ABSTRACT
Epidemiological studies have demonstrated that herpes simplex virus 2 (HSV-2) infection significantly increases the risk of HIV-1 acquisition, thereby contributing to the expanding HIV-1 epidemic. To investigate whether HSV-2 infection directly facilitates mucosal HIV-1 acquisition, we used our transgenic hCD4/R5/cT1 mouse model which circumvents major entry and transcription blocks preventing murine HIV-1 infection by targeting transgenic expression of human CD4, CCR5, and cyclin T1 genes to CD4+ T cells and myeloid-committed cells. Productive infection of mucosal leukocytes, predominantly CD4+ T cells, was detected in all hCD4/R5/cT1 mice intravaginally challenged with an HIV-1 infectious molecular clone, HIV-Du151.2env-NLuc, which expresses an env gene (C.Du151.2) cloned from an acute heterosexually infected woman and a NanoLuc luciferase reporter gene. Lower genital tract HIV-1 infection after HIV-Du151.2env-NLuc intravaginal challenge was increased ∼4-fold in hCD4/R5/cT1 mice coinfected with HSV-2. Furthermore, HIV-1 dissemination to draining lymph nodes was detected only in HSV-2-coinfected mice. HSV-2 infection stimulated local infiltration and activation of CD4+ T cells and dendritic cells, likely contributing to the enhanced HIV-1 infection and dissemination in HSV-2-coinfected mice. We then used this model to demonstrate that a novel gel containing tenofovir disoproxil fumarate (TDF), the more potent prodrug of tenofovir (TFV), but not the TFV microbicide gel utilized in the recent CAPRISA 004, VOICE (Vaginal and Oral Interventions to Control the Epidemic), and FACTS 001 clinical trials, was effective as preexposure prophylaxis (PrEP) to completely prevent vaginal HIV-1 infection in almost half of HSV-2-coinfected mice. These results also support utilization of hCD4/R5/cT1 mice as a highly reproducible immunocompetent preclinical model to evaluate HIV-1 acquisition across the female genital tract.
IMPORTANCE Multiple epidemiological studies have reported that genital herpes simplex virus 2 (HSV-2) infection increases the risk of HIV-1 sexual acquisition by severalfold. Understanding the underlying mechanisms by which HSV-2 facilitates HIV-1 infection and optimizing the efficacy of therapies to inhibit HIV-1 infection during HSV-2 coinfection should contribute to reducing HIV-1 transmission. Using our novel transgenic hCD4/R5/cT1 mouse model infectible with HIV-1, we demonstrated that HSV-2 infection enhances vaginal transmission and dissemination of HIV-1 infection while stimulating recruitment and activation of CD4+ T cells and dendritic cells in the lower genital tract. HIV acquisition by hCD4/R5/cT1 mice vaginally coinfected with HSV-2 could be completely prevented in almost half the mice by preexposure prophylaxis (PrEP) with a novel gel containing tenofovir disoproxil fumarate (TDF), the tenofovir prodrug, but not with the tenofovir microbicide gel utilized in CAPRISA-004, VOICE, and FACTS-001 clinical trials. The hCD4/R5/cT1 mice represent a new preclinical mouse model to evaluate vaginal HIV-1 acquisition.
INTRODUCTION
Infection with herpes simplex virus 2 (HSV-2) is a major cause of sexually transmitted diseases in the world, infecting an estimated 500 million individuals worldwide with a prevalence of up to 70% in sub-Saharan Africa, the epicenter for the human immunodeficiency virus type 1 (HIV-1) pandemic (1–4). Multiple epidemiological studies have indicated that infection with HSV-2 increases the likelihood of acquiring HIV-1 infection by severalfold and thereby contributes to the expansion of the HIV-1 epidemic (5–9). Investigating the molecular and cellular mechanisms underlying the synergistic relationships between HIV-1 and HSV-2 infection has been limited by the absence of an in vivo model to study coinfection with these two viruses. Primary HSV-2 infection creates genital ulcerations that disrupt the epithelial surface and compromise the mucosal barrier; this facilitates HIV-1 passage into the submucosa where it encounters mucosal CD4+ T cells, macrophages, and dendritic cells, infects initial target cells, and forms local foci of infected cells which disseminate throughout the lymphoid tissue (10–13). In addition, HSV-2 infection alters the mucosal microenvironment by increasing the local production of proinflammatory cytokines and chemokines that recruit and activate CD4+ T lymphocytes, macrophages, and dendritic cells, thereby enhancing the probability that HIV-1 will encounter and infect target cells (14–16).
While various humanized mouse models have been used to evaluate mucosal HIV-1 transmission and the efficacy of different preventive therapeutics, including topical or systemic preexposure prophylaxis (PrEP), the exposed mucosal tissues of these chimeric models are composed of mouse epithelial cells while the submucosa consists of a mixture of mouse stromal cells, macrophages, and dendritic cells and various levels of human leukocytes (17). The species differences between mouse and human cells in cytokine and chemokine responsiveness and the binding of lymphocyte homing receptors to their ligands will likely affect the trafficking and recruitment of human leukocytes into the mouse female reproductive tract in response to inflammation induced by infection. In addition, humanized mouse models are constructed by transplanting human hematopoietic stem cells with and without human thymic tissue into highly immunodeficient mice, which are highly susceptible to HSV-2 infection, precluding their use for HIV-1 and HSV-2 coinfection studies. Some of the inherent limitations of humanized mouse models would be circumvented by an immunocompetent mouse model rendered susceptible to HIV-1 infection by the transgenic expression of human genes that overcome murine blocks to HIV-1 replication and entry. We previously constructed hCD4/R5/cT1 mice which are transgenic for CD4 promoter/enhancer-mediated expression of human CD4, CCR5, and cyclin T1 transgenes by mouse CD4+ T cells, monocytes, and dendritic cells (18). Expression of these human transgenes enables CD4-expressing mouse leukocytes to circumvent the HIV-1 entry block in mouse cells due to the inability of mouse CD4 and CCR5 to engage gp120 (19) and to overcome the HIV-1 transcription block, secondary to the inability of Tat to bind mouse cyclin T1 and recruit the positive transcription elongation factor b (P-TEFb) complex to the HIV-1 transactivation response (TAR) RNA target element required to efficiently initiate proviral transcription (20–22). We previously reported that circumventing these inherent murine replication blocks by expression of human CD4, CCR5, and cyclin T1 transgenes enabled the hCD4/R5/cT1 mice to support productive in vivo HIV-1 infection after inoculation with replication-competent HIV-1 expressing a Renilla luciferase (LucR) reporter gene (18).
A major advance in the development of topical PrEP to prevent sexual transmission of HIV-1 infection was the randomized, placebo-controlled CAPRISA 004 trial, which demonstrated that the application of 1% tenofovir (TFV) vaginal gel before and after sexual intercourse reduced HIV acquisition by an estimated 39% in all study participants and by 54% in women highly adherent to the gel application protocol (3). However, a subsequent study of high-risk, predominantly young, unmarried women in sub-Saharan Africa, the Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial, failed to show a protective effect of daily administration of vaginal 1% TFV gel, which, based on analysis of plasma drug levels, was most likely due to low adherence by the study subjects (23). Similarly, the FACTS 001 trial also failed to show any protection, which has also been attributed to poor adherence (24). Because HSV-2 infection facilitates HIV-1 transmission and increases the amount of drug needed to protect against HIV-1 acquisition (13), the efficacy of PrEP for preventing HIV infection could be reduced by the presence of active HSV-2 infection.
The active metabolite of TFV, tenofovir diphosphate, potently inhibits HIV reverse transcriptase by DNA chain termination. TFV was the first antiretroviral drug to be formulated into a microbicide gel due to its excellent safety profile (25). While TFV displays poor oral bioavailability, its prodrug, tenofovir disoproxil fumarate, (TDF; Viread), is efficiently absorbed after oral administration and converted into TFV by serum and tissue esterases (26). Although TFV gel was shown to be effective as a PrEP agent in the CAPRISA 004 trial, TDF gel should prevent HIV-1 acquisition more effectively than TFV gel because it displays ∼100-fold greater in vitro anti-HIV-1 activity than TFV (27), likely a consequence of its more rapid cellular penetration than that of TFV, which enables it to generate higher intracellular levels of the active metabolite, tenofovir diphosphate (4, 28). The long intracellular half-life (12 to 15 h in activated lymphocytes and 33 to 50 h in resting lymphocytes) of tenofovir diphosphate may mitigate intermittent adherence (27, 28). TDF delivered by an intravaginal ring protected 100% of macaques repeatedly exposed to simian immunodeficiency virus (SIV) by 16 weekly vaginal challenges (29), and vaginally administered 0.3% TDF gel was more effective in protecting mice from genital herpes than 1% TFV gel (4). Importantly, ex vivo studies demonstrated that TDF did not adversely affect cell growth or disrupt epithelial cell tight junctions, predictive of TDF not compromising the barrier formed by the vaginal and ectocervical stratified squamous epithelium, which would have the unintended consequence of facilitating HIV-1 infection (28). Therefore, we utilized our novel transgenic mouse mucosal HIV-1 infection model to compare the efficacy of two nucleotide analogue reverse transcriptase inhibitors used as topical PrEP, TFV and its prodrug, TDF (29), to inhibit acquisition of HIV-1 during acute HSV-2 infection (4). In the current study, we demonstrate that the hCD4/R5/cT1 mice are reproducibly infected after mucosal challenge with HIV-1 and that the magnitude of mucosal HIV-1 infection and of dissemination into the lymph nodes is markedly increased when the mice are challenged during acute HSV-2 infection. We also demonstrated that the acquisition of vaginal HIV-1 infection during acute HSV-2 infection can be prevented significantly more effectively by administration of the 0.3% TDF gel than by treatment with 1% TFV gel.
MATERIALS AND METHODS
Generation of HIV-1 infectious molecular clones.
We previously reported on LucR-expressing, replication-competent HIV-1 reporter virus technology (30), which productively infected the hCD4/R5/cT1 mouse model (18) and has well-established utility in other contexts, e.g., to evaluate the HIV-1-inhibitory capacity of antibodies, NK cells, and CD8 T cells (31–38). To further improve upon the sensitivity of HIV-1 reporter virus in vivo detection, we developed a novel reporter virus construct encoding NanoLuciferase (NLuc) (39) in the place of LucR. NLuc is a brighter and more stable bioluminescent reporter with a longer half-life than Renilla luciferase (39), which is particularly important for quantifying low-level HIV replication during the initial stages of infection in the lower genital tract. Furthermore, to provide biological relevance for in vivo studies on acute HIV-1 infection and the efficacy of PrEP to inhibit HIV infection resulting from vaginal exposure, we chose an R5-tropic env gene, C.Du151.2 (GenBank accession number DQ411851), obtained from an acute infection sample of a woman infected in South Africa by heterosexual transmission (40). The new plasmid encoding the replication-competent infectious molecular clone (IMC) was named pNL-NLuc.T2A-C.Du151.2.ecto. Virus derived from it (NL-NLuc.T2A-C.DU151.2.ecto) is referred to herein as HIV-Du151.2env-NLuc. Briefly, pNL-NLuc.T2A-C.Du151.2.ecto was generated by cloning the Env glycoprotein ectodomain coding region of a strain expressing env C.Du151.2 into the pNL-LucR.T2A reporter backbone, essentially as described previously (32, 38), resulting in expression of full-length Env in cis. The LucR coding region was then replaced by that of the NLuc gene (39) present in the vector pNL1.1[Nluc] from Promega (GenBank accession number JQ437370.1), specifically nucleotides (nt) 100 to 615, which span the NLuc start codon to the last codon before the stop codon. of the genetic elements present in NL-LucR.T2A (30), encompassing env and the reporter gene linked in frame to nef by a T2A peptide coding sequence, is preserved, resulting schematically in the following: [env]TAAGctagccaccATG-[Nluc]-GCG---[T2A]tctagaATG[nef], where the stop codon of env is indicated, followed by the naturally occurring G, followed by an additional 5 plus 4 nucleotides (lowercase) to form an NheI site (italic) which overlaps with a Kozak sequence (underlined), followed by the NLuc start codon. The NLuc stop codon (---) is removed to allow for read-through into the T2A peptide sequence, which leads into an XbaI site (lowercase, italic, encoding two additional amino acids) and the nef open reading frame (ORF).
Virus stocks were generated by transient transfection of 293T cells with the plasmid pNL-NLuc.T2A-Du151.2.ecto, and the infectious titer of the virus generated, HIV-Du151.2env-NLuc, was determined by limiting dilution infection of TZM-bl cells (expressed as 1 × 107 to 2 × 107 infectious units [IU]) as described previously (30). We confirmed replication competence in CD4+ T cell lines and in primary CD4+ T cells and confirmed highly sensitive detection of de novo proviral gene expression via the NLuc activity readout (data not shown). Of note, we found that 293T cell-derived virus stocks contained significant levels of NLuc activity, even of the nonsecreted NLuc form utilized here; however, in applications like the one presented here, in which the infection is in vivo and small volumes are applied or wash steps or other diluting circumstances are used, inoculum-derived NLuc activity was not detected when we inoculated wild-type mice (Fig. 1B). Thus, residual NLuc in the inoculum did not pose a barrier to sensitive detection of de novo NLuc gene expression after in vivo inoculation in tissue-derived HIV-infected cells.
FIG 1.
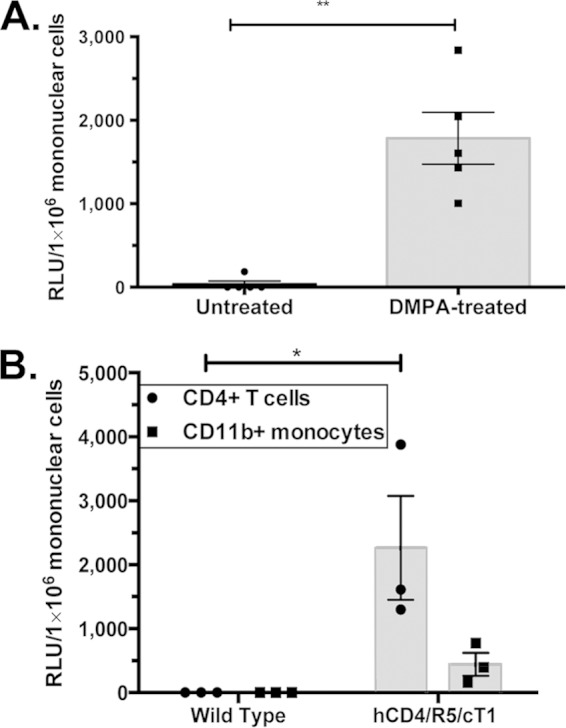
DMPA-treated hCD4/R5/cT1 mice become infected with an infectious molecular HIV-1 clone expressing an NLuc reporter gene. (A) Mice (n = 5 mice/group) were infected by atraumatically introducing HIV-Du151.2env-NLuc (4.5 × 105 IU) into the vagina 5 days after one group of mice were subcutaneously injected with DMPA (2.5 mg). Six days after infection, lower genital tract mononuclear cells were isolated, and the NLuc activity in the cellular lysate was quantified. (B) Wild-type littermates (n = 3) and hCD4/R5/cT1 mice (n = 3) were infected by atraumatic intravaginal introduction of HIV-Du151.2env-NLuc (4.5 × 105 IU) 5 days after subcutaneous injection with DMPA (2.5 mg). Six days after inoculation, CD4+ T cells and CD11b+ monocytes were isolated from the lower genital tract by immunomagnetic sorting. The cells were lysed, and their NLuc activity was quantified. NLuc activity was then determined on the cellular lysates of isolated cells, and values are reported as relative light units (RLU) corresponding to a normalized analysis of 106 mononuclear cells. The average NLuc activity in the cellular lysates from each group ± standard error of the mean and individual data points are shown. Asterisks indicate significance (*,P < 0.05; **, P < 0.01).
Vaginal gels.
TFV (1%) gel and the universal placebo gel, hydroxyethylcellulose (HEC) were provided by CONRAD (41). TDF (Gilead, Foster City, CA) was mixed (0.3%, wt/wt) in HEC (H2O [96.3%, wt/wt], Natrosol 250 HX Pharm HEC [2.7%, wt/wt; Ashland Covington, KY], NaCl [0.85%, wt/wt], and sorbic acid NF [0.1%, wt/wt; Spectrum Chemicals, New Brunswick, NJ]) and stored at −80°C as described previously (4).
The hCD4/R5/cT1 mouse model.
hCD4/R5/cT1 mice were constructed as described previously (18). Briefly, founder mice were generated by injecting fertilized mouse eggs of C57BL/6 mice with two CD4 promoter/enhancer-regulated vectors. One vector expressed the human CD4 and human CCR5 genes linked as a single transcript by the P2A self-cleaving picornavirus-like 2A peptide, which generates equimolar amounts of the CD4 and CCR5 proteins, and the other vector expressed the human cyclin T1 gene. Founder mice with tandem integration of the two vectors were identified by PCR analysis from tail DNA for human CD4, CCR5, and cyclin T1 and crossed with C57BL/6 mice to identify progeny that expressed all three transgenes that were transmitted as a tightly linked single allele.
HIV-1 infection of hCD4/R5/cT1 mice and gel treatment.
hCD4/R5/cT1 mice (8 to 10 weeks old) were either untreated or pretreated with a subcutaneous injection of 2.5 mg of depot medroxyprogesterone acetate (DMPA) (Sicor Pharmaceuticals, Irvine, CA). Five days later, the mice were intravaginally inoculated with 30 μl of HIV-Du151.2env-NLuc (∼4.5 × 105 IU) while in an inverted position, which was maintained for ∼4 min to prevent immediate discharge of virus as described previously (42). Six days after inoculation, vaginal mucosal mononuclear cells were isolated from the lower genital tract (vagina and cervix) as described previously (43) and resuspended in lysis buffer (30 μg/ml DNase I, 0.425 mg/ml collagenase D [Roche], and 0.5 mg/ml Dispase II [Roche]) as described previously (43, 44). HIV-Du151.2env-NLuc infection was quantified by measuring the activity of NLuc in the cellular lysate using a Promega NanoLuc Luciferase Assay (Promega, Madison, WI) according to the manufacturer's instructions. To determine whether CD4+ T cells or macrophages were the predominant population of mucosal cells infected after intravaginal inoculation, mouse CD4+ T cells and CD11b+ monocytes were isolated from the total mononuclear population of the lower genital tract by immunomagnetic sorting. Briefly, lower genital tract mononuclear cells were incubated with anti-mouse CD11b microbeads (Miltenyi Biotec, Cambridge, MA) and passed through a positive-selection AutoMACS separation column using the AutoMACS automated bench-top magnetic cell sorter (Miltenyi Biotec) to obtain purified monocytes. The monocyte-depleted cells were then incubated with anti-mouse CD4 microbeads (Miltenyi Biotec) and passed through the AutoMACS automated bench-top magnetic cell sorter as described above to obtain purified CD4+ T cells. The purity of the immuno-sorted cells was greater than 90%. HIV infection was then quantified by measuring the NLuc activity from the cellular lysate of the purified CD4+ T cells and CD11b+ macrophages.
To test the capacity of PrEP to prevent HIV-1 infection in this model, DMPA-treated hCD4/R5/cT1 mice were treated with daily intravaginal doses of 1% TFV, 0.3% TDF, or HEC gel (30 μl) or with daily intraperitoneal injections of emtricitabine (FTC)/TDF (Truvada; 3.5 mg of FTC and 5.2 mg of TDF) (45), with the first dose administered 1 day prior to intravaginal inoculation with 30 μl of HIV-Du151.2env-NLuc (4.5 × 105 IU). Genital tract tissue was harvested 6 days after inoculation. Alternatively, the mice were treated with a single intravaginal dose of gel (30 μl) at 12 h prior to HIV inoculation and a second dose of gel 12 h after HIV inoculation to simulate the dosing, referred to as BAT24 dosing, used in the CAPRISA 004 trial (3).
Coinfection with HIV and HSV-2.
hCD4/R5/cT1 mice (8 to 10 weeks old) were pretreated with 2.5 mg of DMPA 5 days before intravaginal inoculation of HSV-2 (clinical isolate 4674; 105 PFU), UV-inactivated HSV-2, or phosphate-buffered saline (PBS), as described previously (12). The UV-inactivated HSV-2 virus was produced by exposing the HSV-2 inoculum to the UV light source for 7 min at a distance of 10 cm, as previously described (16). Two days later, a subset of mice from each group was inoculated intravaginally with 30 μl of HIV-Du151.2env-NLuc (4.5 × 105 IU). Six days after HIV-Du151.2env-NLuc inoculation, vaginal mucosal mononuclear cells were isolated from the lower genital tract and draining lymph nodes for quantification of HIV-Du151.2env-NLuc infection by measuring the NLuc activity in the cellular lysate. To measure the phenotypic identity of isolated mononuclear cells in the lower genital tract of hCD4/R5/cT1 mice, mice were DMPA treated and then either mock inoculated or HSV-2 inoculated. Two days later some of the mice from each group were HIV-Du151.2env-NLuc challenged or mock challenged, and mononuclear cells were then isolated from the lower genital tract. To examine the efficacy of PrEP to prevent mucosal HIV-1 infection during acute HSV-2 infection, a group of DMPA-treated hCD4/R5/cT1 mice were inoculated with HSV-2 (105 PFU/mouse). Two days later the mice were treated at 12 h before and 12 h after intravaginal challenge with HIV-Du151.2env-NLuc with 1% TFV, 0.3% TDF, or HEC gel. Six days later, HIV infection was quantified by measuring NLuc activity in mononuclear cells isolated from the lower genital tract as described above and from the draining inguinal/iliac lymph nodes after tissue disassociation through a 70-μm-pore-size cell strainer (BD Biosciences, Bedford, MA). Mice were also evaluated on day 6 post-HIV infection and scored for HSV-mediated epithelial disease (genital ulcers, edema, and erythema) on a scale that ranged from 0 (no disease) to 4 (severe ulceration) as described previously (46, 47).
Flow cytometry analysis.
Mononuclear cells isolated from the lower genital tract tissue, as described above, were incubated with Fc-receptor blocking buffer for 15 min (Miltenyi Biotec, Cambridge, MA) and then stained (5 × 105 cells/sample) with a combination of fluorescently labeled monoclonal antibodies to mouse CD3, CD45, CD4, CD25, and CD69 or to mouse CD45, CD11c, CD14, and major histocompatibility complex class II (MHC-II) (e-Bioscience, San Diego, CA). Expression of these surface molecules was then quantified using an LSRII instrument (BD Biosciences, San Jose, CA) and FlowJo software (Treestar, Ashland, OR). The total number of lymphocyte or myeloid cell phenotypic subpopulations was calculated by multiplying the percentage of cells expressing the phenotypic markers by the total number of viable cells isolated from the lower genital tract.
Statistical analysis of data.
GraphPad Prism statistical software was used for statistical analysis using one-way analysis of variance (ANOVA) with Tukey's test for multiple comparisons or an independent two-tailed Student's t test. Differences were considered statistically different for P values of <0.05.
Study approval.
All the studies were performed under protocols approved by the Institute for Animal Studies at the Albert Einstein College of Medicine in compliance with the human and animal experimentation guidelines of the United States Department of Health and Human Services.
RESULTS
Infection of DMPA-treated hCD4/R5/cT1 mice with HIV-1 after intravaginal inoculation.
We investigated whether DMPA treatment, which thins the vaginal epithelium and facilitates infection of vaginal infection of macaques with SIV and simian-human immunodeficiency virus (SHIV) (48, 49), was required for the development of local HIV-1 infection in the lower genital tract of the hCD4/R5/cT1 mice after intravaginal challenge. The hCD4/R5/cT1 mice express human CD4, CCR5, and cyclin T1 under the control of the CD4 promoter/enhancer, thereby targeting expression of these transgenes and susceptibility to HIV-1 infection to CD4+ T cells and monocyte/macrophages (18). To further increase the sensitivity of HIV-1 detection, we developed a novel NanoLuciferase (NLuc)-expressing replication-competent IMC, NL-NLuc.T2A-C.Du151.2.ecto, expressing an env gene, derived from an HIV strain, C.Du151.2, obtained from a woman soon after acute infection acquired by male-to-female transmission (40). The virus generated by this IMC is referred to as HIV-Du151.2env-NLuc and was chosen for its biological relevance for vaginal transmission studies. DMPA-treated hCD4/R5/cT1 mice developed productive HIV-1 infection after intravaginal inoculation, as indicated by the detection of NLuc activity in the mucosal mononuclear cells of the lower genital tract on day 6 postinfection, whereas infection was not reproducibly detected in the mice not pretreated with DMPA (Fig. 1A). These data confirm results that we previously reported that hCD4/R5/cT1 transgenic mice pretreated with DMPA developed localized infection of cervical-vaginal mucosal leukocytes after intravaginal inoculation with an infectious molecular clone of HIV-1 expressing LucR (18). We next determined which subset(s) of mononuclear cells was most productively infected with HIV-1 by immunomagnetic sorting of mouse CD4+ and CD11b+ cells from the lower genital tract tissues of DMPA-treated hCD4/R5/cT1 mice six days after intravaginal inoculation and measuring NLuc activity in normalized numbers of purified cells (Fig. 1B). The NLuc activity in the purified CD4+ T cells was almost 5-fold higher than that in purified CD11b+ cells, suggesting that at 6 days postchallenge, productive HIV-1 replication predominantly occurred in CD4+ T cells, which is similar to results previously described in vaginally infected macaques (50).
HSV-2 infection enhances HIV-1 infection in the female reproductive tract of hCD4/R5/cT1 mice and increases dissemination of HIV-1-infected cells into draining lymph nodes.
Several epidemiological studies have indicated a strong association between HSV-2 infection and an increased risk for the acquisition of HIV-1 infection (5–9). To directly examine the effect of HSV-2 infection on HIV-1 acquisition, DMPA-treated hCD4/R5/cT1 mice were either mock infected or intravaginally challenged with UV-inactivated HSV-2 or fully infectious HSV-2 (105 PFU), and then 2 days later, subsets of hCD4/R5/cT1 mice were coinfected with HIV-Du151.2env-NLuc (Fig. 2A). The level of HIV-1 infection in mononuclear cells isolated from the lower genital tract 6 days after HIV challenge in the hCD4/R5/cT1 mice first infected with live HSV-2 was ∼4-fold higher (P < 0.05) than that in the mock-infected hCD4/R5/cT1 mice or hCD4/R5/cT1 mice first inoculated with UV-inactivated HSV-2 (Fig. 2B). The enhancing effect of HSV-2 on the level of HIV-1 infection was further indicated by the dissemination of HIV-1 infection, within 6 days after HIV-Du151.2env-NLuc inoculation, to the draining lymph nodes of 9 of 12 hCD4/R5/cT1 mice infected with HSV-2; in contrast, HIV-1 infection was not detectible in the draining lymph nodes of mock-infected hCD4/R5/cT1 mice or the hCD4/R5/cT1 mice that were initially inoculated with UV-inactivated HSV-2 (Fig. 2C).
FIG 2.
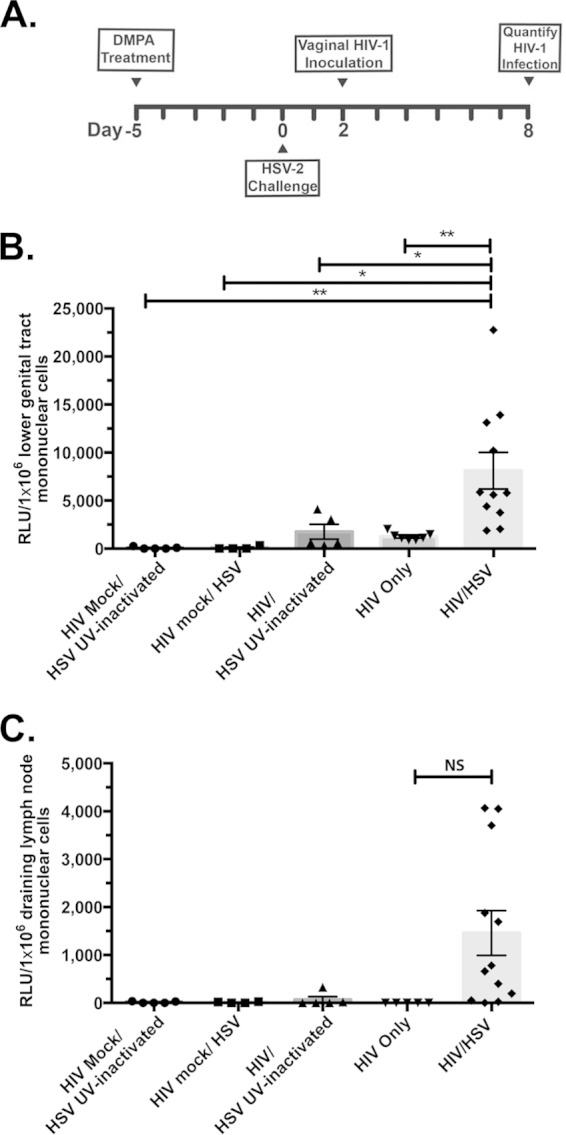
Acute HSV-2 infection increases mucosal HIV-1 infection. hCD4/R5/cT1 mice were intravaginally mock challenged or challenged with infectious HSV-2 or UV-inactivated HSV-2 5 days after subcutaneous injection with DMPA (2.5 mg). Two days after challenge, some mice from each group were then intravaginally mock challenged or challenged with HIV-Du151.2env-NLuc (4.5 × 105 IU). (A) The experimental design. (B) Six days after HIV infection, lower genital tract mononuclear cells were isolated, and NLuc activity in the cellular lysate was quantified. (C) Six days after HIV-1 infection, mononuclear cells were isolated from the draining lymph nodes, and the NLuc activity in the cellular lysate was quantified. The average NLuc activity from two pooled experiments in the cellular lysates from each group ± standard error of the mean and individual data points are shown; values are reported as RLU corresponding to a normalized analysis of 106 mononuclear cells. Asterisks indicate significance using one-way ANOVA with Tukey's test for multiple comparisons (NS, not significant; *, P < 0.05; **, P < 0.01).
HSV-2 infection recruits potential HIV-1 target cells to genital mucosal tissues.
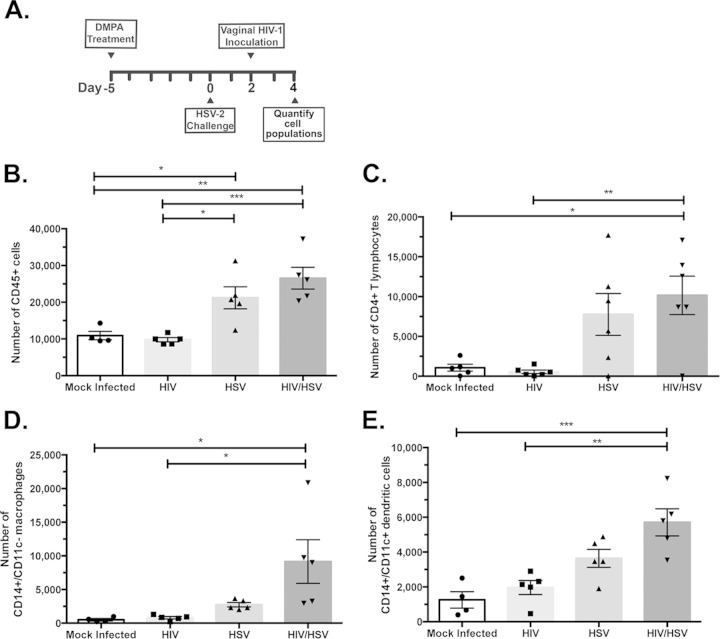
One possible mechanism that may have contributed to the increased HIV-1 infection in HSV-2-infected hCD4/R5/cT1 mice after intravaginal inoculation is HSV-2-mediated secretion of chemokines and cytokines, which recruited CD4+ T cells, dendritic cells, and/or macrophages to the submucosal site of HSV-2 infection (11, 12). An influx of HIV-1-susceptible cells into the submucosal tissues would provide more infectible targets for HIV-1, thereby facilitating both the establishment and dissemination of HIV-1 infection. To investigate this possibility, we intravaginally inoculated DMPA-treated hCD4/R5/cT1 mice with HSV-2 (105 PFU); 2 days later we challenged a subset of these mice with HIV-Du151.2env-NLuc and after another 2 days harvested the lower genital tract for comparative phenotypic analysis (Fig. 3A). HSV-2 infection significantly increased by over 2-fold the number of CD45+ cells in the lower genital tract compared to the numbers in mock-infected and HIV-1-inoculated hCD4/R5/cT1 mice (Fig. 3B), predominantly by markedly increasing the population of CD4+ T cells (Fig. 3C). In addition, HSV-2 infection alone and combined with HIV-Du151.2env-NLuc challenge also significantly increased the number of CD14+ CD11c− monocytes (Fig. 3D) and CD14+/CD11c+ dendritic cells in the lower genital tract compared to the numbers in uninfected hCD4/R5/cT1 mice (Fig. 3E).
FIG 3.
HSV-2 infection induces the influx of CD4+ T cells, monocytes, and dendritic cells into the lower genital tissues of hCD4/R5/cT1 mice. hCD4/R5/cT1 mice were DMPA treated and 5 days later were intravaginally mock challenged or HSV-2 challenged. Two days later, some mice from each group were intravaginally mock inoculated or inoculated with HIV-Du151.2env-NLuc (4.5 × 105 IU). Two days later, lower genital tract mononuclear cells were counted and analyzed by flow cytometry, and the total number of the indicated subpopulation was determined by multiplying the total cell number by the percentage of cells expressing the indicated phenotypic markers. (A) The experimental design. Total numbers of CD45+ cells (B), CD4+ T cells (C), CD14+/CD11c− macrophages (D), and CD14+/CD11c+ dendritic cells (E) are shown. The average number of cells from each group ± standard error of the mean and individual data points are shown. The data represent one of two experiments with 4 to 5 mice per group. Asterisks indicate significance using one-way ANOVA with Tukey's test for multiple comparisons (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Another mechanism by which HSV-2 infection augments HIV-1 infection in the lower genital tract may be by activating CD4+ T cells, thus rendering them more susceptible to productive HIV-1 infection than resting CD4+ T cells. Evaluation of the activation status of mouse CD4+ T cells present in the lower genital tract demonstrated a significant increase in the number of CD4+ CD69+ T cells in HIV-1/HSV-2-coinfected hCD4/R5/cT1 mice compared to that in uninfected mice or mice infected only with HIV-Du151.2env-NLuc (Fig. 4A). HIV-1/HSV-2 coinfection may also activate immature dendritic cells in mucosal tissues, as indicated by increased expression of MHC-II molecules (51), thereby enabling these activated dendritic cells to disseminate HIV-1 infection by transporting HIV-1 into the lymph nodes (52). Therefore, we investigated whether HSV-2/HIV-1 coinfection increased the number of dendritic cells in the lower genital tract that express MHC-II molecules. Two days after DMPA-treated hCD4/R5/cT1 mice were intravaginally mock challenged or HSV-2 challenged, mice from each group were intravaginally challenged with HIV-Du151.2env-NLuc. Two days after HIV-1 inoculation, the lower genital tissues were harvested, and the dendritic cells were analyzed by flow cytometry. The population of activated dendritic cells expressing MHC class II molecules (Fig. 4B) was significantly increased in hCD4/R5/cT1 mice infected with HSV-2 and HIV-1 compared to that in uninfected mice or mice infected only with HIV-Du151.2env-NLuc. This increased population of activated dendritic cells may have facilitated transport of HIV-1 from the lower genital tract to the draining lymph nodes and contributed to the lymph node dissemination observed only in the HSV-2/HIV-1-coinfected hCD4/R5/cT1 mice.
FIG 4.
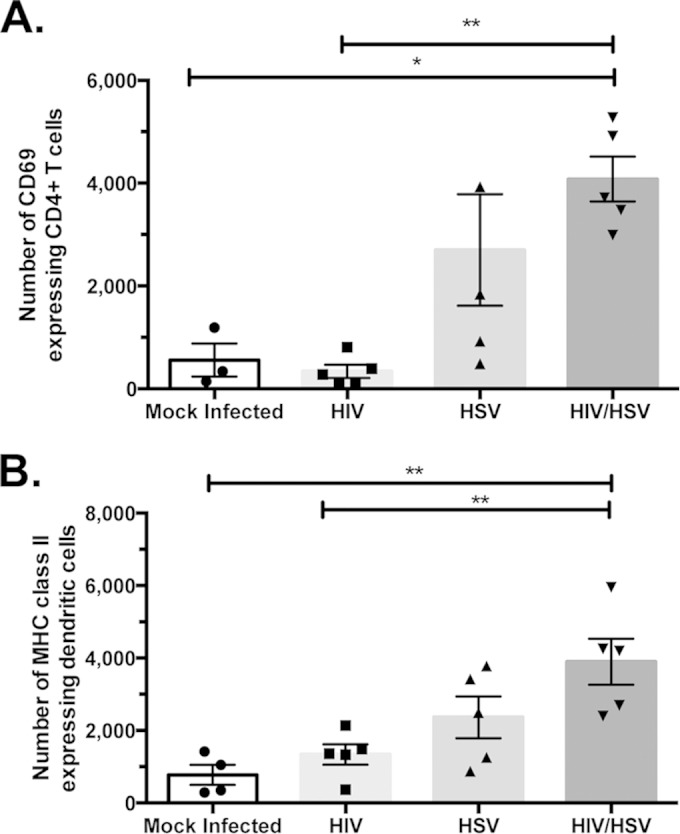
Impact of HSV-2 and/or HIV-1 on the number of activated CD4+ T cells, activated dendritic cells, and CCR7+ dendritic cells. hCD4/R5/cT1 mice were DMPA treated and 5 days later mock challenged or HSV-2 challenged. Two days later, some mice from each group were intravaginally mock inoculated or inoculated with HIV-Du151.2env-NLuc (4.5 × 105 IU). Two days later, lower genital tract mononuclear cells were isolated, counted, and analyzed by flow cytometry, and the total number of the indicated subpopulation was determined by multiplying the total cell number by the percentage of cells expressing the indicated phenotypic markers. (A) Total number of CD69-expressing CD4+ T cells. (B) Total number of MHC class II molecule-expressing dendritic cells. The average number of cells from each group ± standard error of the mean and individual data points are shown. The data represent one of two experiments with 4 to 5 mice/group. Asterisks indicate significance using one-way ANOVA with Tukey's test for multiple comparisons (*, P < 0.05; **, P < 0.01).
Intravaginal TDF gel is more effective than TFV gel in preventing the acquisition of HIV-1 infection in uninfected and HSV-2-coinfected hCD4/R5/cT1 mice.
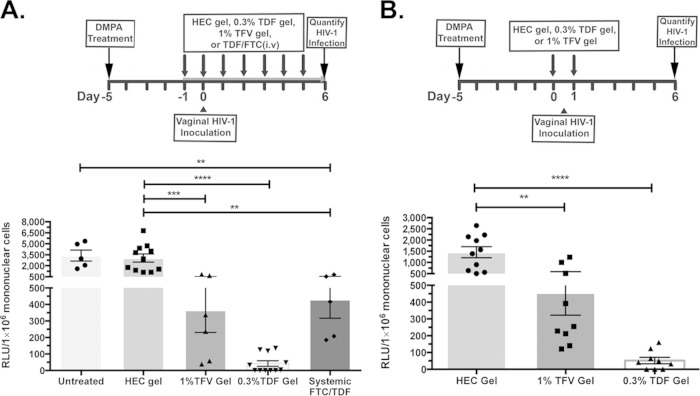
We utilized hCD4/R5/cT1 mice as a preclinical model to evaluate the in vivo efficacy of candidate microbicides during genital HSV-2 infection to prevent vaginal HIV acquisition by comparing the in vivo effectiveness of 1% TFV gel and 0.3% TDF gel vaginally administered according a protocol based on the dosing scheme used in either the CAPRISA 004 trial or VOICE trial. hCD4/R5/cT1 mice were pretreated with 0.3% TDF, 1% TFV, or HEC control gel or intraperitoneally injected with the combination of FTC/TDF 12 h prior to HIV-1 challenge and then daily until the mice were sacrificed at 6 days after HIV-1 inoculation. Daily topical or systemic PrEP significantly inhibited HIV infection in the lower genital tract (Fig. 5A), with the strongest protection observed with 0.3% TDF gel. Complete protection (defined as relative light units [RLU] of <35, which is the maximum background RLU observed in the lower genital tract of mice inoculated with a noninfectious [env-negative] NLuc reporter-expressing control virus) was observed in 7 of 11 TDF gel-treated mice (Fig. 5A). Moreover, when one dose of gel was administered 12 h before and a second dose was administered 12 h after HIV exposure to simulate the pericoital recommended dosing in CAPRISA 004 (designated BAT24), the 0.3% TDF gel completely protected 4 of 9 mice from lower genital tract infection (Fig. 5B). In contrast, while TFV gel significantly inhibited the level of HIV-1 infection, low levels of HIV-1 infection were still detected in the lower genital tracts of almost all of the treated mice.
FIG 5.
The 0.3% TDF gel is significantly more effective in preventing the acquisition of HIV-1 infection than the 1% TFV gel. (A) DMPA-treated hCD4/R5/cT1 mice were treated with intravaginal 1% TFV gel, 0.3% TDF gel, or HEC gel or with an intraperitoneal injection of TDF/FTC starting 24 h prior to infection and then vaginally infected by atraumatically introducing HIV-Du151.2env-NLuc (4.5 × 105 IU). Mice were then treated daily with the indicated compound; 6 days after infection, lower genital tract mononuclear cells were isolated and lysed, and NLuc activity in the cellular lysate was quantified. (B) DMPA-treated hCD4/R5/cT1 mice were treated with either 1% TFV gel, 0.3% TDF gel, or HEC gel 12 h prior to and 12 h after intravaginal inoculation with HIV-Du151.2env-NLuc (4.5 × 105 IU). Six days after challenge, lower genital tract mononuclear cells were isolated and lysed, and NLuc activity in the cellular lysate was quantified; values are reported as RLU corresponding to a normalized analysis of 106 mononuclear cells. The average NLuc activity from two pooled experiments in the cellular lysates of mice from each group (5 to 11 mice/group) ± standard error of the mean and individual data points are shown. Asterisks indicate significance using one-way ANOVA with Tukey's test for multiple comparisons (**, P < 0.01; ***, P < 0.001; ****, P < 0.0001). The experimental design for each experiment is shown above the graph.
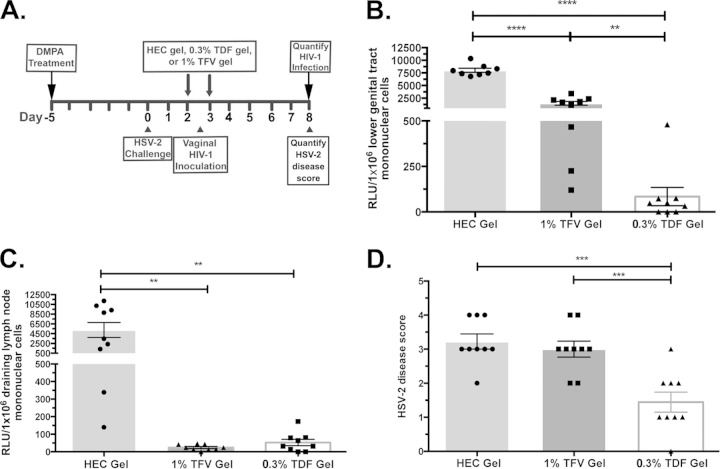
We compared the efficacy of TDF gel and TFV gel to protect hCD4/R5/cT1 mice from the acquisition of HIV-1 infection after acute HSV-2 infection. DMPA-treated hCD4/R5/cT1 mice were mock infected or infected by intravaginal HSV-2 inoculation. After 36 h, groups of mice were treated with 0.3% TDF, 1% TFV, or HEC gel using a dosing similar to BAT24 of 12 h before and 12 h after intravaginal inoculation with HIV-Du151.2env-NLuc. Six days after HIV-1 challenge, NLuc activity was measured in mononuclear cells from the lower genital tract and draining lymph nodes (Fig. 6A). Both TDF and TFV gels significantly protected mice from HIV-1 infection of the lower genital tract, but TDF gel was significantly (P < 0.01) more effective at inhibiting local HIV-1 infection than TFV gel and completely protected 4 of 9 HSV-2-infected mice from acquiring HIV-1 (Fig. 6B). Although treatment with the 1% TFV gel did not completely inhibit lower genital tract HIV-1 infection in any of the HSV-2-infected hCD4/R5/cT1 mice, the marked reduction in the level of initial local infection it provided may have been sufficient to prevent the spread of infection from the mucosal tissues to the draining lymph nodes within 6 days after HIV-1 inoculation, which occurred in 7 out of 9 HEC gel-treated mice (Fig. 6C). Moreover, although the first dose of gel was not administered until 36 h after the mice were HSV-2 challenged, TDF, but not TFV, significantly reduced HSV-2 disease scores (Fig. 6D).
FIG 6.
The 0.3% TDF gel more effectively inhibits vaginal HIV-1 infection in HSV-2-infected mice than the 1% TFV gel. DMPA-treated hCD4/R5/cT1 mice were mock challenged or challenged with HSV-2 (105 PFU/mouse). Two days later, the mice were treated with either HEC gel, 1% TFV gel, or 0.3% TDF gel 12 h before and after they were vaginally infected by atraumatically introducing HIV-Du151.2env-NLuc (4.5 × 105 IU). (A) Experimental design. (B) Six days later, HIV infection was quantified by measuring NLuc activity in mononuclear cells isolated from the lower genital tract, and values are reported as RLU corresponding to a normalized analysis of 106 mononuclear cells. (C) Six days later, HIV infection was quantified by measuring NLuc activity in mononuclear cells isolated from the draining lymph nodes, and values are reported as RLU corresponding to a normalized analysis of 106 mononuclear cells. (D) Six days later, HSV-2 epithelial disease was quantified, and values are presented as the HSV-2 disease score. The average NLuc activity or epithelial disease from two pooled experiments in the cellular lysates from mice from each group (8 to 9 mice/group) ± standard error of the mean and individual data points are shown. Asterisks indicate significance using one-way ANOVA with Tukey's test for multiple comparisons (**, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
DISCUSSION
Evaluation of the in vivo efficacy of HIV-1 PrEP, particularly in the context of coinfection with sexually transmitted infections or other factors that facilitate HIV-1 acquisition, would be greatly facilitated by the expanded availability of preclinical testing models. To this end, we created a preclinical mouse model that reproducibly supports in vivo HIV-1 infection and is highly accessible and cost-effective, hCD4/R5/cT1 mice. These genetically modified mice bypass the murine HIV entry block by expressing human CD4 and human CCR5 transgenes and the murine HIV-1 transcription block by expressing the gene for the human transcription factor, cyclin T1. We previously reported that these hCD4/R5/cT1 mice are susceptible to HIV-1 acquisition by intravaginal challenge using an engineered HIV-1 virus that is replication competent and expresses the Renilla luciferase reporter gene (18). In this report we demonstrated that HIV-1 acquisition after intravaginal challenge was markedly increased by pretreatment with progesterone. This is compatible with macaque studies which reported that vaginal transmission of SIV and SHIV were markedly increased by pretreatment with progesterone due to thinning of the vaginal epithelium (48, 49). We also validated hCD4/R5/cT1 mice as a highly sensitive mouse model that can be used to rapidly screen and evaluate the comparative effectiveness of potential PrEP products to prevent the acquisition of HIV-1 infection. Furthermore, this preclinical model enabled us to begin to evaluate the mechanistic basis by which coinfections with pathogens such as HSV-2 may facilitate HIV-1 acquisition and the comparative efficacy of PrEP to prevent HIV-1 acquisition during coinfection with a sexually transmitted infection that is associated with an increased risk of HIV acquisition (53).
Numerous epidemiological studies have reported a 2- to 4-fold increase in the acquisition of HIV-1 infection in individuals with a history of HSV-2 infection (7). This increased risk for HIV-1 acquisition has been partially attributed to the release of proinflammatory cytokines and chemokines as a result of the interaction between epithelial cells and HSV-2 (10–13). This inflammatory response then recruits to the site of inflammation potential target cells for HIV-1 infection which produce additional cytokines and chemokines, populating the mucosal environment with activated cells that increase the likelihood for the establishment of HIV-1 infection. In our transgenic mouse model, HSV-2-challenged hCD4/R5/cT1 mice displayed increased levels of HIV-1 infection in the lower genital tract mucosa and increased dissemination of HIV-1 infection into the draining lymph nodes. We previously reported that HSV-2 infection of C57BL/6 mice induced an increase in interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-1α, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), and RANTES gene expression in the lower genital tract mucosa 2 days after infection (12). MCP-1, MIP-1β, and RANTES are chemokines that stimulate the recruitment of T cells and monocytes (54, 55). In the current study, we demonstrated that HSV-2 infection induced the cellular recruitment of CD4+ T lymphocytes, monocytes, and dendritic cells into the hCD4/R5/cT1 mouse lower genital tract mucosa, likely due the virus's stimulation of the release of MCP-1, MIP-1β, and RANTES in the lower genital tract. In addition, the HSV-2 infection induced production of TNF-α and IL-1β, activators of T cells, which likely mediated the increased number of CD69+-activated CD4+ T lymphocytes that we observed in the lower genital tract of HSV-2-infected hCD4/R5/cT1 mice (56, 57). HSV-2 infection also increased the population of activated dendritic cells in the lower genital tract, as indicated by the increased number of dendritic cells expressing MHC class II molecules. Expression of MHC class II molecules by dendritic cells following peripheral activation is associated with their maturation into effector dendritic cells that migrate to draining lymph nodes and interact with T cells (51, 58). After vaginal exposure, mouse dendritic cells can transport HIV-1 from the vaginal mucosa to draining lymph nodes (59). Consequently, the increased migration of dendritic cells into draining lymph nodes induced by HSV-2 infection may enable dissemination of HIV-1 infection from the distal genital tissues into the lymph nodes, where they can productively infect resident CD4+ T cells. Although mice have been extensively used to study vaginal transmission of HSV-2 infection, these results need to be interpreted in context because the level of vaginal inflammation after mouse infection is greater than that in humans (60), which may increase the impact of vaginal HSV-2 infection on HIV-1 acquisition. A limitation of this model is the presence of additional blocks in HIV-1 replication in mouse cells that are not corrected by expression of human CD4, CCR5, and cyclin T1 (61), which restricts the use of the model to study infection in the first week after intravaginal HIV-1 inoculation. These additional blocks prevent the development of productive systemic HIV-1 infection and plasma viremia after vaginal inoculation, which impedes the dissemination of HIV-1-infected cells to the draining lymph nodes. We hypothesize that this limitation may be partially overcome by HSV-2-mediated increases in the number of activated CD4+ T cells and dendritic cells expressing MHC class II molecules, which facilitated dissemination of HIV-1 into the draining lymph nodes of HSV-2/HIV-1-infected mice.
Application of TFV-based gels as PrEP has been shown to decrease the acquisition of HIV-1 and HSV-2 infection (3, 4). The CAPRISA 004 trial was the first large-scale study to demonstrate the efficacy of a 1% TFV gel to reduce the heterosexual transmission of HIV-1 infection (39%) and HSV-2 (51%) (3). Studies using humanized bone marrow/liver/thymus (BLT) mice that were treated according to the CAPRISA 004 treatment regime recapitulated these results and showed that a 1% TFV gel inhibited HIV-1 infection by 88% (62). However, the recent FACTS 001 clinical trial showed no protection among women prescribed 1% TFV gel to use within 12 h before and after sexual intercourse. The lack of efficacy was attributed to low adherence, which was assessed by the number of returned empty gel applicators and self-reported sexual acts (24). Nevertheless, despite adhering to the study regimen, some study subjects still acquired HIV infection. In the VOICE trial, 27% of seroconverters in the vaginal TFV gel treatment group had TFV levels detected in their plasma; in the CAPRISA 004 trial, while high adherers displayed a 54% decrease in HIV-1 acquisition, this group was not completely protected from infection (3, 23). These trials support the need to investigate the efficacy of other topical drug formulations with more rapid uptake, increased tissue permeability, and enhanced intracellular concentration or the use of a method for sustained delivery of microbicides, such as an intravaginal ring (29).
We compared the efficacy of 1% TFV gel and 0.3% TDF gel using either a daily dosage treatment regime or treatment 12 h before and after HIV-1 exposure. We previously showed that 0.3% TDF was more effective than 1% TFV gel in preventing HSV-2 infection (4). In this report we expanded upon these studies by comparing the efficacy of 0.3% TDF gel to 1% TFV gel in mice that were either infected with HIV-1 alone or coinfected with HSV-2. Treatment with 0.3% TDF gel protected hCD4/R5/cT1 mice from acquisition of HIV-1 infection after intravaginal exposure more effectively than with 1% TFV gel using both treatment protocols. The increased efficacy of 0.3% TDF gel compared to that of 1% TFV gel in preventing vaginal acquisition of HIV-1 infection was further indicated by its ability to maintain potency even in the context of acute HSV-2 infection. The capacity of TFV to inhibit HIV-1 infection is reduced by HSV-2 infection, as indicated by a recent study using an ex vivo ectocervix model which reported that the presence of HSV-2 infection increased the concentration of TFV required to prevent HIV-1 infection (13). Thus, the hCD4/R5/cT1 mice provided an in vivo model that demonstrated that even in the presence of the inflammation and cellular recruitment induced by acute HSV-2 infection, 0.3% TDF gel provides a sufficient concentration of the active metabolite, tenofovir diphosphate, to prevent the acquisition of HIV-1 infection. HIV-1 infection in the lower genital tract of the hCD4/R5/cT1 mice required pretreatment with DMPA, which was shown to increase the rate of vaginal SIV acquisition in macaques by thinning the vaginal epithelium (48). The use of DMPA treatment may provide a more stringent animal model to test the efficacy of PrEP microbicides because it may reflect the conditions associated with cervical/vaginal inflammation.
A limitation to the use of the hCD4/R5/cT1 mouse model compared to the BLT mouse and other newer humanized mouse models is the diminished capacity of mouse CD4+ T cells to support HIV-1 infection and develop disseminated HIV-1 infection after vaginal exposure. Nevertheless, the mice develop highly reproducible local HIV-1 infection of CD4+ T cells and macrophages after vaginal challenge, HSV-2-induced dissemination of HIV-1 infection into the draining lymph nodes, and the ability to recapitulate a key component in early HIV-1 transmission by predominantly infecting CD4+ T lymphocytes (50). Furthermore, a major advantage of this model is that all of the cells are murine, and therefore this obviates concerns about variable interactions between human cells and mouse epithelial cells crucial for chemokine/cytokine interactions and cellular trafficking. In addition, while humanized mice constructed using highly immunodeficient NSG (NOD-SCID-IL2rΔ−/−) mice would be highly susceptible to HSV-2 infection and likely rapidly killed after infection with this virus, the hCD4/R5/cT1 transgenic mice are fully immunocompetent, enabling them to be used for HSV-2 coinfection studies. Other key advantages of this model compared to humanized mouse models are that these experiments can be performed in a rapid 2-week time frame without a wait of months for engraftment with human cells, that the hCD4/R5/cT1 mice used have the same genetic background in contrast to the multiple human genetic backgrounds of different donors used in humanized mice, and that there is less experimental variability due to differences in levels of engraftment by human cells. In addition, these studies used an infectious molecular clone expressing a clade C Env which is representative of the predominant HIV subtype in sub-Saharan Africa where HSV-2 infection is also endemic (63, 64).
We are currently exploring ways to increase the capacity of mouse CD4+ T cells to support HIV-1 infection by overcoming additional species-specific blocks such as the downregulation of mouse APOBEC3, a host factor that inhibits HIV-1 replication (65). In spite of limitations associated with the use of hCD4/R5/cT1 mice, this preclinical model should provide the field with an immunocompetent, cost-effective, and highly reproducible mouse model to evaluate the efficacy of PrEP microbicides in uninfected mice as well as in mice coinfected with pathogens associated with increased HIV-1 acquisition.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Drug Abuse at the National Institutes of Health (DA033788), the Einstein-Montefiore Center for AIDS Research (P30-AI51519), the University of Alabama at Birmingham Center for AIDS Research (P30-AI277670), the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (AI065309 and T32-AI007501 to K.S.), NIH Center for HIV-1/AIDS Vaccine Immunology (CHAVI) (UO1-AI067854 to J.C.K. and C.O.), the Charles Michael Chair in Autoimmune Diseases (to H.G.), and a VHA Merit Review Award (to J.C.K.).
We thank Gilead for providing tenofovir disoproxil fumarate.
REFERENCES
- 1.Looker KJ, Garnett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 86:805–812, A. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JS, Robinson NJ. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nixon B, Jandl T, Teller RS, Taneva E, Wang Y, Nagaraja U, Kiser PF, Herold BC. 2014. Vaginally delivered tenofovir disoproxil fumarate provides greater protection than tenofovir against genital herpes in a murine model of efficacy and safety. Antimicrob Agents Chemother 58:1153–1160. doi: 10.1128/AAC.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, Mark KE, Casapia M, Mehrotra DV, Buchbinder SP, Corey L, Network NHVT. 2011. Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study). J Acquir Immune Defic Syndr 57:238–244. doi: 10.1097/QAI.0b013e31821acb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Jha P, Stirling B, Sgaier SK, Daid T, Kaul R, Nagelkerke N. 2007. Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One 2:e1001. doi: 10.1371/journal.pone.0001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds SJ, Risbud AR, Shepherd ME, Zenilman JM, Brookmeyer RS, Paranjape RS, Divekar AD, Gangakhedkar RR, Ghate MV, Bollinger RC, Mehendale SM. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis 187:1513–1521. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 9.Wasserheit JN. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 19:61–77. [PubMed] [Google Scholar]
- 10.Feng Z, Qiu Z, Sang Z, Lorenzo C, Glasser J. 2013. Modeling the synergy between HSV-2 and HIV and potential impact of HSV-2 therapy. Math Biosci 245:171–187. doi: 10.1016/j.mbs.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira VH, Kafka JK, Kaushic C. 2014. Influence of common mucosal co-factors on HIV infection in the female genital tract. Am J Reprod Immunol 71:543–554. doi: 10.1111/aji.12221. [DOI] [PubMed] [Google Scholar]
- 12.Nixon B, Fakioglu E, Stefanidou M, Wang Y, Dutta M, Goldstein H, Herold BC. 2014. Genital herpes simplex virus type 2 infection in humanized HIV-transgenic mice triggers HIV shedding and is associated with greater neurological disease. J Infect Dis 209:510–522. doi: 10.1093/infdis/jit472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rollenhagen C, Lathrop MJ, Macura SL, Doncel GF, Asin SN. 2014. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol 7:1165–1174. doi: 10.1038/mi.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelli E, Tharinger H, Frank I, Arthos J, Piatak M Jr, Lifson JD, Blanchard J, Gettie A, Robbiani M. 2011. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS Pathog 7:e1002109. doi: 10.1371/journal.ppat.1002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B, Fowke K, Mazzulli T, Plummer FA, Kaul R. 2007. Negative mucosal synergy between herpes simplex type 2 and HIV in the female genital tract. AIDS 21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 16.Stefanidou M, Ramos I, Mas Casullo V, Trepanier JB, Rosenbaum S, Fernandez-Sesma A, Herold BC. 2013. Herpes simplex virus 2 (HSV-2) prevents dendritic cell maturation, induces apoptosis, and triggers release of proinflammatory cytokines: potential links to HSV-HIV synergy. J Virol 87:1443–1453. doi: 10.1128/JVI.01302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denton PW, Garcia JV. 2012. Mucosal HIV-1 transmission and prevention strategies in BLT humanized mice. Trends Microbiol 20:268–274. doi: 10.1016/j.tim.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seay K, Qi X, Zheng JH, Zhang C, Chen K, Dutta M, Deneroff K, Ochsenbauer C, Kappes JC, Littman DR, Goldstein H. 2013. Mice transgenic for CD4-specific human CD4, CCR5 and cyclin T1 expression: a new model for investigating HIV-1 transmission and treatment efficacy. PLoS One 8:e63537. doi: 10.1371/journal.pone.0063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 20.Imai K, Asamitsu K, Victoriano AF, Cueno ME, Fujinaga K, Okamoto T. 2009. Cyclin T1 stabilizes expression levels of HIV-1 Tat in cells. FEBS J 276:7124–7133. doi: 10.1111/j.1742-4658.2009.07424.x. [DOI] [PubMed] [Google Scholar]
- 21.Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451–462. doi: 10.1016/S0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 22.Wimmer J, Fujinaga K, Taube R, Cujec TP, Zhu Y, Peng J, Price DH, Peterlin BM. 1999. Interactions between Tat and TAR and human immunodeficiency virus replication are facilitated by human cyclin T1 but not cyclins T2a or T2b. Virology 255:182–189. doi: 10.1006/viro.1998.9589. [DOI] [PubMed] [Google Scholar]
- 23.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM. 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rees H, Delany-Moretlwe S, Baron D, Lombard C, Gray G, Myer L, Panchia R, Schwartz J, Doncel G. 2015. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women, abstr 26LB Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 23 to 26 February 2015. [Google Scholar]
- 25.Gengiah TN, Baxter C, Mansoor LE, Kharsany AB, Abdool Karim SS. 2012. A drug evaluation of 1% tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection. Expert Opin Investig Drugs 21:695–715. doi: 10.1517/13543784.2012.667072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazra R, Balis FM, Tullio AN, DeCarlo E, Worrell CJ, Steinberg SM, Flaherty JF, Yale K, Poblenz M, Kearney BP, Zhong L, Coakley DF, Blanche S, Bresson JL, Zuckerman JA, Zeichner SL. 2004. Single-dose and steady-state pharmacokinetics of tenofovir disoproxil fumarate in human immunodeficiency virus-infected children. Antimicrob Agents Chemother 48:124–129. doi: 10.1128/AAC.48.1.124-129.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother 42:612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesquita PM, Rastogi R, Segarra TJ, Teller RS, Torres NM, Huber AM, Kiser PF, Herold BC. 2012. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother 67:1730–1738. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, McNicholl JM, Hendry RM, Dinh CT, Martin A, Herold BC, Kiser PF. 2013. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A 110:16145–16150. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown BK, Wieczorek L, Kijak G, Lombardi K, Currier J, Wesberry M, Kappes JC, Ngauy V, Marovich M, Michael N, Ochsenbauer C, Montefiori DC, Polonis VR. 2012. The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization. PLoS One 7:e29454. doi: 10.1371/journal.pone.0029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chenine AL, Wieczorek L, Sanders-Buell E, Wesberry M, Towle T, Pillis DM, Molnar S, McLinden R, Edmonds T, Hirsch I, O'Connell R, McCutchan FE, Montefiori DC, Ochsenbauer C, Kappes JC, Kim JH, Polonis VR, Tovanabutra S. 2013. Impact of HIV-1 backbone on neutralization sensitivity: neutralization profiles of heterologous envelope glycoproteins expressed in native subtype C and CRF01_AE backbone. PLoS One 8:e76104. doi: 10.1371/journal.pone.0076104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.deCamp A, Hraber P, Bailer RT, Seaman MS, Ochsenbauer C, Kappes J, Gottardo R, Edlefsen P, Self S, Tang H, Greene K, Gao H, Daniell X, Sarzotti-Kelsoe M, Gorny MK, Zolla-Pazner S, LaBranche CC, Mascola JR, Korber BT, Montefiori DC. 2014. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLinden RJ, Labranche CC, Chenine AL, Polonis VR, Eller MA, Wieczorek L, Ochsenbauer C, Kappes JC, Perfetto S, Montefiori DC, Michael NL, Kim JH. 2013. Detection of HIV-1 neutralizing antibodies in a human CD4+/CXCR4+/CCR5+ T-lymphoblastoid cell assay system. PLoS One 8:e77756. doi: 10.1371/journal.pone.0077756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naarding MA, Fernandez N, Kappes JC, Hayes P, Ahmed T, Icyuz M, Edmonds TG, Bergin P, Anzala O, Hanke T, Clark L, Cox JH, Cormier E, Ochsenbauer C, Gilmour J. 2014. Development of a luciferase based viral inhibition assay to evaluate vaccine induced CD8 T-cell responses. J Immunol Methods 409:161–173. doi: 10.1016/j.jim.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. 2012. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pace CS, Song R, Ochsenbauer C, Andrews CD, Franco D, Yu J, Oren DA, Seaman MS, Ho DD. 2013. Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1. Proc Natl Acad Sci U S A 110:13540–13545. doi: 10.1073/pnas.1304985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarzotti-Kelsoe M, Daniell X, Todd CA, Bilska M, Martelli A, LaBranche C, Perez LG, Ochsenbauer C, Kappes JC, Rountree W, Denny TN, Montefiori DC. 2014. Optimization and validation of a neutralizing antibody assay for HIV-1 in A3R5 cells. J Immunol Methods 409:147–160. doi: 10.1016/j.jim.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, Otto P, Zimmerman K, Vidugiris G, Machleidt T, Robers MB, Benink HA, Eggers CT, Slater MR, Meisenheimer PL, Klaubert DH, Fan F, Encell LP, Wood KV. 2012. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol 7:1848–1857. doi: 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol 80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses 21:845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 42.Berges BK, Akkina SR, Folkvord JM, Connick E, Akkina R. 2008. Mucosal transmission of R5 and X4 tropic HIV-1 via vaginal and rectal routes in humanized Rag2−/− γc−/− (RAG-hu) mice. Virology 373:342–351. doi: 10.1016/j.virol.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fidel PL Jr, Wolf NA, KuKuruga MA. 1996. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun 64:3793–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iijima N, Mattei LM, Iwasaki A. 2011. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci U S A 108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, Powell DA, Payne D, Haase AT, Garcia JV. 2008. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendrickson BA, Guo J, Brown I, Dennis K, Marcellino D, Hetzel J, Herold BC. 2000. Decreased vaginal disease in J-chain-deficient mice following herpes simplex type 2 genital infection. Virology 271:155–162. doi: 10.1006/viro.2000.0303. [DOI] [PubMed] [Google Scholar]
- 47.Segarra TJ, Fakioglu E, Cheshenko N, Wilson SS, Mesquita PM, Doncel GF, Herold BC. 2011. Bridging the gap between preclinical and clinical microbicide trials: blind evaluation of candidate gels in murine models of efficacy and safety. PLoS One 6:e27675. doi: 10.1371/journal.pone.0027675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 49.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 50.Haase AT. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 51.Villadangos JA, Cardoso M, Steptoe RJ, van Berkel D, Pooley J, Carbone FR, Shortman K. 2001. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity 14:739–749. doi: 10.1016/S1074-7613(01)00148-0. [DOI] [PubMed] [Google Scholar]
- 52.Wilflingseder D, Mullauer B, Schramek H, Banki Z, Pruenster M, Dierich MP, Stoiber H. 2004. HIV-1-induced migration of monocyte-derived dendritic cells is associated with differential activation of MAPK pathways. J Immunol 173:7497–7505. doi: 10.4049/jimmunol.173.12.7497. [DOI] [PubMed] [Google Scholar]
- 53.Ward H, Ronn M. 2010. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS 5:305–310. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siveke JT, Hamann A. 1998. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol 160:550–554. [PubMed] [Google Scholar]
- 55.Schall TJ, Bacon KB. 1994. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol 6:865–873. doi: 10.1016/0952-7915(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 56.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. 1998. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med 188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, Fauci AS. 1989. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A 86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seth S, Oberdorfer L, Hyde R, Hoff K, Thies V, Worbs T, Schmitz S, Forster R. 2011. CCR7 essentially contributes to the homing of plasmacytoid dendritic cells to lymph nodes under steady-state as well as inflammatory conditions. J Immunol 186:3364–3372. doi: 10.4049/jimmunol.1002598. [DOI] [PubMed] [Google Scholar]
- 59.Masurier C, Salomon B, Guettari N, Pioche C, Lachapelle F, Guigon M, Klatzmann D. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J Virol 72:7822–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kollias CM, Huneke RB, Wigdahl B, Jennings SR. 2015. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol 21:8–23. doi: 10.1007/s13365-014-0302-2. [DOI] [PubMed] [Google Scholar]
- 61.Bieniasz PD, Cullen BR. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J Virol 74:9868–9877. doi: 10.1128/JVI.74.21.9868-9877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denton PW, Othieno F, Martinez-Torres F, Zou W, Krisko JF, Fleming E, Zein S, Powell DA, Wahl A, Kwak YT, Welch BD, Kay MS, Payne DA, Gallay P, Appella E, Estes JD, Lu M, Garcia JV. 2011. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 85:7582–7593. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghebremichael M, Habtzgi D, Paintsil E. 2012. Deciphering the epidemic synergy of herpes simplex virus type 2 (HSV-2) on human immunodeficiency virus type 1 (HIV-1) infection among women in sub-Saharan Africa. BMC research notes 5:451. doi: 10.1186/1756-0500-5-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lihana RW, Ssemwanga D, Abimiku A, Ndembi N. 2012. Update on HIV-1 diversity in Africa: a decade in review. AIDS Rev 14:83–100. [PubMed] [Google Scholar]
- 65.Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nat Rev Microbiol 10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]