1 glycoprotein C protects mice against herpes simplex virus challenge. J Gen Virol 70(Pt 10):2587–2594. [DOI] [PubMed] [Google Scholar]
- 27.Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, Cai M, Brown M, Smith JF, Kowalski R, Swoyer R, Galli J, Copeland V, Rios S, Davidson RC, Salnikova M, Kingsley S, Bryan J, Casimiro DR, Friedman HM. 2014. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J Virol 88:2000–2010. doi: 10.1128/JVI.03163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eing BR, Kuhn JE, Braun RW. 1989. Neutralizing activity of antibodies against the major herpes simplex virus type 1 glycoproteins. J Med Virol 27:59–65. doi: 10.1002/jmv.1890270113. [DOI] [PubMed] [Google Scholar]
- 29.Muggeridge MI, Isola VJ, Byrn RA, Tucker TJ, Minson AC, Glorioso JC, Cohen GH, Eisenberg RJ. 1988. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D using deletion mutants and monoclonal antibody-resistant mutants. J Virol 62:3274–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman HM. 2003. Immune evasion by herpes simplex virus type 1, strategies for virus survival. Trans Am Clin Climatol Assoc 114:103–112. [PMC free article] [PubMed] [Google Scholar]
- 31.Hook LM, Huang J, Jiang M, Hodinka R, Friedman HM. 2008. Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E. J Virol 82:6935–6941. doi: 10.1128/JVI.02599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awasthi S, Belshe RB, Friedman HM. 2014. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of GlaxoSmithKline HSV-2 glycoprotein D2 subunit vaccine. J Infect Dis 210:571–575. doi: 10.1093/infdis/jiu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitbeck JC, Huang ZY, Cairns TM, Gallagher JR, Lou H, Ponce-de-Leon M, Belshe RB, Eisenberg RJ, Cohen GH. 2014. Repertoire of epitopes recognized by serum IgG from humans vaccinated with herpes simplex virus 2 glycoprotein D. J Virol 88:7786–7795. doi: 10.1128/JVI.00544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairns TM, Huang ZY, Whitbeck JC, Ponce de Leon M, Lou H, Wald A, Krummenacher C, Eisenberg RJ, Cohen GH. 2014. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J Virol 88:12612–12622. doi: 10.1128/JVI.01930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol 86:1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muggeridge MI, Wu T-T, Johnson DC, Glorioso JC, Eisenberg RJ, Cohen GH. 1990. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology 174:375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 38.Cairns TM, Fontana J, Huang ZY, Whitbeck JC, Atanasiu D, Rao S, Shelly SS, Lou H, Ponce de Leon M, Steven AC, Eisenberg RJ, Cohen GH. 2014. Mechanism of neutralization of herpes simplex virus by antibodies directed at the fusion domain of glycoprotein B. J Virol 88:2677–2689. doi: 10.1128/JVI.03200-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol 69:4471–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. 2011. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J Virol 85:6175–6184. doi: 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bender FC, Whitbeck JC, Ponce de Leon M, Lou H, Eisenberg RJ, Cohen GH. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J Virol 77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannah BP, Cairns TM, Bender FC, Whitbeck JC, Lou H, Eisenberg RJ, Cohen GH. 2009. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J Virol 83:6825–6836. doi: 10.1128/JVI.00301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen GH, Isola VJ, Kuhns J, Berman PW, Eisenberg RJ. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol 60:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisenberg RJ, Long D, Ponce de Leon M, Matthews JT, Spear PG, Gibson MG, Lasky LA, Berman P, Golub E, Cohen GH. 1985. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol 53:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Concepcion J, Witte K, Wartchow C, Choo S, Yao D, Persson H, Wei J, Li P, Heidecker B, Ma W, Varma R, Zhao LS, Perillat D, Carricato G, Recknor M, Du K, Ho H, Ellis T, Gamez J, Howes M, Phi-Wilson J, Lockard S, Zuk R, Tan H. 2009. Label-free detection of biomolecular interactions using biolayer interferometry for kinetic characterization. Comb Chem High Throughput Screen 12:791–800. [DOI] [PubMed] [Google Scholar]
- 46.Markowski CA, Markowski EP. 1990. Conditions for the effectiveness of a preliminary test of variance. Am Statistician 44:322–326. doi: 10.1080/00031305.1990.10475752. [DOI] [Google Scholar]
- 47.Hollander M, Wolfe DA. 1973. Nonparametric statistical methods, p 68–75. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 48.Royston P. 1982. An extension of Shapiro and Wilk's W test for normality to large samples. Appl Stat 31:115–124. doi: 10.2307/2347973. [DOI] [Google Scholar]
- 49.Core Team R. 2013. R: a language and environment for statistical computing. R-Project, Vienna, Austria: http://www.R-project.org. [Google Scholar]
- 50.Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ. 2010. Bimolecular complementation defines functional regions of herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J Virol 84:3825–3834. doi: 10.1128/JVI.02687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hannah BP, Heldwein EE, Bender FC, Cohen GH, Eisenberg RJ. 2007. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J Virol 81:4858–4865. doi: 10.1128/JVI.02755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bender FC, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J Virol 79:11588–11597. doi: 10.1128/JVI.79.18.11588-11597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson MH, Bird MD, Chu CF, Johnson AJ, Friedrich BM, Allman WR, Milligan GN. 2011. Rapid clearance of herpes simplex virus type 2 by CD8+ T cells requires high level expression of effector T cell functions. J Reprod Immunol 89:10–17. doi: 10.1016/j.jri.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan AA, Srivastava R, Spencer D, Garg S, Fremgen D, Vahed H, Lopes PP, Pham TT, Hewett C, Kuang J, Ong N, Huang L, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from symptomatic and asymptomatic individuals with ocular herpes. J Virol 89:3776–3792. doi: 10.1128/JVI.03419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Schantz A, Schwegler M, Shankar G. 2011. Detection of low-affinity anti-drug antibodies and improved drug tolerance in immunogenicity testing by Octet(R) biolayer interferometry. J Pharm Biomed Anal 54:286–294. doi: 10.1016/j.jpba.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 56.Vratskikh O, Stiasny K, Zlatkovic J, Tsouchnikas G, Jarmer J, Karrer U, Roggendorf M, Roggendorf H, Allwinn R, Heinz FX. 2013. Dissection of antibody specificities induced by yellow fever vaccination. PLoS Pathog 9:e1003458. doi: 10.1371/journal.ppat.1003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jarmer J, Zlatkovic J, Tsouchnikas G, Vratskikh O, Strauss J, Aberle JH, Chmelik V, Kundi M, Stiasny K, Heinz FX. 2014. Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J Virol 88:13845–13857. doi: 10.1128/JVI.02086-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7:e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J Virol 77:8127–8140. doi: 10.1128/JVI.77.14.8127-8140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 61.Lazear E, Carfi A, Whitbeck JC, Cairns TM, Krummenacher C, Cohen GH, Eisenberg RJ. 2008. Engineered disulfide bonds in herpes simplex virus type 1 gD separate receptor binding from fusion initiation and viral entry. J Virol 82:700–709. doi: 10.1128/JVI.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiffer JT, Corey L. 2009. New concepts in understanding genital herpes. Curr Infect Dis Rep 11:457–464. doi: 10.1007/s11908-009-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 26:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]