ABSTRACT
Our earlier studies with pig-tailed macaques demonstrated various simian-human immunodeficiency virus (SHIV) susceptibilities during the menstrual cycle, likely caused by cyclic variations in immune responses in the female genital tract. There is concern that high-dose, long-lasting, injectable progestin-based contraception could mimic the high-progesterone luteal phase and predispose women to human immunodeficiency type 1 (HIV-1) acquisition and transmission. In this study, we adopted a systems biology approach employing proteomics (tandem mass spectrometry), transcriptomics (RNA microarray hybridization), and other specific protein assays (enzyme-linked immunosorbent assays and multiplex chemokine and cytokine measurements) to characterize the effects of hormonal changes on the expression of innate factors and secreted proteins in the macaque vagina. Several antiviral factors and pathways (including acute-phase response signaling and complement system) were overexpressed in the follicular phase. Conversely, during the luteal phase there were factors overexpressed (including moesins, syndecans, and integrins, among others) that could play direct or indirect roles in enhancing HIV-1 infection. Thus, our study showed that specific pathways and proteins or genes might work in tandem to regulate innate immunity, thus fostering further investigation and future design of approaches to help counter HIV-1 acquisition in the female genital tract.
IMPORTANCE HIV infection in women is poorly understood. High levels of the hormone progesterone may make women more vulnerable to infection. This could be the case during the menstrual cycle, when using hormone-based birth control, or during pregnancy. The biological basis for increased HIV vulnerability is not known. We used an animal model with high risk for infection during periods of high progesterone. Genital secretions and tissues during the menstrual cycle were studied. Our goal was to identify biological factors upregulated at high progesterone levels, and we indeed show an upregulation of genes and proteins which enhance the ability of HIV to infect when progesterone is high. In contrast, during low-progesterone periods, we found more HIV inhibitory factors. This study contributes to our understanding of mechanisms that may regulate HIV infection in females under hormonal influences. Such knowledge is needed for the development of novel prevention strategies.
INTRODUCTION
Female acquisition of human immunodeficiency virus type 1 (HIV-1) is not an efficient process, and transmission events are estimated to occur at frequencies of 1/100 to 1/1,000 exposures (1; reviewed in reference 2). Despite this, the heterosexual route is the predominant mode of HIV-1 transmission worldwide (3), and the development of strategies to reduce male-to-female vaginal transmission rates remains a priority. Animal studies have demonstrated that the time between vaginal exposure and virus detection in draining lymph nodes is as little as 1 to 3 days. There is a small window of early protection when the initial innate responses at the mucosal surfaces need to be triggered quickly and effectively to obstruct HIV-1 infection. A gap in knowledge exists in understanding the nature and extent of such responses and their effect on early HIV-1 infection. Defense mechanisms in the mucosal lining of the female genital tract are influenced by menstrual cycle fluctuations in sex hormones, progesterone and estradiol, intended primarily to strike an optimal balance between hostility toward pathogens and tolerance to the allogeneic embryo during reproduction (4, 5). It has been hypothesized that such cycle-dependent changes in immune responses engender variable susceptibilities to sexually transmitted infections (STIs), including HIV-1. We have previously demonstrated using a repeat, low-dose virus challenge model that there are various susceptibilities to simian-human immunodeficiency virus (SHIV) during the different menstrual phases in female pig-tailed macaques (Macaca nemestrina) (6, 7). With menstrual cycling patterns similar to those in humans, and the previously reported success in infecting the animals at low virus doses without hormonal intervention, pig-tailed macaques have become unique animal models for HIV studies; their importance is especially underscored in testing menstrual phase-dependent susceptibility to HIV, considering the difficulty in conducting such studies with normally cycling women. The luteal phase is characterized by higher levels of progesterone, which have been associated with depressed immune responses, vaginal epithelial thinning (less in humans than in macaques), and other factors that increase risk of HIV-1 acquisition (7–9). Although this has not been directly tested, there are public health implications to the possibility that women on progestin-based contraception might exhibit a lack of immune responses akin to those seen in the luteal phase, thus increasing risk of HIV-1 acquisition. In this study, which, to our knowledge, is the first of its kind using nonhuman primate models, we took a systems biology approach that used proteomic analyses of secreted factors and specific regulatory protein assays to extensively explore proteins that are at play in the genital tracts of normally cycling uninfected pig-tailed macaques. To complement analysis of secreted, soluble immune factors, we also used genomic analyses of vaginal tissues to determine gene expression in epithelial tissues and resident cells in the vaginal canal that may impact immunity in situ, regardless of the secretion of protein factors. Past studies have shown fluctuations in the thickness of the vaginal epithelium based on the phase of the menstrual cycle (10, 11), in concert with changes in infiltration of HIV-1 target cells, and cellular junctions. These significant tissue architecture modifications might result in gene expression changes which have not been documented for pig-tailed macaques to date. Using proteomic analyses, it was shown that there exists an abundance of soluble proteins in different compartments of the human female genital tract (12). Thus, our study goal was to not only catalogue menstrual phase-related changes but also help compare and contrast the pigtail model to women whose fluctuations in susceptibility to HIV during the cycle are unknown. Our results might provide insight into effects of hormonal contraception by providing a frame of reference for susceptibility changes in women and identify signature factors during high-susceptibility or resistant phases that may become targets for HIV intervention strategies. We demonstrated profound menstrual phase-related changes in the overall abundance of innate immune factors and proteins, which might play a role in susceptibility to infection.
(Data were presented in part and published as an abstract at the 12th Congress of the International Society for Immunology Reproduction [ISIR] hosted by the American Society for Reproductive Immunology [ASRI], 28 May to 1 June 2013, Boston, MA; the AIDS Vaccine 2011 Conference, 12 to 15 September 2011, Bangkok, Thailand; and the 29th Annual Symposium on Nonhuman Primate Models for AIDS, 25 to 28 October 2011, Seattle, WA.)
MATERIALS AND METHODS
Study samples.
All procedures were approved by the Centers for Disease Control and Prevention Institutional Animal Care and Use Committee. The uninfected macaques were naturally cycling and not treated with depot medroxyprogesterone acetate (Depo-Provera) or synchronized for menstrual cycle. All analyses were done on archived samples (vaginal biopsy tissues and cervicovaginal lavage samples [CVLs]) collected from 27 normally cycling female pig-tailed macaques (unknown reproductive history prior to purchase) that were part of various other published and unpublished studies: proteomics analyses (6 macaques [13]), genomic analyses (11 macaques [14]), and Luminex or enzyme-linked immunosorbent assays (ELISA) (10 macaques [unpublished data]). The menstrual status of each monkey was determined by measuring blood progesterone levels (Wisconsin National Primate Research Center, Madison, WI) (15) at least once a week. Day 1 of the menstrual cycle was defined as the time point following the steepest progesterone decline (7). The cycle was divided based on the rise in progesterone levels into follicular (first part) and luteal (second part) phases.
Tandem mass spectrometry.
For a proteomics-based approach to identify differentially expressed innate immune factors, CVLs were collected twice a week by washing the vaginal cavity with 8 ml of sterile phosphate-buffered saline (PBS) from six macaques (collected 20 s after a single PBS infusion) and were pooled from at least one complete menstrual cycle based on plasma progesterone measurements to represent the luteal or follicular phase. Samples contaminated with blood were not used. The number of samples pooled ranged from three to nine (same animal) depending on the progesterone readings, presence of blood, and length of the cycle, all of which varied from macaque to macaque. Equal amounts of protein from each sample (one from each menstrual phase for every animal) were then digested with trypsin and differentially labeled with isobaric tags for relative and absolute quantitation (iTRAQ), according to the manufacturer's instructions (Applied Biosystems, Foster City, CA), as previously described (16). An internal reference was created with equal amounts of protein from every sample to normalize between experimental runs. Equal amounts of labeled peptides were then combined and analyzed by tandem mass spectrometry (16). Protein searches were performed against the International Protein Index (IPI) human database (v3.78).
RNA purification and microarray hybridization.
Vaginal pinch biopsy specimens (1- to 2-mm samples) were collected from 12 pig-tailed macaques at both the follicular and luteal phases using a rigid biopsy punch (EuroMed, Tuttlingen, Germany). For each macaque, the biopsy specimens were pooled. No samples were collected from the cervix. Total RNA extraction was performed using the RNeasy minikit (Qiagen, Hilden, Germany). The integrity and quantity of the extracted RNA were assessed with an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA) and NanoDrop 2000 spectrophotometer (Thermo Scientific Inc., Wilmington, DE). Samples were hybridized to Affymetrix GeneChip rhesus macaque genome arrays (Affymetrix, Santa Clara, CA), which contain over 52,000 individual probe-sets that assay over 47,000 transcripts. Briefly, cDNA synthesis and amplification were performed using the NuGEN Ovation Pico WTA V2 system (NuGEN, San Carlos, CA). Five micrograms of the amplified cDNA was fragmented, biotinylated, and hybridized to arrays using the NuGEN Ovation Encore biotin module according to kit instructions. Arrays were washed, stained, and scanned as described in the Affymetrix GeneChip expression analysis technical manual, and the .CEL files were extracted from the raw scanned images using the Affymetrix GeneChip command console software.
Multiplex chemokine and cytokine assays.
CVLs were collected weekly from another set of 10 pig-tailed macaques and stored immediately at −80°C in the absence of protease inhibitors. For detection of soluble cytokines and chemokines, undiluted CVLs were analyzed utilizing Luminex bead technology. Multiplex bead kits were purchased from the following manufacturers: BioSource International, Inc. (now Life Technologies Corporation, Carlsbad, CA; for interleukin 2 [IL-2], IL-1β, IL-6, IL-15, monocyte chemoattractant protein 1 [MCP-1], tumor necrosis factor alpha [TNF-α], MIP-1α, RANTES, and eotaxin), Bio-Rad Laboratories, Inc. (Hercules, CA; for granulocyte colony-stimulating factor [G-CSF], MIP-1β, and IL-7), R&D Systems, Inc. (Minneapolis, MN; for the IL-1Ra kit), and Upstate Laboratories, Inc. (East Syracuse, NY; for IL-8). Supernatants of phorbol myristate acetate (PMA)-ionomycin-stimulated pigtail peripheral blood mononuclear cells (PBMCs) were internal positive controls in the Luminex-based assays throughout the study.
ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) were performed on the same weekly specimens (10 macaques) to determine the presence of innate proteins, including kallikrein-like (KLK) peptidases (Antibodies Online, Atlanta, GA), trappin-2, secretory leukocyte protease inhibitor (SLPI), thymic stromal lymphopoietin (R&D Systems Inc., Minneapolis, MN), α-defensins (human neutrophil proteins 1 to 3 [HNP1 to HNP3]), surfactant protein-D (Cell Sciences Inc., Canton, MA), β-defensin 2, and β-defensin 3 (Phoenix Pharmaceuticals Inc., Burlingame, CA).
Statistical analyses.
Proteins identified as differentially abundant by proteomics were those with P values below the level of significance (0.05) based upon the use of the Student t test. The statistical correction for multiple comparisons was not applied in this protein analysis to avoid excessive stringency that might lead to omission of potentially relevant proteins. This exclusion would not serve the exploratory purpose of this study. Hierarchical clustering of differentially abundant proteins was then generated by unsupervised centroid linkage hierarchical clustering using the Pearson correlation coefficient as the distance metric. The two major branches were further analyzed by Ingenuity Pathway Analysis software (Ingenuity Systems, Redwood City, CA). The association between proteins in the data set and the canonical pathways in the Ingenuity Pathway knowledgebase was studied by considering the number of molecules from the data that map to a pathway and the total number of molecules that map to a known canonical pathway. A right-tailed Fisher exact test (with Benjamini-Hochberg multiple testing correction) was used to assess whether there was a higher proportion of proteins in the data set associated with the known canonical pathway. Pathways with a P value of <0.05 and with at least 2 proteins were selected as potential pathways associated with each branch in the cluster analysis. For the ELISA and multiplex analyses, we used random-effects models (using a P value of <0.002 to identify significant effects based on the Bonferroni adjustment.) with left-censoring of observations below the limit of detection to identify differentially expressed proteins; this model compared proteins levels in the two menstrual phases.
Microarray data analysis.
Background adjustment, normalization, and median polish summarization of .CEL files were performed with the robust multichip average (RMA) algorithm using the “affy” Bioconductor package. The quality of hybridized chips was assessed after normalization by examining their NUSE and RLE plots. The data from one animal suggested low RNA quality and were excluded. Downstream analyses were performed using Partek Genomics Suite software version 6.5 (Partek Inc., St. Louis, MO). To determine whether genes were differentially expressed between phases, we used a paired t test and corrected for multiple tests using the q-value method (17) calculation with a false-discovery rate of 0.1; the effective P value was 0.0152. We further required a ≥1.5-fold difference. To test the robustness of the data, we also used the LIMMA package (18), which employs Bayesian statistics to analyze the microarray results. While there is a large amount of literature that has demonstrated that Bayesian modeling can improve sensitivity of statistical testing in microarray analyses, classical statistics like t tests are also widely accepted (19). Moreover, the primary advantage of Bayesian modeling has been shown to be in small sample sizes (n = 2 or 3), but when sample sizes are larger (n > 5), Bayesian and t tests perform similarly (20). As our sample size (n = 12) was quite large, we thought that using the actual per-gene error estimates would be more appropriate than modeling. Hence, our downstream analysis and discussion involve results from our paired t tests.
GSEA of microarray data.
Gene set enrichment analysis (GSEA) was performed using the desktop module available from the Broad Institute (http://www.broadinstitute.org/gsea/). While conventional microarray statistics determine significance based on variance on a single-gene basis, GSEA employs a cumulative statistic based on multiple genes within a biological pathway, and these multiple genes are grouped together based on their common function or characteristic or empirical data (21). Gene rank was calculated for the normalized expression table using the signal-to-noise metric. The ranked data set was screened against the Broad Institute's curated (C2) and immunologic signatures (C7) gene sets, from the curated pathways maintained at the NCI Pathway Interaction Database (http://pid.nci.nih.gov/), as well as against an interferon-stimulated gene (ISG) gene set determined using previous data sets from our laboratory (22).
Comparison of genomic and proteomic data.
For a side-by-side comparison of immune-related pathways associated significantly with both the genomic and proteomic data sets, gene ontology and pathway associations were determined using the Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.7) and Qiagen's Ingenuity Pathway Analysis software. Right-tailed Fisher's exact tests were used to calculate the probability that the association between each protein in the data set and the biological function or pathway was random. Significant pathway criteria were a P value of <0.05 and a minimum of two proteins associated.
Microarray data accession number.
Microarray data are available at the NCBI Gene Expression Omnibus (GEO) repository under accession number GSE68079.
RESULTS
To study the effects of the two menstrual phases on innate mucosal immunity, we applied various techniques that evaluated expression of both genes and secreted proteins in the cervicovaginal environment believed to be a critical early deterrent against STIs. Table S1 in the supplemental material shows dates of birth for the macaques. The purpose of a systems biology approach was to look at different biologic compartments to get a comprehensive catalogue of genes and expressed proteins in the cervicovaginal canal.
Detection of innate immune proteins and pathways in vaginal secretions.
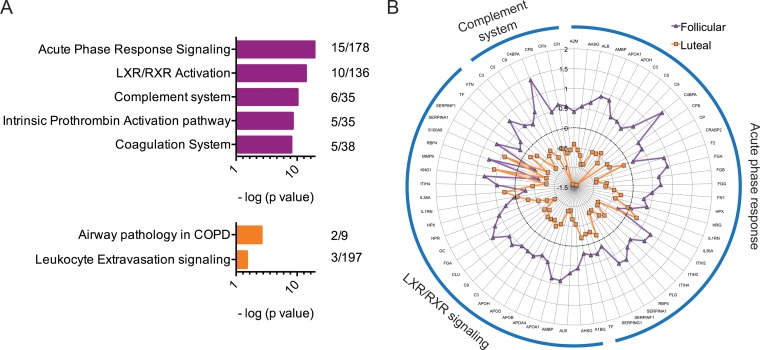
Cataloguing of the CVL proteome in secretions over complete menstrual cycles allows better understanding of the roles the two menstrual phases play in influencing the levels of soluble protein factors that, in turn, contribute to host susceptibility to pathogens. We assessed CVLs from the follicular and luteal phases collected from six macaques and selected 50 proteins (of 327 [15.3%]) that were differentially expressed in the two phases (Fig. 1; Tables 1 and 2). The luteal-phase samples from macaque CV85 and follicular-phase samples from macaque PHQ1 showed data inconsistent with the rest of the samples (Fig. 1), which could be attributed to an incorrect assignment to a menstrual phase that was based solely on progesterone readings measured twice per week. Several immune response pathways were overexpressed in the follicular phase relative to luteal phase (Fig. 2A), including acute-phase response signaling (P < 0.0001), complement system (P < 0.0001), and liver X receptor/retinoid X receptor (LXR/RXR) activation pathways (P < 0.0001). When all proteins belonging to these three pathways were plotted (spider plot [Fig. 2B]), upregulation was observed in the follicular phase for the majority of these factors, although not all factors were statistically significant. Proteins that were significantly overexpressed in the follicular phase (Table 1) included complement component 3 (P = 0.03), a critical regulator of innate immunity; isoforms of complement factors B (P = 0.04), H (P = 0.01), and I (P = 0.02) that are crucial components of the alternate complement pathway; vitronectin, a cell adhesion mediator with HIV-inhibitory properties; apolipoproteins A-I (P = 0.03) and D (P = 0.03); and analogues of serpins, inter-α (globulin) inhibitor H2 (P = 0.009), α-2-HS-glycoprotein (P < 0.0464), and inter-α-trypsin inhibitor (P = 0.009). Among the proteins that were significantly overexpressed in the luteal phase (Table 2) were moesin (P = 0.008), a cross-linking protein involved in the interplay between actin filaments and plasma membrane; vimentin (P = 0.03) and isoform of heterogeneous nuclear ribonucleoproteins C1/C2 (P < 0.05), which are known to facilitate HIV entry; and proteases (matrix metalloproteinase [P = 0.02], collagenase [P = 0.02], and cathepsins [P = 0.04]). IPA analysis also identified two associated pathways with these factors, including airway pathology in chronic obstructive pulmonary disease (COPD) (P = 0.002) (MMP8 and MMP9) and leukocyte extravasation signaling (P = 0.04) (MMP8, MMP9, and MSN). Both of these pathways are involved with general inflammation and migration of leukocytes and immune cells.
FIG 1.
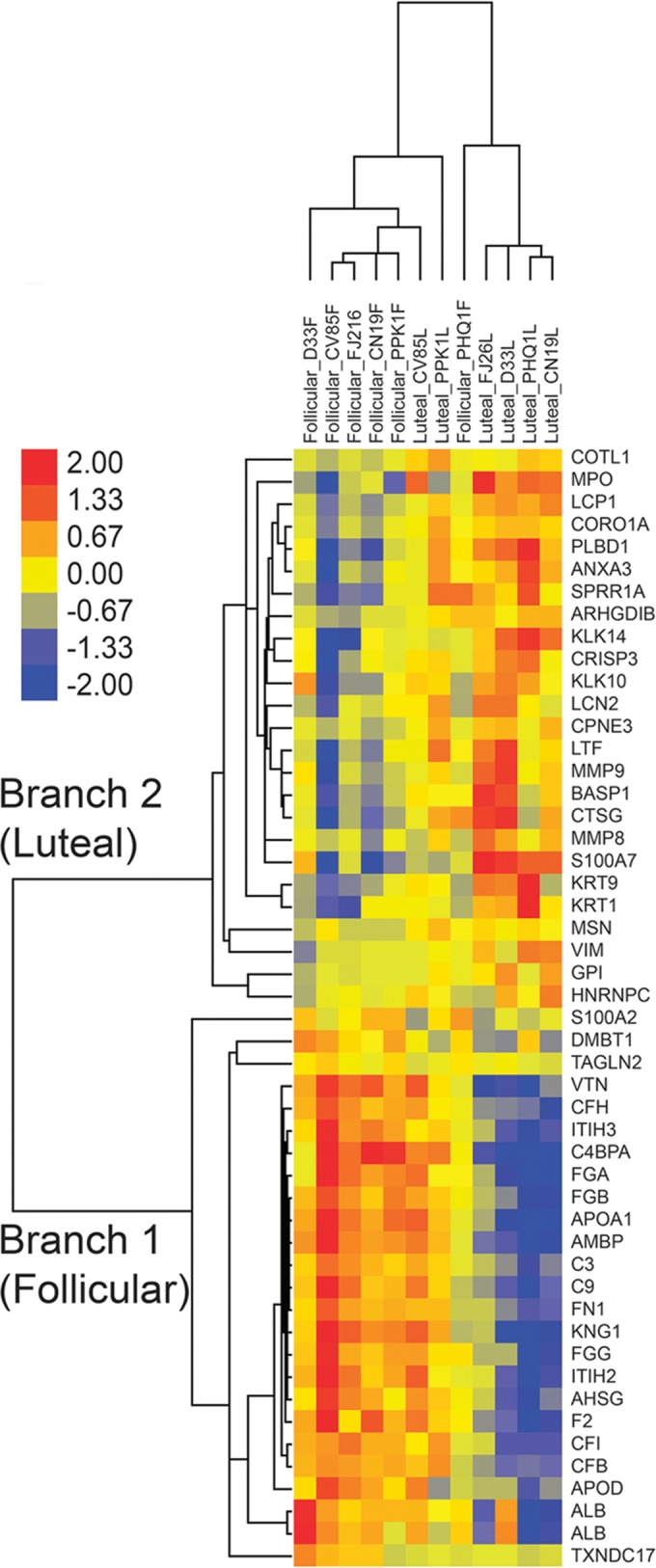
Hierarchical clustering analysis of differentially expressed proteins from cervicovaginal lavage samples obtained from the luteal or follicular phase of the menstrual cycle. Proteins (n = 50) which were differentially abundant between menstrual phase samples according to the Student t test (P < 0.05) are illustrated. Protein details can be obtained at http://www.uniprot.org; hierarchical clustering of proteins was generated by unsupervised centroid linkage using the Pearson correlation as the distance metric. The abundance of each protein is shown in color (red color denotes overabundant proteins, yellow unchanged, and blue underabundant compared to the mean). Two trees (top) are observed distinguishing menstrual-phase samples (follicular grouping on left side, luteal on the right), with only 3 of the samples having heterogeneous protein abundance patterns (CV85 luteal; PPK1 and PHQ1 follicular). The follicular and luteal phases are distinguished by two distinct branches; the top branch (branch 2) groups overabundant proteins from the luteal phase, and the bottom branch (branch 1) shows those overabundant in the follicular phase. The macaque identifiers (example: CV85) are indicated at the top.
TABLE 1.
Proteins significantly overexpressed in the follicular phase of the menstrual cycle
| Protein name | IPI database accession no. | P value (t test) |
|---|---|---|
| Isoform 1 of deleted in malignant brain tumors 1 protein | IPI00099110 | 0.0035 |
| Transgelin-2 | IPI00550363 | 0.0042 |
| Protein S100-A2 | IPI00019869 | 0.0091 |
| Isoform 1 of inter-α-trypsin inhibitor heavy chain H3 | IPI00028413 | 0.0092 |
| Isoform 1 of complement factor H | IPI00029739 | 0.0130 |
| C4b-binding protein alpha chain | IPI00021727 | 0.0165 |
| Glucose-6-phosphate isomerase | IPI00027497 | 0.0209 |
| Complement factor I | IPI00291867 | 0.0216 |
| Vitronectin | IPI00298971 | 0.0216 |
| Protein AMBP | IPI00022426 | 0.0217 |
| Isoform gamma-B of fibrinogen gamma chain | IPI00021891 | 0.0227 |
| Isoform 1 of fibrinogen alpha chain | IPI00021885 | 0.0246 |
| Apolipoprotein A-I | IPI00021841 | 0.0249 |
| Apolipoprotein D | IPI00006662 | 0.0263 |
| Prothrombin (fragment) | IPI00019568 | 0.0291 |
| Complement C3 (fragment) | IPI00783987 | 0.0291 |
| Thioredoxin domain-containing protein 17 | IPI00646689 | 0.0318 |
| Isoform 1 of fibronectin | IPI00022418 | 0.0367 |
| cDNA FLJ55673 (highly similar to complement factor B) | IPI00019591 | 0.0372 |
| Uncharacterized protein | IPI00022434 | 0.0421 |
| Complement component C9 | IPI00022395 | 0.0428 |
| Inter-α (globulin) inhibitor H2, isoform CRA_a | IPI00305461 | 0.0464 |
| cDNA FLJ55606 (highly similar to α-2-HS-glycoprotein) | IPI00022431 | 0.0469 |
| Isoform HMW of kininogen-1 | IPI00032328 | 0.0482 |
| Uncharacterized protein | IPI00022434 | 0.0493 |
TABLE 2.
Proteins significantly overexpressed in the luteal phase of the menstrual cycle
| Protein name | IPI database accession no. | P value (t test) |
|---|---|---|
| Plastin-2 | IPI00010471 | 0.0004 |
| Copine-3 | IPI00024403 | 0.0008 |
| Coactosin-like protein | IPI00017704 | 0.0015 |
| Coronin-1A | IPI00010133 | 0.0030 |
| Isoform H17 of myeloperoxidase | IPI00007244 | 0.0061 |
| Phospholipase B-like 1 | IPI00016255 | 0.0077 |
| Moesin | IPI00219365 | 0.0082 |
| Isoform 1 of neutrophil gelatinase-associated lipocalin | IPI00299547 | 0.0099 |
| Keratin, type I cytoskeletal 9 | IPI00019359 | 0.0117 |
| cDNA FLJ78440 (highly similar to human lactoferrin) | IPI00298860 | 0.0136 |
| Annexin A3 | IPI00024095 | 0.0140 |
| Rho GDP-dissociation inhibitor 2 | IPI00003817 | 0.0163 |
| Matrix metalloproteinase-9 | IPI00027509 | 0.0175 |
| Isoform 1 of brain acid soluble protein 1 | IPI00299024 | 0.0183 |
| Kallikrein-10 | IPI00480121 | 0.0190 |
| Fibrinogen beta chain | IPI00298497 | 0.0191 |
| Neutrophil collagenase | IPI00027846 | 0.0236 |
| Protein S100-A7 | IPI00219806 | 0.0236 |
| Keratin, type II cytoskeletal 1 | IPI00220327 | 0.0245 |
| Kallikrein-14 | IPI00000700 | 0.0264 |
| Cysteine-rich secretory protein 3 isoform 1 precursor | IPI00942117 | 0.0269 |
| Vimentin | IPI00418471 | 0.0311 |
| Cornifin-A | IPI00017987 | 0.0377 |
| Cathepsin G | IPI00028064 | 0.0380 |
| Isoform C1 of heterogeneous nuclear ribonucleoproteins C1/C2 | IPI00216592 | 0.0470 |
FIG 2.
The major canonical pathways associated with differentially abundant proteins observed in the follicular and luteal phase. (A) The top two branches identified by hierarchical clustering were further analyzed using IPA pathway software. The major canonical pathways associated with branch 1 (magenta; top: follicular overabundant proteins) and branch 2 (orange; bottom: luteal overabundant proteins) are shown in decreasing order by significance value (P < 0.05). Numbers adjacent to charts (right side) indicate numbers of proteins overexpressed out of the total number of factors involved in this pathway as characterized by the IPA knowledgebase. A right-tailed Fisher's exact test (Benjamini-Hochberg corrected; horizontal axis represents −log10 P value) was used to assess the association between each protein appearing in the data set and a known canonical pathway. Only the top pathways (and those with a −log10 P value of 2.0 and at least 2 proteins/pathway) are shown for vertical sizing. (B) Spider plot illustrating the expression patterns of the top three associated pathways. The average expression profile of all proteins identified in the mass spectrometry data set belonging to these three pathways is shown. Each concentric circle represents the average log2-fold change of the specific protein for each menstrual phase (follicular phase, magenta; luteal phase, orange). The black dotted circle represents mean expression across both phases. Overexpression of these three pathways is shown in the follicular phase compared to the luteal phase.
Measurement of specific innate proteins, including cytokines, chemokines, and other immune proteins.
A few innate immune proteins with known ability to orchestrate innate immunity pathways were chosen for an in-depth analysis to gain more insight into what drives the differential regulation of proteins. Selections were based on the availability of reagents to study macaque immunity and were not necessarily expected to be detectable in the proteomic catalogue identified above. The results of these analyses demonstrated significantly higher levels of a potent immunomodulatory chemokine, monocyte chemoattractant protein 1 (MCP-1; P < 0.05), in the follicular phase (Fig. 3). The median level of MCP-1 in the follicular phase was 54.25 pg/ml, compared to a level of 11.83 pg/ml in the luteal phase. Levels of other proteins, including TNF-α, IL-1β, IL-1Ra, IL-2, IL-6, IL-7, IL-8, MCP-1, MIP-1α, MIP-1β, RANTES, eotaxin, G-CSF, kallikreins, trappin-2, SLPI, thymic stromal lymphopoietin, surfactant protein D, α-defensins (HNP1 to HNP3), and β-defensins 2 and 3 were not significantly different by menstrual cycle phase (data not shown).
FIG 3.
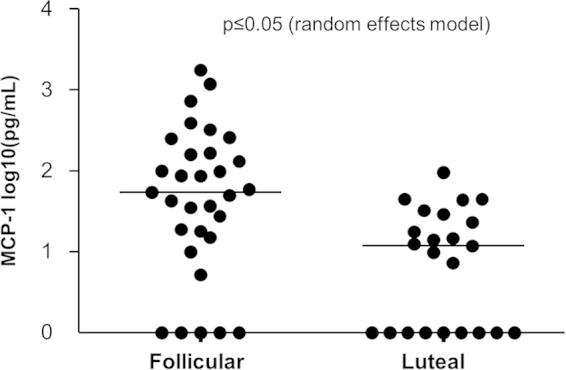
Comparison of MCP-1 levels in the two menstrual phases of pig-tailed macaques, determined by Luminex technology; we used a random-effects model to evaluate differential expression (P < 0.05). A left-censoring mechanism was used in statistical analyses to handle observations below the limit of detection, shown here as zero.
Analyses of the vaginal epithelium transcriptome.
To obtain more information on changes in cell infiltration and function, and how they might contribute to the reduced or enhanced infectivity observed during the two phases, we obtained paired vaginal biopsy specimens from 11 pig-tailed macaques during the two menstrual phases and assessed gene expression using microarrays. Of the 47,000 probesets represented on the GeneChip Rhesus Macaque Genome Array, 763 were differentially expressed between the two menstrual phases (Fig. 4). In the volcano plot in Fig. 4A, the red data points indicate genes differentially expressed (P < 0.05) with ≥1.5-fold differences between phases. Among these, 402 genes had higher expression in follicular phase, while 361 exhibited higher expression in the luteal phase (see Tables S2 and S3 in the supplemental material). Hierarchical clustering of the differentially expressed genes (Fig. 4B) demonstrated that the gene expression patterns were fairly consistent among individual animals except animals PPY1 and 96PO47, which demonstrated noticeable dissimilarities. Shown in Table S4 in the supplemental material are results from our LIMMA analyses that were performed to test the robustness of the data, and their comparison to results from paired t tests.
FIG 4.
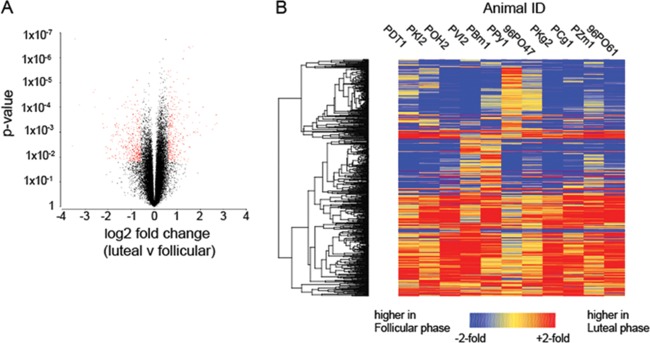
(A) Volcano plot depicting distribution of fold change and statistical significance in the microarray data set. Data points in red indicate genes defined to be differentially expressed based on a 1.5-fold difference between phases and a P value of <0.05. (B) Heat map of 763 DEGs. The color scale is shown at the bottom; red indicates genes with higher expression in the luteal phase relative to the paired follicular-phase sample, and blue indicates higher relative expression in the follicular phase.
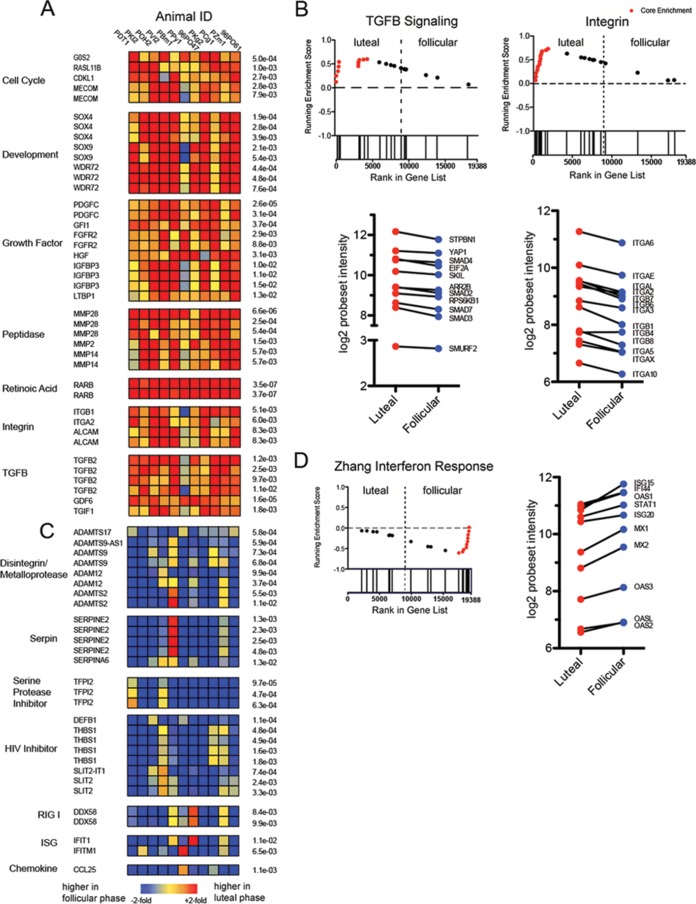
Several genes were consistently upregulated during the luteal phase (Fig. 5A; see also Table S2 in the supplemental material), including those regulating cellular growth and development, and many matrix metalloproteinases. Of significance, we also noted strong upregulation of retinoic acid receptor beta (RARB; P < 0.0001), a receptor for retinoic acid 15, and concordant upregulation of the integrin genes for ITGB1 and ITGA2. To increase the sensitivity of our analysis, we further queried our microarray data using GSEA and observed enrichment of several notable pathways in luteal samples, particularly of integrin and transforming growth factor (TGF) genes (Fig. 5B). Of note, in keeping with the enhanced expression of RARB, we observed that the transcripts for multiple integrin genes were higher in samples from the luteal phase (Fig. 5B), although they did not come up in our list of differentially expressed genes (DEGs) using conventional statistics. Additionally, we noted that there was upregulated expression of two probesets for TGF-β signaling; the immunosuppressive properties and elevated presence of TGF-β in HIV infections have been described before (16). In line with the upregulated pathways seen in the proteomic analyses (COPD and leukocyte signaling), our genomic results also showed an upregulation of genes for activated leukocyte cell adhesion molecule (ALCAM) (Fig. 5A), which is found on both dendritic cells and activated leukocytes, and SDC, syndecans, and cell surface heparan sulfate proteoglycans (data not shown).
FIG 5.
(A) Heat maps of genes showing luteal phase overexpression, organized into functional families as identified in the literature. The order of macaques is the same as that seen in Fig. 4B; red indicates genes with higher expression in the luteal phase relative to the paired follicular sample, and blue indicates higher relative expression in the follicular phase. (B) Gene set enrichment plots (GSEA) of two gene families enriched in the data set, TGF-β signaling and integrins, showing luteal-phase overexpression. The running enrichment score (y axis) is plotted by each gene's individual rank (x axis); bars below the x axis indicate individual gene ranks in the whole data set. The ranking is based on relative gene expression in the two menstrual phases. Genes in the leading edge (contributing the most to the enrichment score) are shown in red. The mean log2 probeset intensity of selected leading-edge genes is shown in the lower graphs. (C) Heat maps of genes showing follicular-phase overexpression, organized into functional families from the literature. (D) GSEA plot of interferon-stimulated gene set enriched in the data set, showing follicular phase overexpression. The mean log2 probeset intensity of selected leading-edge genes is shown on the right. The gene set chosen (Zhang interferon response) for GSEA was from the Broad Institute database.
Distinct from those detected in the luteal phase, we noted upregulated expression of several transcripts for growth factors and cell cycle regulatory genes in the follicular phase (see Table S3 in the supplemental material). We observed upregulation of several members of the ADAM disintegrin/metalloproteinase family and many serpin and nonserpin protease inhibitors (Fig. 5C). Additionally, several transcripts were upregulated with reported inhibitory effects on HIV replication: thrombospondin 1 (THBS1), a mucosal peptide found in saliva and cervical secretions, has been demonstrated to exhibit anti-HIV properties (23), and several of the upregulated ADAM family genes contained thrombospondin motifs; also found were transcripts for SLIT2, a membrane-associated glycoprotein; β-defensin 1 (DEFB1), which has antiviral properties; and chemokine ligand 25 (CCL25), a chemokine known to attract IgA-secreting cells (24). Also intriguing is the overexpression of the antiviral gene for DDX58 (DEAD [Asp-Glu-Ala-Asp] box polypeptide 58), also known as RIG-1, retinoic acid-inducible gene 1. We extracted numerous gene sets in this study associated with viral infection or interferon production from the Molecular Signatures Database (MSigDB; http://www.broadinstitute.org/gsea/msigdb/index.jsp) and used GSEA to test for enrichment in the two phases. We observed that several gene sets representing genes upregulated by viral infection, alpha interferon (IFN-α), and/or viral Toll-like receptor (TLR) ligands were enriched in follicular samples (Fig. 5D). Of note, we found MX2, which is described classically as ISG within the enriched gene sets representing interferon responses and has been demonstrated to potently inhibit HIV in vitro (25).
Overlap of genomic and proteomic data.
There was little overlap when we looked at a side-by-side comparison of immune-related pathways that were associated significantly with both the genomic and proteomic data sets (see Fig. S1 in the supplemental material). In the follicular phase, there was overlap only in the intrinsic prothrombin activation pathway; in the luteal phase, overlap was observed in seven pathways, including granulocyte adhesion and diapedesis, HIF-1α signaling, leukocyte extravasation signaling, IL-8 signaling, Rho family GTPase signaling, inhibition of matrix metalloproteases, and agranulocyte adhesion and diapedesis. The modest overlap was also observed when we performed GSEA (see Fig. S2 in the supplemental material) on the 50 proteins determined by proteomic analyses as differentially expressed; the notable proteins in the core enriched ones (see Table S5 in the supplemental material) included apolipoproteins, complement component 9, and serpins (follicular phase).
DISCUSSION
Female pig-tailed macaques are relevant animal models for examining HIV infection in women because of their similarities to the human reproductive tract and susceptibility to human STIs (26). In this study, we used uninfected macaques to document the many biological changes that may be associated with changing susceptibility to infection. We previously showed that susceptibility to SHIV infection varies by menstrual phases in pig-tailed macaques (6, 7), likely the consequence of differential expression of innate factors influenced by sex hormones, progesterone and estradiol. Also, since the vaginal epithelium is known to thin during the luteal phase (11), it is conceivable that changes in cellular expression affect the levels and nature of immune factors. Adopting a systems biology approach, we demonstrated what Wira and Fahey have suggested (4): a host of genes and proteins are differentially regulated in the menstrual phases, all of which could directly or indirectly influence susceptibility to infection. Such an approach allowed us to study various samples in the female genital tract, including secreted proteins in CVLs (composites from the cervix and vagina) and tissue samples from the vagina and underlying stroma which reflect changes in epithelial thickness.
Our study demonstrates higher expression of antiviral proteins and genes and pathways in the follicular phase than in the luteal phase. Some of the notable follicular-phase-overexpressed pathways observed in the proteomic analyses include acute-phase response signaling, complement system, and LXR/RXR activation pathways. Acute-phase response proteins help block spread of HIV-1 in the eclipse phase (27); this response is a critical component of early host defense, since acute-phase proteins are generated before the first signs of viremia, and some of them might trigger subsequent antiviral cytokine cascades. Kramer et al. also demonstrated that the plasma components during acute HIV-1 infection included complement factors, alluding to their early role in host defense (27). The LXR/RXR activation pathway might impede HIV-1 replication (28) by effecting cholesterol efflux from infected cells.
Some of the specific innate immunity-related proteins significantly overexpressed in the follicular phase include complement component 3 and analogues of complement factors B, H, and I, which are pivotal components for viral suppression (reviewed in reference 29). Other proteins significantly overexpressed in the follicular phase are vitronectin and apolipoproteins (A-I and D), which have HIV-1-inhibitory properties (23, 30). It is intriguing to note that apoliprotein A-I (Apo A-I) was one of the abundantly expressed proteins (31) in the cervicovaginal mucosa of highly exposed seronegative (HESN) sex workers. Overexpressed proteins in these individuals included many serpins (31), several of which were also overexpressed in the follicular phase in our study, as determined by both proteomic and genomic analyses, consistent with earlier findings (32). Serpins are reported to possess anti-inflammatory properties (33) and are important players in mucosal defense against HIV (32), especially in the eclipse phase (27). However, it is noteworthy that not all serine protease inhibitors we tested in this study (including SLPI and trappin-2) showed significantly different expression in the two menstrual phases. This is consistent with a recent ex vivo study (34) in which the authors showed no significant upregulation of β-defensins, SLPI, or proinflammatory cytokines in the follicular phase. However, they concluded that tissues from the luteal phase had a better capacity to support HIV-1 infection than those from the follicular phase, suggesting the presence of other innate factors in the follicular phase that might thwart infection. DDX58/RIG-I, Slit2, and CCL25 are other anti-HIV-1 genes (24, 35, 36, 37) of interest whose expression is enhanced in the follicular phase. We demonstrated enhanced DDX58/RIG-I expression during acute SIV infection in both rhesus macaques and sooty mangabeys (38); its elevated presence in the follicular phase may therefore suggest a heightened antiviral environment. Although the individual genes were not statistically significant on a gene-by-gene level, the GSEA demonstrated that collectively, there was significant enrichment of ISGs and antiviral genes in follicular samples.
GSEA is a powerful tool that considers cumulative statistics to quantify gene expression, in a way that is less sensitive to menstrual cycle synchronization issues that impact gene-to-gene statistics. The flexibility of the GSEA algorithm is ideal since cumulative statistics from multiple genes frequently outweigh the increase in variance exhibited by a minority of individual genes. It is important to note that the MSIGDB gene sets containing ISGs that we used to query our data sets in GSEA were derived experimentally from in vitro treatment of cells using IFN-α, viral TLRs, or viral infections rather than assembled from a priori knowledge. Therefore, the enhanced enrichment of these gene sets indicates that the follicular environment, even in the absence of overt viral stimuli, shares a transcriptional signature with cells induced to an antiviral state. One possible explanation for the elevated expression of immune factors in follicular phase is that it is simply due to the influx of leukocytes during the thickening of the vaginal wall. However, the lack of detection of highly expressed markers of immune cell populations (e.g., CD3, CD4, CD8, and CD14) suggests that this is unlikely.
Our proteomic studies found specific luteal-phase-overexpressed proteins that might be involved in enhancing HIV-1 infection: moesin (39), cathepsin G (40, 41), myeloperoxidase isoform (42), an isoform of heterogeneous nuclear ribonucleoproteins C1/C2 (43), vimentin (44), and kallikreins (45, 46). Interestingly, cathepsin G, a protease, is known to be inhibited by the follicular phase-overexpressed serpins (reviewed in reference 47), which alludes to a fine balance between proteases and antiproteases in the two menstrual phases, an aspect critical to the innate mucosal immune response. In 2012, it was reported for HESN (32) that serpins were overexpressed in the follicular phase of normally cycling women and suppressed in women on progestin-based contraception. This balance is key in tissue remodeling, thus making it pertinent that our study showed upregulation of antiproteases in the follicular phase, when the vaginal epithelium is at its thickest, and showed protease upregulation in the luteal phase, when the epithelium is much thinner. Additionally, our genomic analyses showed overexpression in the luteal phase of genes for HIV-1-enhancing proteins: syndecans (48, 49), TGF-β (50), and a retinoic acid receptor, RARB, which facilitates upregulation of important integrins, including α4β7, and other cell receptors involved in gut homing of T cells, thus enabling enhanced acquisition and dissemination of HIV-1 (51). By showing that MCP-1 was overexpressed in the follicular phase, our study supports the existence of dual roles for proteins like those reported for MIP-3α, present in the female genital tract (52). Past reports suggest a correlation between MCP-1 levels present in the cellular environment and the ability of MCP-1 to suppress or enhance HIV-1 (53, 54).
Using gene ontology, pathway associations, and GSEA, we saw little overlap in the genomic and proteomic data, especially looking at HIV-related protein pathways. This is not surprising considering the difference in the nature of samples used in the two techniques (soluble proteins versus tissue biopsy specimens). In fact, the purpose of this systems biology approach was to employ diverse techniques that would allow us to measure discrete innate immune factors.
Study limitations include the small number of animals tested for each assay; we designed our analyses so that they utilized leftover specimens from studies with other primary purposes. This limited our ability to optimally design sample collection schedules and techniques. More frequent sampling would have led to an improved detection of menstrual phases. At the time of sample collection, other methods that we currently use (sex swelling and daily menstrual blood monitoring) which would have given us a more accurate assignment of menstrual phases could not be implemented. Our evaluation was confined to the vaginal canal and did not include the uterus or other sites of potential HIV-1 acquisition. Also, this was a descriptive study that did not clarify the functional importance of protein pathways. The study lacked the capacity to identify the critical pathway regulators and specific interventions. As our coverage of proteins and genes is not exhaustive, it is possible that we overlooked other pathways of pathogen control.
A local environment in the female genital tract that exhibits a predominance of antimicrobial factors while minimizing target cells would be ideally suited to obstruct STIs. Our results indicate that in contrast to the luteal phase, the follicular phase of the menstrual cycle exhibits a plethora of innate pathways and factors that in various combinations could influence susceptibility to HIV-1 infection, the effects of which are likely cumulative. These results are meaningful considering that women on hormonal contraception might exhibit luteal-phase-like immune responses. Our study provides baseline data for future studies on hormonal contraception, to enable research on whether progestin-based contraceptives induce biochemical signatures similar to those found in the luteal phase of the cycle. Our results can be used to identify novel intervention targets for HIV prevention efforts, e.g., factors associated with natural vulnerability to SHIV infection modulated in the luteal phase of the cycle. The study thus provides critical data for future design of innovative strategies that seek to prevent viral acquisition, replication, or spread via the mucosal environment of the female genital tract.
Supplementary Material
ACKNOWLEDGMENTS
We thank James Mitchell, Shanon Ellis, and Frank Deyounks for animal technical assistance and our veterinarians and colleagues in the Animal Resources Branch (ARB) for help with macaque-related work.
This work was supported by the CDC and by an Interagency Agreement (Y1-AI-0681-02) between the CDC and NIH.
All authors report no association that might pose a conflict of interest.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00263-15.
REFERENCES
- 1.Powers KA, Poole C, Pettifor AE, Cohen MS. 2008. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis 8:553–563. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. 2014. Estimating per-act HIV transmission risk: a systematic review. AIDS 28:1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat Rev Immunol 8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wira CR, Fahey JV. 2008. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickey DK, Patel MV, Fahey JV, Wira CR. 2011. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol 88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kersh EN, Henning T, Vishwanathan SA, Morris M, Butler K, Adams DR, Guenthner P, Srinivasan P, Smith J, Radzio J, Garcia-Lerma JG, Dobard C, Heneine W, McNicholl J. 2014. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol 43:310–316. doi: 10.1111/jmp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. 2011. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr 57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 8.Sodora DL, Gettie A, Miller CJ, Marx PA. 1998. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses 14(Suppl 1):S119–S123. [PubMed] [Google Scholar]
- 9.Sheffield JS, Wendel GD Jr, McIntire DD, Norgard MV. 2009. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci 16:20–31. doi: 10.1177/1933719108325510. [DOI] [PubMed] [Google Scholar]
- 10.Hadzic SV, Wang X, Dufour J, Doyle L, Marx PA, Lackner AA, Paulsen DB, Veazey RS. 2014. Comparison of the vaginal environment of Macaca mulatta and Macaca nemestrina throughout the menstrual cycle. Am J Reprod Immunol 71:322–329. doi: 10.1111/aji.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. 2006. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol 190:829–835. doi: 10.1677/joe.1.06873. [DOI] [PubMed] [Google Scholar]
- 12.Burgener A, Tjernlund A, Kaldensjo T, Abou M, McCorrister S, Westmacott GR, Mogk K, Ambrose E, Broliden K, Ball B. 2013. A systems biology examination of the human female genital tract shows compartmentalization of immune factor expression. J Virol 87:5141–5150. doi: 10.1128/JVI.03347-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henning TR, Butler K, Hanson D, Sturdevant G, Ellis S, Sweeney EM, Mitchell J, Deyounks F, Phillips C, Farshy C, Fakile Y, Papp J, Evan Secor W, Caldwell H, Patton D, McNicholl JM, Kersh E. 2014. Increased susceptibility to vaginal simian/human immunodeficiency virus transmission in pig-tailed macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis. J Infect Dis 210:1239–1247. doi: 10.1093/infdis/jiu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins LT, Hanson D, Heneine W, Garcia-Lerma JG. 2014. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS 28:1431–1439. doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 15.Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. 1994. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol Behav 56:801–810. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 16.Burgener A, Mogk K, Westmacott G, Plummer F, Ball B, Broliden K, Hasselrot K. 2012. Salivary basic proline-rich proteins are elevated in HIV-exposed seronegative men who have sex with men. AIDS 26:1857–1867. doi: 10.1097/QAD.0b013e328357f79c. [DOI] [PubMed] [Google Scholar]
- 17.Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth GK, Michaud J, Scott HS. 2005. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- 19.Nadon R, Shoemaker J. 2002. Statistical issues with microarrays: processing and analysis. Trends Genet 18:265–271. doi: 10.1016/S0168-9525(02)02665-3. [DOI] [PubMed] [Google Scholar]
- 20.Baldi P, Long AD. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, Sandler NG, Roque A, Liebner J, Battegay M, Bernasconi E, Descombes P, Erkizia I, Fellay J, Hirschel B, Miro JM, Palou E, Hoffmann M, Massanella M, Blanco J, Woods M, Gunthard HF, de Bakker P, Douek DC, Silvestri G, Martinez-Picado J, Telenti A. 2011. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest 121:2391–2400. doi: 10.1172/JCI45235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. 2011. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol 65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman EP, Kuklin NA, Youngman KR, Lazarus NH, Kunkel EJ, Pan J, Greenberg HB, Butcher EC. 2002. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med 195:269–275. doi: 10.1084/jem.20010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, Schoggins JW, Rice CM, Yamashita M, Hatziioannou T, Bieniasz PD. 2013. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502:563–566. doi: 10.1038/nature12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veazey RS. 2008. Microbicide safety/efficacy studies in animals: macaques and small animal models. Curr Opin HIV AIDS 3:567–573. doi: 10.1097/COH.0b013e32830891bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer HB, Lavender KJ, Qin L, Stacey AR, Liu MK, di Gleria K, Simmons A, Gasper-Smith N, Haynes BF, McMichael AJ, Borrow P, Kessler BM. 2010. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog 6:e1000893. doi: 10.1371/journal.ppat.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow MP, Grant A, Mujawar Z, Dubrovsky L, Pushkarsky T, Kiselyeva Y, Jennelle L, Mukhamedova N, Remaley AT, Kashanchi F, Sviridov D, Bukrinsky M. 2010. Stimulation of the liver X receptor pathway inhibits HIV-1 replication via induction of ATP-binding cassette transporter A1. Mol Pharmacol 78:215–225. doi: 10.1124/mol.110.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunkelberger JR, Song WC. 2010. Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 30.Elia C, Cassol E, Sidenius N, Blasi F, Castagna A, Poli G, Alfano M. 2007. Inhibition of HIV replication by the plasminogen activator is dependent on vitronectin-mediated cell adhesion. J Leukoc Biol 82:1212–1220. doi: 10.1189/jlb.0407251. [DOI] [PubMed] [Google Scholar]
- 31.Burgener A, Rahman S, Ahmad R, Lajoie J, Ramdahin S, Mesa C, Brunet S, Wachihi C, Kimani J, Fowke K, Carr S, Plummer F, Ball TB. 2011. Comprehensive proteomic study identifies serpin and cystatin antiproteases as novel correlates of HIV-1 resistance in the cervicovaginal mucosa of female sex workers. J Proteome Res 10:5139–5149. doi: 10.1021/pr200596r. [DOI] [PubMed] [Google Scholar]
- 32.Rahman S, Rabbani R, Wachihi C, Kimani J, Plummer FA, Ball TB, Burgener A. 2013. Mucosal serpin A1 and A3 levels in HIV highly exposed sero-negative women are affected by the menstrual cycle and hormonal contraceptives but are independent of epidemiological confounders. Am J Reprod Immunol 69:64–72. doi: 10.1111/aji.12014. [DOI] [PubMed] [Google Scholar]
- 33.Aboud L, Ball TB, Tjernlund A, Burgener A. 2014. The role of serpin and cystatin antiproteases in mucosal innate immunity and their defense against HIV. Am J Reprod Immunol 71:12–23. doi: 10.1111/aji.12166. [DOI] [PubMed] [Google Scholar]
- 34.Saba E, Origoni M, Taccagni G, Ferrari D, Doglioni C, Nava A, Lisco A, Grivel JC, Margolis L, Poli G. 2013. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol 6:1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solis M, Nakhaei P, Jalalirad M, Lacoste J, Douville R, Arguello M, Zhao T, Laughrea M, Wainberg MA, Hiscott J. 2011. RIG-I-mediated antiviral signaling is inhibited in HIV-1 infection by a protease-mediated sequestration of RIG-I. J Virol 85:1224–1236. doi: 10.1128/JVI.01635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Yu J, Kuzontkoski PM, Zhu W, Li DY, Groopman JE. 2012. Slit2/Robo4 signaling modulates HIV-1 gp120-induced lymphatic hyperpermeability. PLoS Pathog 8:e1002461. doi: 10.1371/journal.ppat.1002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand AR, Zhao H, Nagaraja T, Robinson LA, Ganju RK. 2013. N-terminal Slit2 inhibits HIV-1 replication by regulating the actin cytoskeleton. Retrovirology 10:2. doi: 10.1186/1742-4690-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest 119:3556–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrero-Villar M, Cabrero JR, Gordon-Alonso M, Barroso-Gonzalez J, Alvarez-Losada S, Munoz-Fernandez MA, Sanchez-Madrid F, Valenzuela-Fernandez A. 2009. Moesin is required for HIV-1-induced CD4-CXCR4 interaction, F-actin redistribution, membrane fusion and viral infection in lymphocytes. J Cell Sci 122:103–113. doi: 10.1242/jcs.035873. [DOI] [PubMed] [Google Scholar]
- 40.Lim JK, Lu W, Hartley O, DeVico AL. 2006. N-terminal proteolytic processing by cathepsin G converts RANTES/CCL5 and related analogs into a truncated 4-68 variant. J Leukoc Biol 80:1395–1404. doi: 10.1189/jlb.0406290. [DOI] [PubMed] [Google Scholar]
- 41.Moriuchi H, Moriuchi M, Fauci AS. 2000. Cathepsin G, a neutrophil-derived serine protease, increases susceptibility of macrophages to acute human immunodeficiency virus type 1 infection. J Virol 74:6849–6855. doi: 10.1128/JVI.74.15.6849-6855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klebanoff SJ. 2005. Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 43.Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. 2010. Vimentin interacts with heterogeneous nuclear ribonucleoproteins and dengue nonstructural protein 1 and is important for viral replication and release. Mol Biosyst 6:795–806. doi: 10.1039/b923864f. [DOI] [PubMed] [Google Scholar]
- 44.Thomas EK, Connelly RJ, Pennathur S, Dubrovsky L, Haffar OK, Bukrinsky MI. 1996. Anti-idiotypic antibody to the V3 domain of gp120 binds to vimentin: a possible role of intermediate filaments in the early steps of HIV-1 infection cycle. Viral Immunol 9:73–87. doi: 10.1089/vim.1996.9.73. [DOI] [PubMed] [Google Scholar]
- 45.Shaw JL, Petraki C, Watson C, Bocking A, Diamandis EP. 2008. Role of tissue kallikrein-related peptidases in cervical mucus remodeling and host defense. Biol Chem 389:1513–1522. [DOI] [PubMed] [Google Scholar]
- 46.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. 2006. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J 20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 47.Pham CT. 2006. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 48.Bobardt MD, Saphire AC, Hung HC, Yu X, Van der Schueren B, Zhang Z, David G, Gallay PA. 2003. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18:27–39. doi: 10.1016/S1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 49.de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P. 2007. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc Natl Acad Sci U S A 104:19464–19469. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima RG, Van Weyenbergh J, Saraiva EM, Barral-Netto M, Galvao-Castro B, Bou-Habib DC. 2002. The replication of human immunodeficiency virus type 1 in macrophages is enhanced after phagocytosis of apoptotic cells. J Infect Dis 185:1561–1566. doi: 10.1086/340412. [DOI] [PubMed] [Google Scholar]
- 51.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, Wang X, Li J, Price AA, Little DM, Zaidi M, Lyles R, Villinger F. 2011. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol 186:1044–1059. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. 2010. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One 5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Modi WS, Goedert JJ, Strathdee S, Buchbinder S, Detels R, Donfield S, O'Brien SJ, Winkler C. 2003. MCP-1-MCP-3-Eotaxin gene cluster influences HIV-1 transmission. AIDS 17:2357–2365. doi: 10.1097/00002030-200311070-00011. [DOI] [PubMed] [Google Scholar]
- 54.Vicenzi E, Alfano M, Ghezzi S, Gatti A, Veglia F, Lazzarin A, Sozzani S, Mantovani A, Poli G. 2000. Divergent regulation of HIV-1 replication in PBMC of infected individuals by CC chemokines: suppression by RANTES, MIP-1alpha, and MCP-3, and enhancement by MCP-1. J Leukoc Biol 68:405–412. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.