ABSTRACT
Chronic wasting disease (CWD) is an emergent, rapidly spreading prion disease of cervids. Shedding of infectious prions in saliva and urine is thought to be an important factor in CWD transmission. To help to elucidate this issue, we applied an in vitro amplification assay to determine the onset, duration, and magnitude of prion shedding in longitudinally collected saliva and urine samples from CWD-exposed white-tailed deer. We detected prion shedding as early as 3 months after CWD exposure and sustained shedding throughout the disease course. We estimated that the 50% lethal dose (LD50) for cervidized transgenic mice would be contained in 1 ml of infected deer saliva or 10 ml of urine. Given the average course of infection and daily production of these body fluids, an infected deer would shed thousands of prion infectious doses over the course of CWD infection. The direct and indirect environmental impacts of this magnitude of prion shedding on cervid and noncervid species are surely significant.
IMPORTANCE Chronic wasting disease (CWD) is an emerging and uniformly fatal prion disease affecting free-ranging deer and elk and is now recognized in 22 U.S. states and 2 Canadian provinces. It is unique among prion diseases in that it is transmitted naturally through wild populations. A major hypothesis to explain CWD's florid spread is that prions are shed in excreta and transmitted via direct or indirect environmental contact. Here we use a rapid in vitro assay to show that infectious doses of CWD prions are in fact shed throughout the multiyear disease course in deer. This finding is an important advance in assessing the risks posed by shed CWD prions to animals as well as humans.
INTRODUCTION
Chronic wasting disease (CWD) is an emergent transmissible spongiform encephalopathy affecting free-ranging populations of mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), elk (Cervus canadensis), and moose (Alces alces) in North America (1, 2). CWD is the only known prion disease to spread horizontally through wild populations, in which it continues to expand in prevalence and range in North America (3). As a prion disease, CWD is caused by a pathogenic, misfolded conformation of the normal, natively folded cellular protein PrPC to a pathogenic prion conformer (variously designated PrPCWD, PrPSC, or PrPD) (2, 4–7).
A leading hypothesis for the facile spread of CWD in wild populations is that the accumulation and excretion of CWD prions in bodily fluids facilitate both direct animal-to-animal transfer and substantial environmental contamination leading to indirect infection (8–10). Infectious CWD prions have been identified in urine, saliva, blood, and feces by bioassay of deer or cervid PrPC-expressing transgenic mice (11–16). Prions bound to soil are remarkably stable, retaining infectivity even after a decade (9, 17–20). Moreover, some evidence suggests that prions bound to soil may increase infectivity through an unknown mechanism (21). Understanding the kinetics and magnitude of CWD prion shedding into the environment and assessing the risks to humans and other species remain significant yet unmet challenges.
Recent advances in the detection of prions at minute quantities and in diverse biological fluids, such as saliva, urine, and blood, allow for a thorough analysis of the shedding of CWD prions during the disease course (11, 14, 22, 23). In the present study, we used a rapid in vitro real-time prion protein conversion assay (real-time quaking-induced conversion [RT-QuIC]) (24) and an unprecedented number of longitudinal saliva and urine samples from white-tailed deer exposed to CWD prions by various routes (aerosol, oral, and environmental) to track the kinetics and magnitude of prion seeding activity and to estimate accrued prion shedding over the course of infection.
MATERIALS AND METHODS
Sourcing and inoculation of white-tailed deer.
The longitudinal shedding kinetics of CWD in excreta were analyzed in three experimentally exposed cohorts of CWD-naive, hand-raised, indoor-adapted white-tailed deer (n = 22). Our long-time collaborators at the Warnell School of Forestry and Natural Resources, University of Georgia, provided CWD-free white-tailed deer fawns that were housed in the indoor CWD research facility at Colorado State University. All appropriate institutional protocols for animal handling and treatment were properly followed. Inoculation methods and protocols to prevent cross contamination among study cohorts have been described previously (15, 25, 26). In short, aerosol-exposed deer received two 1.0-ml doses of a 5% CWD+ brain homogenate via aerosolization (deer A-1 to A-6), the per os (p.o.)-exposed deer received a single, 1-g dose of CWD+ brain orally (deer PO-1 to PO-10), and the environmental group was exposed to fomites from feed buckets, bedding, and water from CWD+ deer suites every day for 19 months (E-1 and E-2), without any direct contact of the two animal groups. Sham-inoculated deer were exposed to negative brain homogenates by the aerosol or oral route and were housed in separate suites in the same building.
Sample collection.
Body fluids and excreta (saliva, blood, urine, and feces) were collected along with tonsil and recto-anal mucosa-associated lymphoid tissue (RAMALT). Biopsy specimens were serially collected from all exposed deer cohorts at intervals of 3 months or less from the study start to termination (up to 2 years) (Tables 1 to 3). Due to sample tissue or body fluid availability, however, not all samples were collectable at each time point (Tables 1 to 3). All animals were monitored for CWD infection by immunohistochemistry (IHC) of tonsil and RAMALT biopsy specimens at each collection interval, as well as by clinical disease scoring (Table 4). At study termination, all deer were necropsied and multiple tissues collected for an array of assays.
TABLE 1.
Summary of IHC and RT-QuIC results for orally inoculated CWD-exposed deera
| Parameter | Value or description | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Animal no. | PO-1 | PO2 | PO-3 | PO-4 | PO-5 | PO-6 | PO-7 | PO-8 | PO-9 | PO-10 |
| Sex | M | M | M | M | F | M | F | M | F | F |
| Genotype | G/S | G/S | G/G | G/S | G/G | G/G | G/S | G/G | G/G | G/G |
| Time to positive biopsy specimen (mo p.i.) | ||||||||||
| Tonsil | 6 | 9 | 9 | 9 | 9 | 6 | 6 | 9 | 6 | 6 |
| RAMALT | 6 | 15 | 9 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| No. of positive specimens/total no. of specimens tested | ||||||||||
| 3 mo p.i. | ||||||||||
| Saliva | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 1/8 | 0/8 | 0/8 | 0/8 |
| Urine | 0/8 | NA | NA | 0/8 | NA | NA | 0/8 | NA | 0/8 | 0/8 |
| 6 mo p.i. | ||||||||||
| Saliva | 0/8 | 0/8 | NA | 0/8 | 0/8 | 0/8 | 1/8 | 0/8 | 0/8 | 1/8 |
| Urine | 0/8 | 0/8 | 5/8 | 0/8 | 1/8 | 0/8 | 2/8 | NA | 0/8 | 1/8 |
| 9 mo p.i. | ||||||||||
| Saliva | NA | 0/8 | 2/8 | NA | 0/8 | 8/8 | 3/8 | 8/8 | 0/8 | 4/8 |
| Urine | 4/8 | NA | NA | 1/8 | 0/8 | 1/8 | NA | NA | 0/8 | 0/8 |
| 10 mo p.i. | ||||||||||
| Saliva | 0/8 | 1/8 | 1/8 | 4/8 | NA | NA | 4/8 | 5/8 | NA | NA |
| Urine | 1/8 | NA | 3/8 | 4/8 | NA | 0/8 | NA | NA | NA | 0/8 |
| 12 mo p.i. | ||||||||||
| Saliva | 0/8 | 0/8 | 5/8 | 3/8 | 0/8 | 1/8 | 0/8 | 5/8 | 1/8 | 3/8 |
| Urine | 7/8 | NA | 2/8 | NA | 0/8 | NA | NA | NA | 0/8 | 0/8 |
A total of 22 indoor-adapted white-tailed deer were inoculated with CWD+ brain homogenate via either aerosol, oral, or environmental exposure, the latter of which was done with feed, water, and bedding harvested from a separate suite containing CWD-infected deer. Data on aerosol- and environmentally exposed deer are shown in Tables 2 and 3. The sex of the deer and the genotype at PrP position 96 (G/G or G/S) are noted. Animals were monitored for CWD throughout the disease course by IHC of tonsil and RAMALT biopsy specimens. All aerosol and orally inoculated deer were PrPCWD positive by tonsil biopsy between 6 and 9 months postinoculation. For the aerosol-inoculated deer, saliva and urine were collected at 3-month intervals during the subclinical phase of disease and more frequently in the clinical phase of the disease (i.e., after 15 months). Saliva and urine RT-QuIC results are reported as numbers of positive replicates among the total number of replicates, representing a minimum of 2 experiments. Results for positive samples are shown in bold. M, male; F, female; NA, not available.
TABLE 3.
Summary of IHC and RT-QuIC results for environmentally exposed CWD-exposed deera
| Parameter | Value or description | |
|---|---|---|
| Animal no. | E-1 | E-2 |
| Sex | M | M |
| Genotype | G/S | G/G |
| No. of positive specimens/total no. of specimens tested | ||
| Saliva | ||
| 0 mo p.i. | 0/12 | 0/12 |
| 3 mo p.i. | 1/12 | 3/12 |
| 6 mo p.i. | 2/12 | 6/12 |
| 12 mo p.i. | 2/12 | 12/12 |
| 15 mo p.i. | 2/12 | 9/12 |
| Urine | ||
| 6 mo p.i. | 0/8 | 1/8 |
| 12 mo p.i. | 0/8 | 0/8 |
| 15 mo p.i. | 2/8 | 8/8 |
| 18 mo p.i. | 0/8 | 3/8 |
See the footnote to Table 1 for further details.
TABLE 4.
Clinical disease stage scoringa
| Time of scoring (mo p.i.) | Score for indicated animal |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orally inoculated group |
Aerosol-inoculated group |
|||||||||||||||
| PO-1 | PO-2 | PO-3 | PO-4 | PO-5 | PO-6 | PO-7 | PO-8 | PO-9 | PO-10 | A-1 | A-2 | A-3 | A-4 | A-5 | A-6 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Termination of study | 3 | 3 | 3 | 3 | 0 | 2 | 2 | 3 | 3 | 2 | ||||||
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 19 | 1 | 1 | 1 | 1 | 2 | 3 | ||||||||||
| 20 | 2 | 1 | 2 | 1 | 2 | † | ||||||||||
| 21 | 2 | 2 | 2 | 1 | † | † | ||||||||||
| 22 | 2 | 2 | 2 | 1 | † | † | ||||||||||
| 23 | 2 | 3 | 3 | 2 | † | † | ||||||||||
| 25 | 2 | † | † | 3 | † | † | ||||||||||
| 26 | 3 | † | † | † | † | † | ||||||||||
Deer were given a score of 0 to 4 for clinical disease at each time point when excreta samples were taken. 0, normal behavior; 1, subtle behavioral changes (diurnal rhythms and patterns of sleeping, feeding, and activity are altered); 2, mild but observable neurological deficits, commonly mild ataxia in the hindquarters; 3, early stage, behavioral changes continue, with early signs of deterioration and continued progression of ataxia; 3, late stage, gait abnormalities become pronounced; 4, neurological deficit progression, wide-legged stance, low-hanging head, piloerection, obvious signs of muscle wasting, and increased ataxia. The appetite and ability to eat and drink are intact, with dramatic increases often seen (2 to 3 times normal volumes). †, deer that died during the study.
TABLE 2.
Summary of IHC and RT-QuIC results for aerosol-inoculated CWD-exposed deera
| Parameter | Value or description | |||||
|---|---|---|---|---|---|---|
| Animal no. | A-1 | A-2 | A-3 | A-4 | A-5 | A-6 |
| Sex | M | F | M | F | M | M |
| Genotype | G/G | G/G | G/G | G/G | G/G | G/G |
| Time to positive biopsy specimen (mo p.i.) | ||||||
| Tonsil | 6 | 9 | 6 | 9 | 6 | 6 |
| RAMALT | 12 | 6 | 6 | 9 | 6 | 6 |
| No. of positive specimens/total no. of specimens tested | ||||||
| 3 mo p.i. | ||||||
| Saliva | NA | NA | NA | NA | 7/8 | 0/8 |
| Urine | NA | 0/8 | 0/8 | NA | 0/8 | NA |
| 6 mo p.i. | ||||||
| Saliva | 0/8 | 2/8 | 0/8 | NA | 4/8 | NA |
| Urine | 0/8 | NA | NA | NA | 3/8 | NA |
| 9 mo p.i. | ||||||
| Saliva | NA | 8/8 | NA | 2/8 | NA | 1/8 |
| Urine | 5/8 | NA | NA | NA | 5/8 | NA |
| 12 mo p.i. | ||||||
| Saliva | 0/8 | 8/8 | 0/8 | NA | 2/8 | 1/8 |
| Urine | 2/8 | NA | 0/8 | NA | 8/8 | 0/8 |
| 15 mo p.i. | ||||||
| Saliva | 1/8 | 8/8 | 4/8 | NA | 1/8 | 6/8 |
| Urine | 0/8 | 0/8 | 0/8 | NA | 0/8 | 0/8 |
| 16 mo p.i. | ||||||
| Saliva | 0/8 | 4/8 | 3/8 | 1/8 | 5/8 | 6/8 |
| Urine | 0/8 | NA | 0/8 | NA | 4/8 | NA |
| 19 mo p.i. | ||||||
| Saliva | 0/8 | 4/8 | 8/8 | 0/8 | NA | NA |
| Urine | 0/8 | 0/8 | 0/8 | 1/8 | NA | 0/8 |
| 20 mo p.i. | ||||||
| Saliva | 0/8 | 8/8 | NA | 8/8 | 0/8 | † |
| Urine | 1/8 | 1/8 | 0/8 | NA | 6/8 | † |
| 21 mo p.i. | ||||||
| Saliva | 0/8 | 3/8 | 5/8 | 4/8 | 7/8 | † |
| Urine | 0/8 | 3/8 | 0/8 | 2/8 | NA | † |
| 22 mo p.i. | ||||||
| Saliva | NA | 0/8 | 0/8 | 2/8 | † | † |
| Urine | 0/8 | 1/8 | 0/8 | 0/8 | † | † |
| 23 mo p.i. | ||||||
| Saliva | 0/8 | 0/8 | 0/8 | 3/8 | † | † |
| Urine | 0/8 | 1/8 | 0/8 | 0/8 | † | † |
| 25 mo p.i. | ||||||
| Saliva | 0/8 | † | † | 3/8 | † | † |
| Urine | 2/8 | † | † | 0/8 | † | † |
| 26 mo p.i. | ||||||
| Saliva | 8/8 | † | † | † | † | † |
| Urine | NA | † | † | † | † | † |
See the footnote to Table 1 for further details. †, deer that died during the study.
Preparation of RT-QuIC reagents and recombinant SH-rPrP(90-231).
Syrian hamster recombinant PrP containing amino acids 90 to 231 [SH-rPrP(90-231)] was prepared as described previously (14, 27). In summary, protein expression in 1-liter cultures was induced using Over Night Express (EMD-Millipore) autoinduction medium, and inclusion bodies were harvested by using Lysonase (EMD-Millipore) according to the manufacturer's protocol. Inclusion bodies were solubilized in 8.0 M guanidine hydrochloride (GuHCl) with 100 mM NaPO4 for 1 h with rotation at room temperature. The solubilized rPrP was bound to superflow Ni resin (Qiagen) and refolded on the Ni column by using a 180-ml linear gradient from 6.0 M GuHCl, 100 mM NaPO4, 10 mM Tris, pH 8.0, to the same buffer without the GuHCl, flowing at 0.75 ml/min. rPrP was eluted with a linear gradient from 100 mM NaPO4, 10 mM Tris, pH 8.0, to 0.5 M imidazole in 100 mM NaPO4, 10 mM Tris, pH 5.5, at 2.0 ml/min. The eluted protein was dialyzed in two changes of 4.0 liters of 20 mM NaPO4 at pH 5.5. The concentration of SH-rPrP(90-231) was determined by measuring the A280, and the protein was stored at 4°C.
RT-QuIC assay conditions.
RT-QuIC reaction mixtures contained 20 mM NaPO4, 1 mM EDTA, 320 mM NaCl, 0.1 mg/ml SH-rPrP(90-231), and 10 μM thioflavin T (ThT; Sigma). Shaking and reading settings were as previously reported (14). RT-QuIC reactions were deemed positive when the ThT fluorescence value reached a level beyond 5 standard deviations from the initial fluorescence value.
Preparation of samples for RT-QuIC.
Saliva was thawed at room temperature and vortexed, and then 100 μl of undiluted saliva was transferred for further concentration of CWD prions as previously reported (14). A 4% solution of freshly prepared phosphotungstic acid (PTA; Sigma) was added to 100 μl saliva, to a final concentration of 0.3%. Samples were incubated for 60 min at 37°C, with shaking at 1,700 rpm, and were then centrifuged at 17,000 × g for 30 min. PTA-precipitated pellets were resuspended in 10 μl 1× phosphate-buffered saline (PBS) (20 mM NaPO4, 150 mM NaCl, pH 7.4) with 0.1% sodium dodecyl sulfate (SDS). Two microliters of each sample was added in quadruplicate to a prepared RT-QuIC reaction mixture.
Urine samples were thawed at room temperature and vortexed, and 500 μl was transferred to a fresh tube and then centrifuged for 30 min at 17,000 × g. Supernatants were removed, and cell pellets were resuspended in 100 μl of 1× PBS. Seven microliters of a freshly prepared 4% solution of sodium phosphotungstic acid (NaPTA; Sigma) was added to the 100-μl suspension, to a final concentration of 0.3%. Samples were incubated for 60 min at 37°C, with shaking at 1,700 rpm, and then centrifuged at 17,000 × g for 30 min. Supernatants were removed, and cell pellets were resuspended in 16 μl of 0.05% SDS. Four microliters of each sample was added in quadruplicate to a prepared RT-QuIC reaction mixture.
Immunohistochemistry.
Tissues from biopsy and necropsy specimen collections were fixed in paraformaldehyde-lysine-periodate (PLP) for 1 to 3 days and then transferred to 60% ethanol for long-term storage. Sections of obex, retropharyngeal lymph node, and tonsil were routinely processed and embedded in paraffin, and 5-μm sections were placed on positively charged slides. Slides were processed for PrPCWD detection as previously described (28). Briefly, deparaffinized and dehydrated tissue sections were treated with 88% formic acid for 30 min prior to hydrated autoclaving antigen retrieval in a citrate buffer. The antigen signal was detected with an anti-prion antibody (F99/97.6.1) at a concentration of 10 μg/ml followed by an alkaline phosphatase-conjugated anti-mouse secondary antibody and was visualized with an alkaline phosphatase red kit, using an automated stainer (Ventana Medical Systems). Positive- and negative-control slides containing obex and retropharyngeal lymph node sections were run in parallel.
Calculation of infectivity.
To determine the infectivity of excreted samples, the threshold for positivity was set as the average baseline fluorescence plus 5 standard deviations. Only samples with more than 6 positive results among the total of 8 replicates were analyzed. The threshold cycle (CT) value was calculated for each sample by determining the time at which the reaction crossed the threshold. The amyloid formation rate could then be defined as the inverse of the CT (1/CT). Additionally, a standard curve was developed from the amyloid formation rates from an endpoint-bioassayed brain sample from a CWD+ animal and fit to a log-linear line of best fit [y = mlog(x) + b; calculated for 3 experiments with 4 replicates in each experiment] (27). The amyloid formation rates from the saliva and urine samples were interpolated on the standard curve to estimate the infectivity of the sample relative to the bioassayed reference brain homogenate. With the line of best fit, amyloid formation rates of saliva and urine samples were used to calculate the micrograms of CWD brain equivalents. The latter were translated to 50% lethal dose (LD50) values by being divided by the LD50 of the reference bioassayed brain homogenate (27). Saliva and urine amyloid formation rates were calculated based on at least 2 experiments with at least 4 replicates each.
RESULTS
Kinetics of CWD prion shedding in saliva and urine.
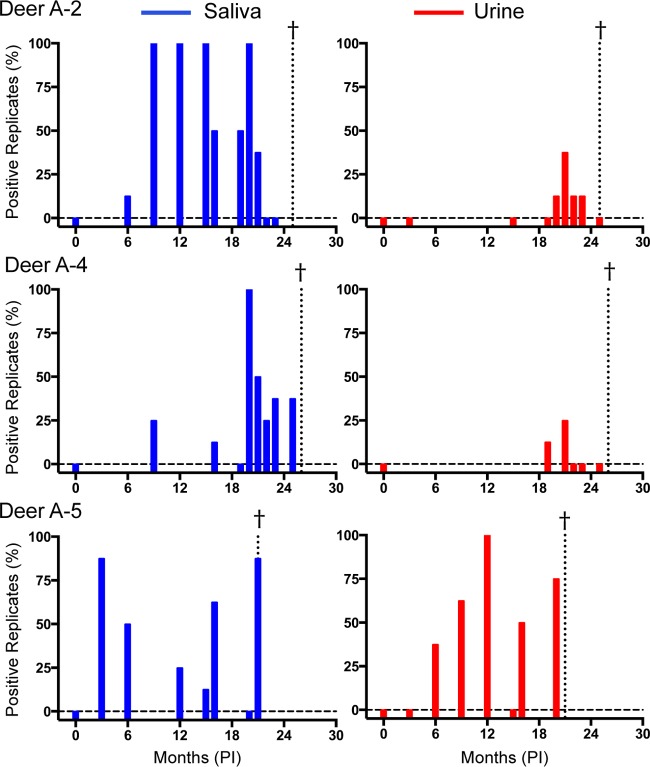
To better understand the onset and persistence of prion shedding over time, we analyzed longitudinally collected saliva and urine samples from deer exposed to CWD by aerosolization of CWD (deer A-1 to A-6), p.o. administration (deer PO-1 to PO-10), and environmental fomite contact (E-1 and E-2). Each saliva (n = 94) and urine (n = 65) sample was analyzed in at least two experiments, with four replicates for each experiment. Blinded analysis of saliva and urine from CWD-negative animals resulted in a test specificity of 97.2% for saliva (n = 104 replicates) and 99.3% for urine (n = 280 replicates). No individual saliva or urine samples from CWD-naive deer had more than one false-positive replicate in two experiments with eight total replicates. Therefore, any saliva or urine sample analyzed in at least two experiments with more than one positive replicate was considered positive. Positive RT-QuIC wells were deemed positive when the thioflavin T fluorescence reached a value that was 5 standard deviations higher than the initial fluorescence value. Prion seeding activity was observed in saliva and urine from all infected deer at points throughout the long disease course, although we found considerable variation in detectable prion shedding among longitudinal sampling dates (Fig. 1 and Tables 1 to 3). Prion seeding activity in saliva was detected as early as 3 months postinoculation (p.i.) in an aerosol-exposed deer (Fig. 1, deer A5). Seeding activity in urine was detected at 6 months p.i. and later in the disease course (>12 months p.i.) (Fig. 1 and Tables 1 to 3). In all aerosol- and orally exposed deer, CWD prion seeding activities were relatively similar in terminal brain samples, indicating relatively similar endpoints of disease (Fig. 2).
FIG 1.
Shedding of CWD prions in aerosol-exposed deer. Saliva results are shown in blue (left), and urine results are shown in red (right). RT-QuIC results for saliva and urine are from two experiments with eight total replicates. The data for three deer of six from the aerosol-inoculated group are shown to illustrate the trends observed. Each sample tested is represented by a bar, with between 0 and 100% replicates testing positive. Negative samples are expressed as a bar that meets the zero line (dashed horizontal line); samples that were not available have no bar. The dotted vertical line marked with a cross represents the terminal sample for the indicated animal.
FIG 2.
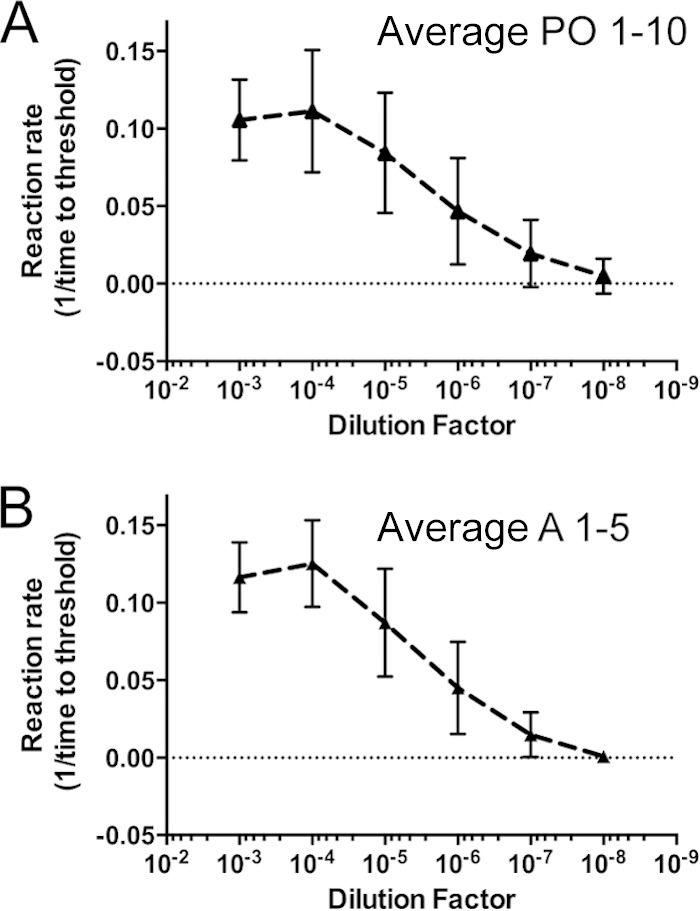
Similar levels of CWD seeding activity were observed in terminal obex samples from orally and aerosol-inoculated deer. The average amyloid formation rate (1/CT) was plotted for the orally inoculated group (A) and the aerosol-inoculated group (B). The reaction rate (1/CT) was determined by dividing by the time in hours until an RT-QuIC reaction crossed the experimental threshold (5 standard deviations [SD] from the initial fluorescence value) (y axis). Larger numbers signify higher amyloid formation rates. The dilution factor represents a series of 10-fold dilutions of a 10% homogenate of a terminal obex/brain stem sample (x axis). Error bars represent 1 SD for all averaged rates for all animals in each inoculation group. Amyloid formation rates were calculated for at least two experiments and at least four replicates (per experiment) of each serial dilution from 10−2 to 10−8 for each brain sample.
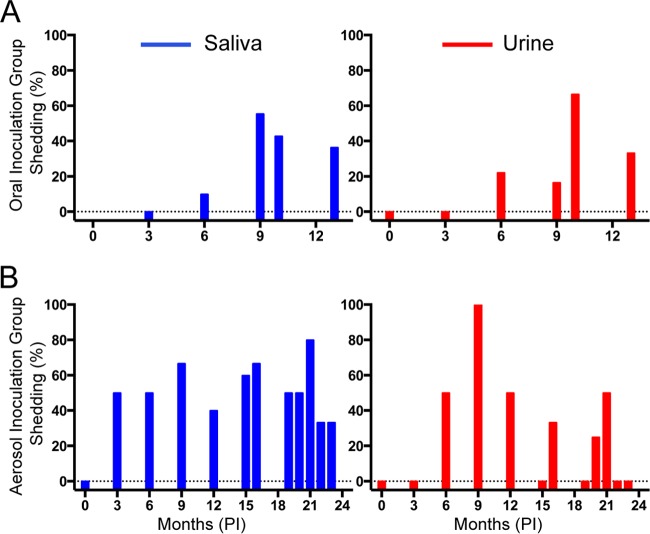
Frequency of prion seeding activity in saliva and urine of deer exposed by mucosal routes.
The temporal nature of prion shedding in CWD pathogenesis is pertinent to understanding the spread of the disease in cervids by direct and indirect environmental contact. Prion seeding activity was detected in ∼50% of saliva samples collected from deer exposed by the aerosol or oral route (Fig. 3). Overall, there were significantly more positive test replicates for saliva samples from the aerosol-inoculated deer than for those from the orally inoculated deer (P = 0.0067), suggesting that higher prion loads are shed in saliva from deer exposed to CWD by that route. Urinary amyloid seeding activity was detected in ∼25% of all samples tested in both the aerosol and oral exposure groups (24% for the p.o. group and 27% for the aerosol group). Four of the orally exposed deer had the 96G/S PRNP genotype, which was previously linked to a longer survival period than that for animals with the more frequent 96G/G genotype (30). However, the percentages of positive saliva and urine samples between 96G/S and 96G/G deer were not statistically different (P = 0.20 for urine and P = 0.23 for saliva) (Tables 1 to 3). All deer in the aerosol and oral exposure groups were IHC positive by 9 months p.i., by either tonsil or RAMALT biopsy. However, heterogeneity is often seen in biopsy specimens from live animals due to sampling difficulties and the availability of lymphatic tissues after multiple biopsy specimen samples have been taken (Tables 1 to 3).
FIG 3.
Frequencies of prion shedding in CWD-exposed deer. Saliva and urine data are shown in blue and red, respectively. (A) Percentages of total positive samples in which shedding was detected for the orally inoculated deer (≥2 of 8 replicates were positive). (B) Percentages of samples wherein shedding was detected for the aerosol-inoculated deer (≥2 of 8 replicates were positive). Bars that meet zero (horizontal dotted line) are time points where no shedding was detected, and samples that were not available have no bar.
Prion shedding in environmentally exposed deer.
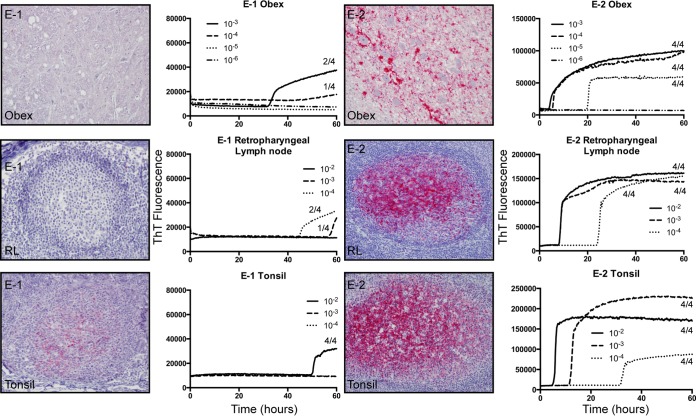
Two deer were exposed to CWD by a somewhat more natural route, i.e., transfer of used bedding, water, and feed from separate suites containing CWD-infected deer, with no direct contact between the two animal groups (26). Both environmentally exposed deer (E-1 and E-2) developed CWD infection, although both the time of detection and prion seeding loads at termination varied between them (Fig. 4). PrPCWD and RT-QuIC seeding activity were readily detected in the terminal brain (obex region of the medulla), retropharyngeal lymph node, and tonsil from animal E-2 (Fig. 4). In contrast, in deer E-1, PrPCWD was detected only in three tonsil lymphoid follicles at terminal collection, and low levels of prion seeding activity were detected in the tonsil, retropharyngeal lymph node, and brain (Fig. 4). Additionally, deer E-2 showed slightly lower amyloid formation rates in terminal obex/brain stem samples than those of the aerosol-exposed group of deer, and as shown above, deer E-1 showed only marginal seeding activity in the terminal obex/brain stem (Fig. 5). Thus, deer E-1, which had the 96 G/S genotype, was likely at an earlier stage of infection progression (Table 3 and Fig. 5). Prion amyloid seeding activity was detected in saliva and urine from both of the environmentally exposed deer, beginning as early as 3 months in deer E-2, which, interestingly, had the most consistent prion shedding observed in this study despite having less seeding activity in terminal brain tissue (Fig. 6A and B and Table 3).
FIG 4.
Analysis of environmentally exposed animal terminal disease state. IHC results and representative RT-QuIC data for deer E-1 and E-2 are displayed. Results are for one replicate each for serial dilutions of obex (10−3 to 10−6), retropharyngeal lymph node (10−2 to 10−4), and tonsil (10−2 to 10−4) samples. Each serial dilution was repeated in two experiments, with four replicates in each experiment. The number of total positive replicates for each dilution is noted. IHC staining for PrPCWD is characterized by red granular deposits in the neuropil of the obex and in germinal centers of lymphoid follicles in the tonsil and retropharyngeal lymph node. IHC staining was performed with the antibody F99/97.6.1. Magnification, ×20.
FIG 5.
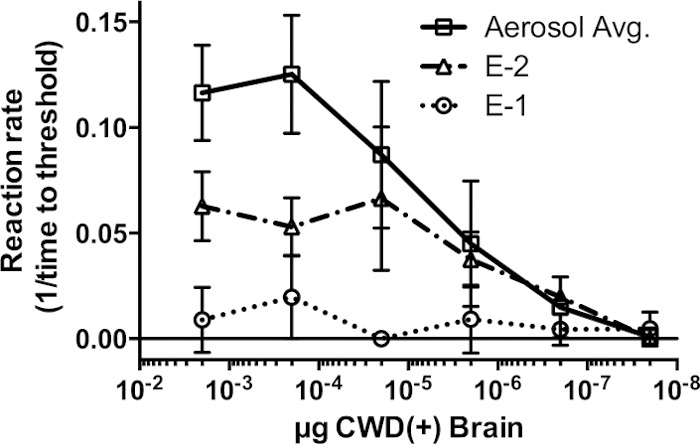
Quantitation of obex sample data for environmentally exposed deer. Amyloid formation rates in serially diluted obex samples from deer E-1 (dotted line) and E-2 (dashed-dotted line) and an average amyloid formation rate for the entire CWD+ aerosol-inoculated group (solid line) are compared. The amyloid formation rate was calculated as previously described. Larger numbers signify higher amyloid formation rates. Error bars represent 1 standard deviation from the mean.
FIG 6.
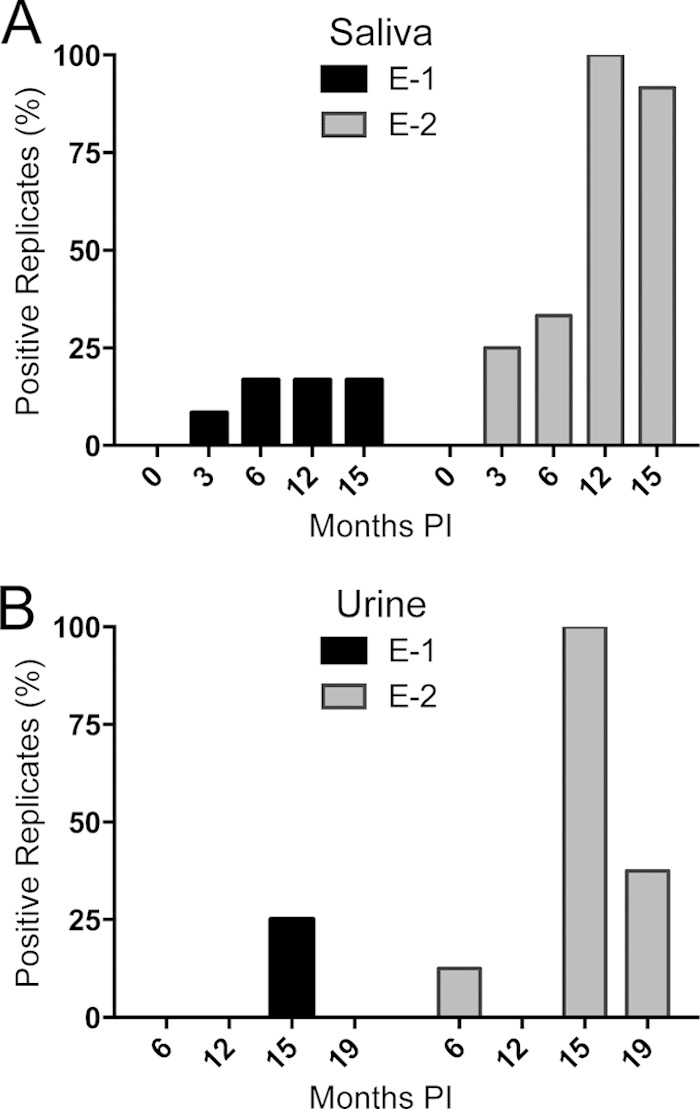
Longitudinal analysis of salivary and urinary shedding in environmentally exposed deer. (A) For saliva analysis, the percentage of positive replicates among a total of 12 replicates representing 3 independent experiments was plotted for each time point. (B) Urine RT-QuIC results for 2 experiments with 4 replicates each, with 8 replicates total for each time point.
Estimation of infectivity in saliva and urine samples.
To better characterize the magnitude of prion shedding, we applied a previously described approach based on relating the amyloid formation rate to a bioassayed reference brain homogenate to estimate the relative level of lethality (LD50) in excreta samples (27). Amyloid formation rates assayed by RT-QuIC were expressed as 1/time for ThT fluorescence emission to cross the threshold (CT). Thus, a higher amyloid formation rate (1/CT) indicates a greater concentration of amyloid seeds, analogous to the results of quantitative real-time PCR, wherein lower CT values indicate higher initial concentrations of DNA seeds (27, 31). We determined that the amyloid formation rates of saliva and urine samples producing ≥6 positive results among 8 replicates (Fig. 7A) were equivalent to the rates produced by 10−6 to 10−7 dilutions of a reference (10% [wt/vol]) CWD+ brain homogenate (Fig. 7A).
FIG 7.
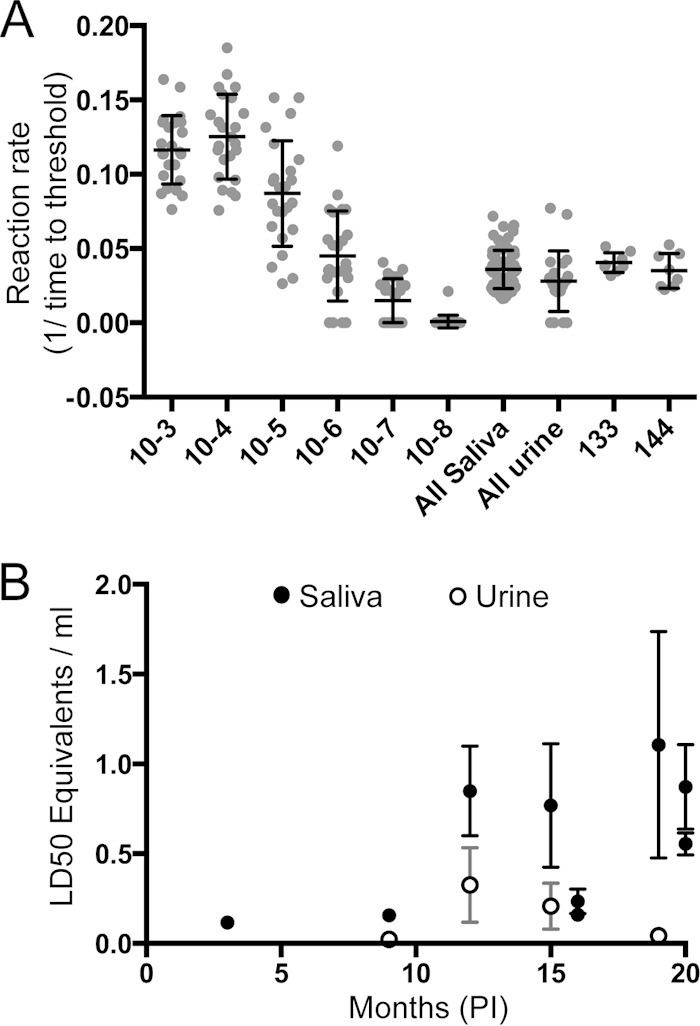
Comparison of amyloid formation rates and LD50 equivalents in saliva and urine during the CWD disease course. (A) Amyloid formation rates were plotted for 10-fold dilution series of terminal obex samples and saliva and urine samples from deer in this study where at least 6 of 8 total replicates were positive and for bioassayed saliva samples from deer 133 and 144. (B) The Tg(cerPrP)mouse LD50 was calculated for 1 ml of either saliva or urine and plotted over time of disease. Data for saliva samples are shown by filled circles, and those for urine samples are shown by open circles. Error bars represent 1 standard error of the mean.
To help substantiate this approach, we also analyzed the amyloid formation rates of two historical saliva samples (from deer 133 and 144) that had previously been bioassayed in cervidized transgenic mice (13, 14, 32) (Fig. 7A). The rates for each of these previously bioassayed saliva samples were similar to the rates found in the present longitudinal study (Fig. 7A). Samples of 300 μl of saliva from deer 133 and 144 produced 500-day attack rates of ∼50% (14). When the same samples were analyzed by quantitative RT-QuIC, the extrapolated LD50s for saliva of deer 133 and 144 were estimated to be 494 ± 202 μl and 411 ± 168 μl, respectively, thus resembling the volume of saliva producing ∼1 LD50 in cervidized mice.
The LD50 values for saliva and urine samples collected at time points of more than or close to 1 year p.i. exhibited higher amyloid formation rates, implying that higher concentrations of prion infectivity are shed later in disease progression (Fig. 7B and Tables 1 to 3). While the volume of saliva shed is surely much smaller than that of urine, the level of extrapolated infectivity was ∼10-fold greater than that in urine (i.e., ∼1.0 versus 0.1 transgenic mouse LD50/ml in urine). Nevertheless, given that the average volume of urine excreted daily by a 100-kg deer is ∼1 liter, a CWD-infected deer would deposit an estimated 100 (cervidized mouse) LD50 daily into the environment (33).
DISCUSSION
The geographic region in which CWD has been detected has continued to expand in the last decade (http://www.nwhc.usgs.gov). While the factors that influence CWD spread remain incompletely understood, direct and indirect/environmental exposure to shed prions remains the leading hypothesis. To better understand the magnitude and mechanisms of CWD spread, we analyzed the longitudinal shedding of prions in saliva and urine of white-tailed deer exposed to CWD by mucosal exposure routes. We documented prion shedding as early as 3 months postexposure and estimated that an infected deer would excrete thousands of prion infectious doses over its disease course. We also found little difference in prion shedding between deer of the more susceptible 96G/G and more resistant 96G/S genotypes (30) (although our sample size was small). However, after CWD infection was established in G/S deer, they displayed shedding kinetics and levels similar to those of G/G deer. Perhaps due to selective pressures imposed by CWD in nature, G/S deer are more prominently represented in older age classes (34). In theory, a slower disease progression combined with a larger population fraction could lead to a larger environmental contamination impact attributable to CWD-infected G/S deer (34).
Interestingly, we observed that persistent environmental exposure to presumed low levels of excreted CWD prions was associated with prominent prion seeding activity detected in the saliva and urine of a deer so exposed (Fig. 4 to 6 and Tables 1 to 3). Perhaps exposure to repeated low prion doses in nature may in turn lead to more consistent prion shedding by animals so infected, as has previously been inferred for subinfectious doses of scrapie (35), although substantially more data would be needed to support this extrapolation. Additional information is needed to assess the infectivity of excreta deposited in the environment; however, prion shedding of this magnitude in a free-ranging species would seem to pose a substantial challenge to eradication of CWD. The risk to humans and other species posed by florid dissemination of CWD prions into the environment, while unclear, cannot be discounted in light of more recent evidence that barriers to cross-species infection may not be absolute (36–39).
We have made significant progress in detection of prion amyloid seeding activity in excreta, saliva, and blood (11, 14, 27); however, assay inhibitors of these complex biological materials may yet remain. Thus, our quantitative estimation of prion shedding may be understated (Fig. 1 to 4 and Tables 1 to 3). Moreover, due to the presence of inhibitors, our sampling of excreta was restricted to small volumes (100 μl for saliva and 500 μl for urine) compared to what is actually shed in the environment. It seems likely that temporal gaps in our detection of CWD prions in excreta reflect limits in our ability to detect seeding activity rather than natural oscillations in prion shedding. Thus, we continue to explore more practical and effective means of enrichment and/or enhancement to better address the needle-in-the-haystack aspect of prion detection in excreted and environmental samples.
Prion shedding from mucosal surfaces is not limited to CWD. Prion seeding activity has been detected in body fluids or excreta of scrapie-infected sheep and hamsters (40–44), as well as bovine spongiform encephalopathy (BSE)-infected cattle (45). Amyloid seeding activity has also been detected in cerebrospinal fluid of human patients with sporadic Creutzfeldt-Jakob disease (sCJD) (46), highlighting the potential mucosal egress of prions in humans with sCJD.
While directly comparable assay methods have not been applied to the study of sheep scrapie, our estimations of shed infectivity in saliva and urine in CWD appear to be consistent with the studies of Maddison et al. (40), Gough et al. (47), and others, using serial protein misfolding cyclic amplification (sPMCA) and bioassays. Again, estimating the infectious prion loads deposited in the environment is complicated by both the potential intermittent nature of shedding and uncertainties about the stability of prion infectivity in environmental niches and surfaces (9, 20). Nevertheless, the importance of environmental contamination in CWD is supported by the studies of Miller et al. (18, 48), wherein naive deer repopulating pastures that previously housed prion-infected deer also became infected. Evidence that soil-bound prions retain infectivity has been supplied by the studies of Seidel et al. (49).
The species barrier limiting transmission of CWD prions to humans appears to be substantial (50, 51), as no case of human prion disease has yet been linked to CWD (52, 53). However, works by Castilla et al. (38), Barria et al. (36, 39), Cassard et al. (37), and others show that the species barrier may be more dynamic than previously estimated. It remains unknown whether natural passage of excreted CWD prions through generations of outbred cervids in nature may ultimately alter its species/transmission barrier. Thus, a more complete understanding of the transmission, excretion, environmental contamination, and species barrier for this emergent prion disease is warranted.
ACKNOWLEDGMENTS
This work was supported by NIH grant R01 NS-061902 and by grant D12ZO-045 from the Morris Animal Foundation.
We thank Jeanette Hayes-Klug and Kelly Anderson for their excellent observation and care of and sample collection from the deer in this study. We thank Kristen Davenport for a critical review of the manuscript. We thank Wilfred Goldman for CWD PRNP genotyping of the deer used. Finally, we appreciate the continued collaboration of Sallie Dahmes, David Osborn, Carl Miller, Robert Warren, and the University of Georgia for the rearing of the CWD-free, indoor-adapted white-tailed deer used in these studies.
REFERENCES
- 1.Sigurdson CJ. 2008. A prion disease of cervids: chronic wasting disease. Vet Res 39:41. doi: 10.1051/vetres:2008018. [DOI] [PubMed] [Google Scholar]
- 2.Williams ES. 2005. Chronic wasting disease. Vet Pathol 42:530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- 3.Saunders SE, Bartelt-Hunt SL, Bartz JC. 2012. Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerg Infect Dis 18:369–376. doi: 10.3201/eid1803.110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colby DW, Prusiner SB. 2011. Prions. Cold Spring Harb Perspect Biol 3:a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prusiner SB, Groth DF, Bolton DC, Kent SB, Hood LE. 1984. Purification and structural studies of a major scrapie prion protein. Cell 38:127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- 6.Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 7.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Almberg ES, Cross PC, Johnson CJ, Heisey DM, Richards BJ. 2011. Modeling routes of chronic wasting disease transmission: environmental prion persistence promotes deer population decline and extinction. PLoS One 6:e19896. doi: 10.1371/journal.pone.0019896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders SE, Bartz JC, Telling GC, Bartelt-Hunt SL. 2008. Environmentally-relevant forms of the prion protein. Environ Sci Technol 42:6573–6579. doi: 10.1021/es800590k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamguney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. 2013. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS One 8:e80203. doi: 10.1371/journal.pone.0080203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. 2011. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol 85:6309–6318. doi: 10.1128/JVI.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B, Hoover EA. 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS One 8:e74377. doi: 10.1371/journal.pone.0074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 16.Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, Letessier F, Dearmond SJ, Prusiner SB. 2008. Transmission and detection of prions in feces. J Infect Dis 198:81–89. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgsson G, Sigurdarson S, Brown P. 2006. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol 87:3737–3740. doi: 10.1099/vir.0.82011-0. [DOI] [PubMed] [Google Scholar]
- 18.Miller MW, Williams ES, Hobbs NT, Wolfe LL. 2004. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders SE, Bartelt-Hunt SL, Bartz JC. 2012. Resistance of soil-bound prions to rumen digestion. PLoS One 7:e44051. doi: 10.1371/journal.pone.0044051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders SE, Bartz JC, Vercauteren KC, Bartelt-Hunt SL. 2010. Enzymatic digestion of chronic wasting disease prions bound to soil. Environ Sci Technol 44:4129–4135. doi: 10.1021/es903520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols TA, Spraker TR, Gidlewski T, Powers JG, Telling GC, VerCauteren KC, Zabel MD. 2012. Detection of prion protein in the cerebrospinal fluid of elk (Cervus canadensis nelsoni) with chronic wasting disease using protein misfolding cyclic amplification. J Vet Diagn Invest 24:746–749. doi: 10.1177/1040638712448060. [DOI] [PubMed] [Google Scholar]
- 23.Orru CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, Caughey B. 2011. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. mBio 2:e00078–11. doi: 10.1128/mBio.00078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atarashi R, Sano K, Satoh K, Nishida N. 2011. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion 5:150–153. doi: 10.4161/pri.5.3.16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mathiason CK, Hoover EA. 2013. Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 87:1890–1892. doi: 10.1128/JVI.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hoover EA. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. doi: 10.1371/journal.pone.0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA Jr. 2015. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol 96:210–219. doi: 10.1099/vir.0.069906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, Spowart RA, O'Rourke KI, Miller JM, Merz PA. 1997. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis 33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Johnson CJ, Herbst A, Duque-Velasquez C, Vanderloo JP, Bochsler P, Chappell R, McKenzie D. 2011. Prion protein polymorphisms affect chronic wasting disease progression. PLoS One 6:e17450. doi: 10.1371/journal.pone.0017450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson UE, Heid CA, Williams PM. 1996. A novel method for real time quantitative RT-PCR. Genome Res 6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 32.Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, Miller MW, Williams ES, Hoover E, Telling GC. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol 78:13345–13350. doi: 10.1128/JVI.78.23.13345-13350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dukes HH, Reece WO. 2004. Dukes' physiology of domestic animals, 12th ed Comstock Publishing Associates, Ithaca, NY. [Google Scholar]
- 34.Robinson SJ, Samuel MD, Johnson CJ, Adams M, McKenzie DI. 2012. Emerging prion disease drives host selection in a wildlife population. Ecol Appl 22:1050–1059. doi: 10.1890/11-0907.1. [DOI] [PubMed] [Google Scholar]
- 35.Jacquemot C, Cuche C, Dormont D, Lazarini F. 2005. High incidence of scrapie induced by repeated injections of subinfectious prion doses. J Virol 79:8904–8908. doi: 10.1128/JVI.79.14.8904-8908.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barria MA, Telling GC, Gambetti P, Mastrianni JA, Soto C. 2011. Generation of a new form of human PrP(Sc) in vitro by interspecies transmission from cervid prions. J Biol Chem 286:7490–7495. doi: 10.1074/jbc.M110.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassard H, Torres JM, Lacroux C, Douet JY, Benestad SL, Lantier F, Lugan S, Lantier I, Costes P, Aron N, Reine F, Herzog L, Espinosa JC, Beringue V, Andreoletti O. 2014. Evidence for zoonotic potential of ovine scrapie prions. Nat Commun 5:5821. doi: 10.1038/ncomms6821. [DOI] [PubMed] [Google Scholar]
- 38.Castilla J, Gonzalez-Romero D, Saa P, Morales R, De Castro J, Soto C. 2008. Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell 134:757–768. doi: 10.1016/j.cell.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krejciova Z, Barria MA, Jones M, Ironside JW, Jeffrey M, Gonzalez L, Head MW. 2014. Genotype-dependent molecular evolution of sheep bovine spongiform encephalopathy (BSE) prions in vitro affects their zoonotic potential. J Biol Chem 289:26075–26088. doi: 10.1074/jbc.M114.582965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddison BC, Rees HC, Baker CA, Taema M, Bellworthy SJ, Thorne L, Terry LA, Gough KC. 2010. Prions are secreted into the oral cavity in sheep with preclinical scrapie. J Infect Dis 201:1672–1676. doi: 10.1086/652457. [DOI] [PubMed] [Google Scholar]
- 41.Murayama Y, Yoshioka M, Okada H, Takata M, Yokoyama T, Mohri S. 2007. Urinary excretion and blood level of prions in scrapie-infected hamsters. J Gen Virol 88:2890–2898. doi: 10.1099/vir.0.82786-0. [DOI] [PubMed] [Google Scholar]
- 42.Okada H, Murayama Y, Shimozaki N, Yoshioka M, Masujin K, Imamura M, Iwamaru Y, Matsuura Y, Miyazawa K, Fukuda S, Yokoyama T, Mohri S. 2012. Prion in saliva of bovine spongiform encephalopathy-infected cattle. Emerg Infect Dis 18:2091–2092. doi: 10.3201/1812.120528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry LA, Howells L, Bishop K, Baker CA, Everest S, Thorne L, Maddison BC, Gough KC. 2011. Detection of prions in the faeces of sheep naturally infected with classical scrapie. Vet Res 42:65. doi: 10.1186/1297-9716-42-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vascellari M, Nonno R, Mutinelli F, Bigolaro M, Di Bari MA, Melchiotti E, Marcon S, D'Agostino C, Vaccari G, Conte M, De Grossi L, Rosone F, Giordani F, Agrimi U. 2007. PrPSc in salivary glands of scrapie-affected sheep. J Virol 81:4872–4876. doi: 10.1128/JVI.02148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orrù CD, Hughson AG, Race B, Raymond GJ, Caughey B. 2012. Time course of prion seeding activity in cerebrospinal fluid of scrapie-infected hamsters after intratongue and intracerebral inoculations. J Clin Microbiol 50:1464–1466. doi: 10.1128/JCM.06099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orru CD, Bongianni M, Tonoli G, Ferrari S, Hughson AG, Groveman BR, Fiorini M, Pocchiari M, Monaco S, Caughey B, Zanusso G. 2014. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med 371:519–529. doi: 10.1056/NEJMoa1315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gough KC, Baker CA, Rees HC, Terry LA, Spiropoulos J, Thorne L, Maddison BC. 2012. The oral secretion of infectious scrapie prions occurs in preclinical sheep with a range of PRNP genotypes. J Virol 86:566–571. doi: 10.1128/JVI.05579-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller MW, Williams ES. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 49.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. 2007. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, Yuan J, Zheng M, Bai H, Deng H, Chen K, Jenny AL, O'Rourke K, Belay ED, Schonberger LB, Petersen RB, Sy MS, Chen SG, Gambetti P. 2005. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 25:7944–7949. doi: 10.1523/JNEUROSCI.2467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raymond GJ, Bossers A, Raymond LD, O'Rourke KI, McHolland LE, Bryant PK III, Miller MW, Williams ES, Smits M, Caughey B. 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J 19:4425–4430. doi: 10.1093/emboj/19.17.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson CA, Bosque P, Filley CM, Arciniegas DB, Kleinschmidt-Demasters BK, Pape WJ, Tyler KL. 2007. Colorado surveillance program for chronic wasting disease transmission to humans: lessons from 2 highly suspicious but negative cases. Arch Neurol 64:439–441. doi: 10.1001/archneur.64.3.439. [DOI] [PubMed] [Google Scholar]
- 53.Mawhinney S, Pape WJ, Forster JE, Anderson CA, Bosque P, Miller MW. 2006. Human prion disease and relative risk associated with chronic wasting disease. Emerg Infect Dis 12:1527–1535. doi: 10.3201/eid1210.060019. [DOI] [PMC free article] [PubMed] [Google Scholar]