Abstract
Herpesviruses are unusual among enveloped viruses because they bud twice yet acquire a single envelope. They are also the only known viruses that bud into the nuclear envelope. We discovered that the herpesvirus nuclear egress complex could bud membranes without the help of other proteins by forming a coat-like hexagonal scaffold inside the budding membrane. This finding raises the possibility that a phenotypically similar nuclear export of large RNAs is cargo driven.
INTRODUCTION
Most enveloped viruses acquire their envelopes by capsid budding into a cellular membrane. Some viruses, such as HIV or influenza virus, bud into the plasma membrane while other viruses, such as flaviviruses, bud into intracellular membranes such as the endoplasmic reticulum, the Golgi compartment, or others, depending on the virus. Herpesviruses represent an unusual case. Despite having a single envelope, these double-stranded DNA viruses undergo two rounds of budding; they bud first into the inner nuclear membrane (INM) and later into cytoplasmic membranes derived from the trans-Golgi network or early endosomes (1). This also makes them the only known viruses to use the nuclear membrane for budding. Yet, the envelope acquired during the first budding event at the INM does not end up in the mature viral particle. Only the second and final round of budding in the cytosol generates the single-layer envelope of the mature virus. Instead, the unusual nuclear budding allows the viral capsid to escape from the nucleus.
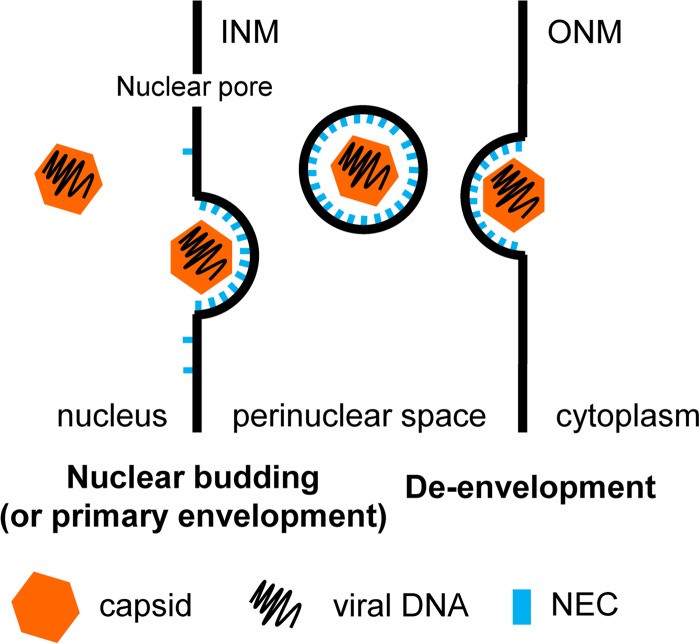
Herpesvirus genomes are replicated and encapsidated in the nucleus. The nucleus is surrounded by the INM and outer nuclear membrane (ONM), and most traffic in and out of the nucleus occurs through the nuclear pores. The diameter of herpesvirus capsids (125 nm in HSV-1) is considerably larger than that of the nuclear pore channels (52 nm), and thus, the capsids cannot fit through them. To escape from the nucleus, nucleocapsids bud into the INM, forming perinuclear immature viral particles—intermediates that are different from the mature, infectious viruses—that then fuse with the ONM, releasing the naked capsids into the cytosol (Fig. 1). As a result of this process, termed nuclear egress, nucleocapsids are translocated from the nucleus into the cytoplasm, where they mature into final, infectious virions.
FIG 1.
Schematic model of herpesvirus nuclear egress. The mature capsid is recruited to the INM, where the NEC is located via the UL34 TM region. Upon capsid binding, the NEC oligomerizes into a hexameric lattice and deforms the INM around the viral capsid. After scission of the bud, which may be facilitated by cellular factors, the enveloped capsid resides in the perinuclear space. Disassembly of the hexameric NEC lattice may allow fusion of the viral particle with the ONM, termed de-envelopment. The naked capsid is then released into the cytosol, where it undergoes further maturation.
Efficient exit of nascent capsids from the nucleus requires the virus-encoded nuclear egress complex (NEC) (reviewed in reference 1). The NEC consists of conserved viral proteins UL31 and UL34. UL34 is anchored to the INM by a C-terminal transmembrane (TM) helix with three residues extending into the perinuclear space (3). UL31 is a nuclear phosphoprotein that localizes to the INM through interaction with UL34 (4, 5). Formation of the NEC is a prerequisite for efficient nuclear egress. In the absence of either UL31 or UL34, viral replication is impaired and most capsids are retained in the nucleus (6, 7). The NEC is also sufficient to drive the formation of perinuclear vesicles in transfected cells (8, 9), which demonstrated that UL31 and UL34 are the only viral proteins necessary for vesiculation. But these experiments left the function of the NEC in membrane budding unclear. Does the NEC function as an adaptor that recruits (as-yet-unidentified) host proteins, or does it mediate membrane budding directly?
To address the exact role of the NEC in membrane budding, we expressed the HSV-1 NEC lacking the TM anchor of UL34 in Escherichia coli, purified it to homogeneity, and added it to synthetic lipid vesicles (10). This NEC is referred to here as the soluble NEC. We first showed that the soluble NEC could bind membranes even in the absence of the TM anchor but required acidic membranes. This means that the soluble NEC relies on electrostatic interactions between basic residues and acidic lipid head groups to get recruited to the membrane. In support of this idea, the requirement for acidic lipids becomes less important once the NEC is recruited to membranes by means of an artificial membrane anchor, as was shown by adding a C-terminal His tag to soluble UL34 (NEC-His) and using mildly acidic membranes with a nuclear-envelope-like composition and containing Ni-chelating lipid.
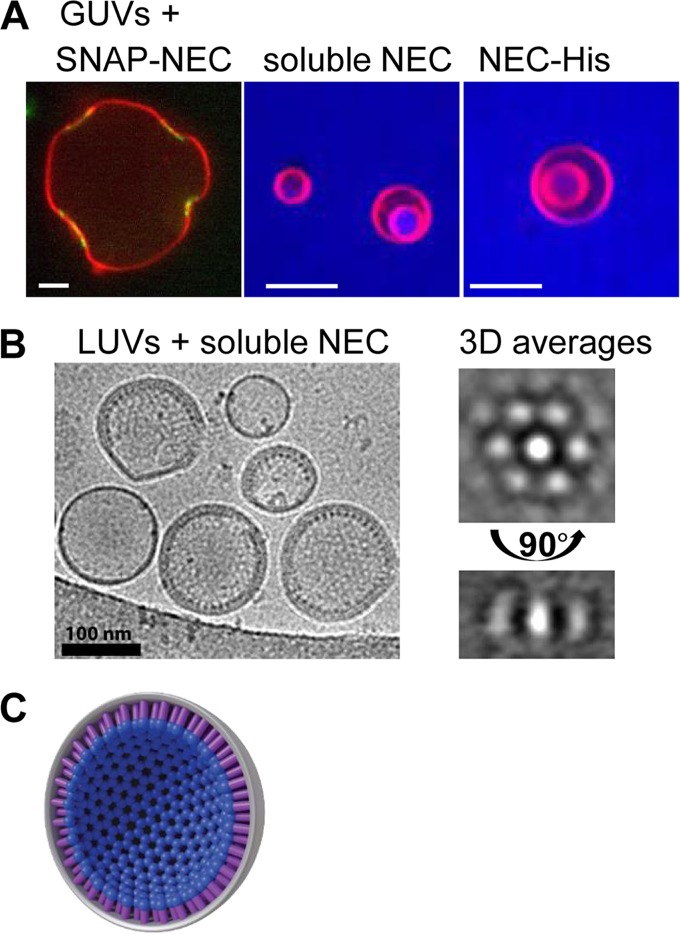
To study the effect of soluble NEC on synthetic vesicles, we turned to confocal microscopy. Experiments with fluorescently labeled NEC (SNAP-NEC) and giant unilamellar vesicles (GUVs) showed that NEC binding generated negative curvature at the binding site (Fig. 2A). Moreover, by using the aqueous dye Cascade Blue and the unlabeled NEC, we could demonstrate that the soluble NEC efficiently vesiculated fluorescently labeled GUVs, resulting in the appearance of intraluminal vesicles (ILVs) filled with Cascade Blue. Cascade Blue cannot permeate membranes, so its presence inside ILVs shows that both membrane budding and scission have taken place. Thus, soluble NEC alone could mediate membrane budding and scission in the absence of any other factors. The membrane budding efficiency was similar regardless of whether we used the soluble NEC and acidic membranes versus the His-tagged NEC and Ni-containing membranes, which confirmed that the UL34 TM region does not play an active role in budding beyond anchoring the NEC into the INM. By reconstituting the budding process in vitro using the purified NEC and synthetic liposomes, we showed that the HSV-1 NEC has an intrinsic ability to mediate budding and scission (10). This finding was subsequently confirmed for the pseudorabies virus NEC (11).
FIG 2.
The NEC deforms and buds membranes in vitro by forming a honeycomb coat. (A) Green fluorescently labeled NEC (SNAP-NEC) binds to GUVs (red) and induces negative curvature at the binding site. The untagged NEC (soluble NEC) or artificially anchored NEC (NEC-His) buds GUVs, resulting in the appearance of ILVs. Bars, 5 μm. (B) Cryo-EM images showing circular structures on the inside of large unilamellar vesicles (LUVs) and spikes emanating from the rim. Three-dimensional (3D) averaging allowed us identify the circular structures as a hexagonal honeycomb pattern in a view of the spikes from the top. (C) Model of hexagonal coat on the inside of budded vesicles. All of the images shown are adapted from reference 10.
Although the in vitro budding experiments do not provide direct information on the NEC-mediated budding in infected cells, the negative, or inward, curvature generated by the NEC in vitro has the same directionality as capsid budding into the INM during HSV-1 infection and nuclear vesiculation in NEC-transfected cells. To relate the in vitro observations to the in vivo budding phenotype, we constructed a soluble NEC that contains a double point mutation in UL34 that has a nonbudding phenotype and is impaired in membrane deformation around the capsid (12). As expected, the mutant NEC showed reduced budding in vitro despite being able to bind membranes, thus linking the in vitro observations to the in vivo phenotype.
We next turned our attention to the mechanism of NEC-mediated budding. Using cryoelectron microscopy, we found that the NEC, although heterodimeric in solution, forms an ordered array on the inner surface of the budded vesicles (Fig. 2B) (10). Images showed that the NEC formed spikes emanating from the membrane toward the interior of the vesicle, as well as a hexagonal honeycomb lattice that is related to the spikes by a 90° rotation. Averaging confirmed that the two arrays represent different projections of honeycomb coats on the inner surface of budded vesicles. The NEC coats form rapidly; no energy in the form of ATP is required, and NEC-NEC and NEC-membrane interactions appear sufficient to drive coat formation. We hypothesize that the NEC drives budding by the rapid and likely cooperative formation of this coat-like hexagonal lattice that efficiently scaffolds the membrane from the inside (Fig. 2C). Although the scaffold has hexagonal symmetry, to form a sphere, this symmetry needs to be slightly disrupted, for example, by having a few pentagons. Higher-resolution images are needed in order to visualize such flaws in symmetry, although we have seen occasional heptagons in cryo-electron microscopy (cryo-EM) images. Taken together, these results suggest that the NEC vesiculates membranes by oligomerizing on the membrane and creating a hexagonal scaffold inside the bud.
While in vitro the NEC mediates not only membrane budding but also scission, it is unclear whether efficient scission in cells requires additional host factors. One may envision that although the NEC represents the minimal budding machinery, cellular budding factors may, perhaps, be recruited to affect budding efficiency, for example, the efficiency of neck scission. Budding of most enveloped viruses relies on cellular ESCRT proteins (13). In principle, ESCRT proteins could fulfill this function, and very recently, ESCRT proteins were shown to play a role in nuclear envelope remodeling after mitosis (2). However, nuclear egress of HSV-1 is not sensitive to Vps4 dominant negative mutation, which is a well-accepted test of ESCRT involvement (14). This is only one report, however, and reproducing it would help settle the question of potential ESCRT involvement in nuclear egress.
Our results explain why nuclear budding by herpesviruses may not depend on ESCRT proteins. We showed that the NEC forms ordered coats on the inner surface of budded vesicles, suggesting that it mediates scission by scaffolding the membrane bud and constricting the neck to the point of scission. The only other example of fully virus-encoded membrane-budding machinery is found in influenza virus. However, unlike influenza virus, which encodes two independently acting proteins for bud formation and scission, M1 and M2, respectively (15), the NEC appears to mediate both functions, indicating that the NEC is a complete membrane-budding machine that functions by a novel mechanism. In addition, the NEC is the only viral or cellular machinery currently known to mediate budding of the nuclear, as opposed to cytoplasmic, membrane. This scission mechanism also differs from that of the ESCRT-III proteins, which localize only to the neck but not to the nascent vesicle itself. They probably cleave the neck from its exterior end (16), although more recent data show that during HIV budding, ESCRT-III moves into the interior of the viral bud and may scaffold it (17).
Both in vitro and in transfected cells, the NEC has powerful membrane vesiculation activity. Yet, empty perinuclear vesicles are not observed during infection (1), which means that in infected cells, the intrinsic budding potential of the NEC is likely controlled to ensure productive budding. Given that NEC oligomerization is the driving force for vesiculation, formation of the NEC lattice must be inhibited until triggered. Although neither the inhibitory nor the triggering mechanism is yet understood, several possibilities can be considered. For example, dynamic modifications, such as phosphorylation of the NEC (e.g., by HSV-1 kinase US3), may play a role in regulation of its budding activity, especially inhibition (1). Further, it is predominantly mature nucleocapsids that bud into the INM (1), which suggests that during infection, proteins present on mature but not immature capsids may trigger oligomerization either by binding the NEC directly or by inactivating an inhibitor that blocks NEC oligomerization.
The hexagonal honeycomb lattice on the inside of budded vesicles represents a stable structure that needs to be disassembled for the de-envelopment step in nuclear egress. How this lattice is dismantled remains to be shown, but one potential candidate involved in the process may be the HSV-1 kinase US3. US3 is thought to be essential for the de-envelopment process (5). Phosphorylation of the NEC after primary budding may lead to structural rearrangements that disrupt the hexameric lattice, thereby enabling de-envelopment. In this way, by interfering with oligomerization, phosphorylation of the NEC could both inhibit budding in the absence of the capsid and disassemble the NEC coat during de-envelopment.
Recent studies showed that the process of nuclear egress is not unique to herpesviruses and also observed during the export of large synaptic ribonucleoparticles (RNPs) in Drosophila (18), which are too large to exit the nucleus through nuclear pores. It is unclear how common nuclear envelope budding is in host cells, for example, whether it occurs in mammalian cells and whether it is restricted to certain cell types, for example, nondividing cells. The existence of nuclear egress in uninfected cells suggests that herpesviruses may have hijacked this pathway. Indeed, ATPase torsin, required during the de-envelopment step of large RNPs (19), has also been implicated in HSV-1 de-envelopment (20). Nevertheless, no cellular protein(s) has yet been implicated in nuclear budding of large RNPs. Considering our finding that the NEC can vesiculate membranes without the help of other factors, one interesting possibility is that nuclear budding in general does not utilize an common endogenous cellular process but is instead cargo driven. If this is true, it would mean that the large RNPs may contain a factor(s) capable of membrane budding rather than utilize other endogenous budding machinery such as ESCRT proteins. One tantalizing possibility is that RNA rather than protein could be such a factor, although no RNA has ever been implicated in the process of membrane deformation by viruses or otherwise. Further studies are clearly necessary to delineate the contributions of host factors to nuclear egress of herpesviruses versus large RNPs and mechanistic similarities and differences between these two processes. Whether herpesviruses hijack or mimic eukaryotic host processes or have invented a new strategy, increasing our understanding of this critical aspect of viral replication and host cellular function could identify new antiviral strategies and mechanisms of human disease.
ACKNOWLEDGMENTS
We apologize to colleagues whose work was not explicitly acknowledged in this article because of the strict limitation on the number of references that can be cited. We thank Melissa Moore for stimulating discussions on the nuclear export of large RNAs.
This work was funded by NIH grants 1R21AI097573 and 1R01GM111795 (E.E.H.), the Burroughs Wellcome Fund (E.E.H.), and a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft GZ: BI 1658/1-1 (J.M.B.).
REFERENCES
- 1.Johnson DC, Baines JD. 2011. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol 9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 2.Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. 2015. ESCRT-III controls nuclear envelope reformation. Nature 522:236–239. doi: 10.1038/nature14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiba C, Daikoku T, Goshima F, Takakuwa H, Yamauchi Y, Koiwai O, Nishiyama Y. 2000. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J Gen Virol 81:2397–2405. [DOI] [PubMed] [Google Scholar]
- 4.Chang YE, Roizman B. 1993. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J Virol 67:6348–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol 76:8939–8952. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs W, Klupp BG, Granzow H, Osterrieder N, Mettenleiter TC. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions. J Virol 76:364–378. doi: 10.1128/JVI.76.1.364-378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roller RJ, Zhou YP, Schnetzer R, Ferguson J, DeSalvo D. 2000. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J Virol 74:117–129. doi: 10.1128/JVI.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klupp BG, Granzow H, Fuchs W, Keil GM, Finke S, Mettenleiter TC. 2007. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc Natl Acad Sci U S A 104:7241–7246. doi: 10.1073/pnas.0701757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai PJ, Pryce EN, Henson BW, Luitweiler EM, Cothran J. 2012. Reconstitution of the Kaposi's sarcoma-associated herpesvirus nuclear egress complex and formation of nuclear membrane vesicles by coexpression of ORF67 and ORF69 gene products. J Virol 86:594–598. doi: 10.1128/JVI.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bigalke JM, Heuser T, Nicastro D, Heldwein EE. 2014. Membrane deformation and scission by the HSV-1 nuclear egress complex. Nat Commun 5:4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz M, Vollmer B, Unsay JD, Klupp BG, Garcia-Saez AJ, Mettenleiter TC, Antonin W. 2015. A single herpesvirus protein can mediate vesicle formation in the nuclear envelope. J Biol Chem 290:6962–6974. doi: 10.1074/jbc.M114.627521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roller RJ, Bjerke SL, Haugo AC, Hanson S. 2010. Analysis of a charge cluster mutation of herpes simplex virus type 1 UL34 and its extragenic suppressor suggests a novel interaction between pUL34 and pUL31 that is necessary for membrane curvature around capsids. J Virol 84:3921–3934. doi: 10.1128/JVI.01638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Votteler J, Sundquist WI. 2013. Virus budding and the ESCRT pathway. Cell Host Microbe 14:232–241. doi: 10.1016/j.chom.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crump CM, Yates C, Minson T. 2007. Herpes simplex virus type 1 cytoplasmic envelopment requires functional Vps4. J Virol 81:7380–7387. doi: 10.1128/JVI.00222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossman JS, Lamb RA. 2011. Influenza virus assembly and budding. Virology 411:229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. 2009. Membrane scission by the ESCRT-III complex. Nature 458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Engelenburg SB, Shtengel G, Sengupta P, Waki K, Jarnik M, Ablan SD, Freed EO, Hess HF, Lippincott-Schwartz J. 2014. Distribution of ESCRT machinery at HIV assembly sites reveals virus scaffolding of ESCRT subunits. Science 343:653–656. doi: 10.1126/science.1247786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, Moore MJ, Budnik V. 2012. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell 149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jokhi V, Ashley J, Nunnari J, Noma A, Ito N, Wakabayashi-Ito N, Moore MJ, Budnik V. 2013. Torsin mediates primary envelopment of large ribonucleoprotein granules at the nuclear envelope. Cell Rep 3:988–995. doi: 10.1016/j.celrep.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maric M, Shao J, Ryan RJ, Wong CS, Gonzalez-Alegre P, Roller RJ. 2011. A functional role for TorsinA in herpes simplex virus 1 nuclear egress. J Virol 85:9667–9679. doi: 10.1128/JVI.05314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]