ABSTRACT
Foot-and-mouth disease (FMD) is a highly contagious viral disease affecting biungulate species. Commercial vaccines, formulated with inactivated FMD virus (FMDV), are regularly used worldwide to control the disease. Here, we studied the generation of antibody responses in local lymphoid tissues along the respiratory system in vaccinated and further aerosol-infected cattle. Animals immunized with a high-payload monovalent FMD vaccine developed high titers of neutralizing antibodies at 7 days postvaccination (dpv), reaching a plateau at 29 dpv. FMDV-specific antibody-secreting cells (ASC), predominantly IgM, were evident at 7 dpv in the prescapular lymph node (LN) draining the vaccination site and in distal LN draining the respiratory mucosa, although in lower numbers. At 29 dpv, a significant switch to IgG1 was clear in prescapular LN, while FMDV-specific ASC were detected in all lymphoid tissues draining the respiratory tract, mostly as IgM-secreting cells. None of the animals (n = 10) exhibited FMD symptoms after oronasal challenge at 30 dpv. Three days postinfection, a large increase in ASC numbers and rapid isotype switches to IgG1 were observed, particularly in LN-draining virus replication sites already described. These results indicate for the first time that systemic FMD vaccination in cattle effectively promotes the presence of anti-FMDV ASC in lymphoid tissues associated with the respiratory system. Oronasal infection triggered an immune reaction compatible with a local anamnestic response upon contact with the replicating FMDV, suggesting that FMD vaccination induces the circulation of virus-specific B lymphocytes, including memory B cells that differentiate into ASC soon after contact with the infective virus.
IMPORTANCE Over recent decades, world animal health organizations as well as national sanitary authorities have supported the use of vaccination as an essential component of the official FMD control programs in both endemic and disease-free settings. Very few works studied the local immunity induced by FMD vaccines at the respiratory mucosa, and local responses induced in vaccinated animals after aerosol infection have not been described yet. In this work, we demonstrate for the first time that systemic FMD vaccination (i) induced the early presence of active antigen-specific ASC along the respiratory tract and (ii) prompted a rapid local antibody response in the respiratory mucosa, triggered upon oronasal challenge and congruent with a memory B-cell response. This information may help to understand novel aspects of protective responses induced by current FMD vaccines as well as to provide alternative parameters to establish protection efficiency for new vaccine developments.
INTRODUCTION
Foot-and-mouth disease (FMD) is a highly contagious and acute viral disease affecting a wide range of economically important livestock species (1). All domestic biungulates are susceptible to infection with the FMD virus (FMDV); in addition, a number of wildlife species may act as reservoirs for the virus under particular ecological conditions (2). Lethality has been described for young animals and certain FMDV strains (3). However, the main disruptive potential of this transboundary disease is the high morbidity rate and the numerous indirect losses associated with its incursions into territories with susceptible populations. Consequently, FMD outbreaks may result in severe and far-reaching economic losses due to the interruption of regional and international trade in developed countries (4, 5), but also importantly, due to the loss of animals, production efficiency, and genetic diversity in developing regions (6).
Cattle are highly susceptible to FMDV, and virus usually gains entry through the respiratory tract of the animals (3). The soft palate and pharynx were identified as primary sites of FMDV replication and persistence in bovines infected through the oronasal route (7, 8). FMDV infection progresses through replication in pneumocytes, permitting the virus to accomplish an extensive distribution in the lungs which in turn allows the establishment of a sustained viremia (9).
Disease outbreaks are managed using a spectrum of possible strategies, including vaccination and/or the sacrifice of infected and exposed animals. Starting in the early 2000s, however, several social, environmental, and economic concerns were raised by the scientific community and public opinion regarding approaches involving mass culling of livestock and favoring the use of FMD vaccination as a control measure (10, 11). Currently, vaccination is used in both disease-free settings and those where the virus is endemic around the world, and it is recognized as an essential tool throughout the FMD Progressive Control Pathway (PCP), endorsed by the Food and Agriculture Organization of the United Nations (FAO) and the World Organization for Animal Health (OIE) (12).
Commercial FMD vaccines are based on chemically inactivated whole-virus particles, manufactured as aqueous or oil formulations, formulated with aluminum hydroxide/saponin, or as single or double emulsions for oil adjuvants (13). During the last several decades, extensive use of FMD vaccines has prevented the development and transmission of the disease, as well as decreased the incidence of persistently infected animals (14–16). Protection against the disease is closely related to the induction of specific antibodies (17), and humoral responses generated by FMD vaccines are serotype restricted and, in some cases, even strain specific (13).
The vast majority of studies examining the induction of antibodies in response to FMD vaccination has been at the systemic level. Despite the importance of understanding the local antibody response against the virus within the respiratory mucosa, there are practically no reports addressing the mucosal responses induced by FMD vaccination in cattle, except for two early reports working with oronasal secretions (18, 19).
Following our previous study on the induction of local adaptive responses in naive bovines infected through the oronasal route (20), here we analyzed the local antibody production induced after systemic FMD vaccination and the responses triggered in these animals after oronasal challenge with virulent FMDV. Our results demonstrate that soon after systemic immunization with high-payload FMD vaccines, it is possible to detect virus-specific antibody-secreting cells (ASC) in lymphoid tissues distal from the inoculation spot. This phenomenon was extended and enhanced at 29 dpv, observing a progression and dynamics which differed from those of the FMDV-specific systemic antibody responses and from the lymph node draining the vaccination site. Furthermore, oronasal infection with virulent FMDV did not produce disease in vaccinated steers, characterized by an immune response that included a rapid isotype switch in lymphoid tissues located along the entire respiratory tract, which, for some lymph nodes, occurred simultaneously with an IgM-driven virus-specific response. Here, we discuss possible mechanisms associated with these findings, analyze their role in pathogenesis during aerogenous FMDV infection, and compare immune responses at distal sites with the concurrent antibody responses observed in the vaccine-draining lymph tissues and at the systemic level.
MATERIALS AND METHODS
Experimental animals.
A total of 16 14- to 18-month-old Hereford steers were purchased from a livestock breeder located in an FMDV-free region without vaccination in Argentina (Patagonia). All of the animals were checked for the absence of FMDV-specific antibodies by liquid-phase blocking enzyme-linked immunosorbent assay (LPB-ELISA) (21) before their arrival. Three additional 18- to 20-month-old steers from the experimental field of CICVyA–INTA, which have received three FMD vaccinations (every 6 months) in the frame of the official FMD vaccination campaigns in Argentina, also were utilized in B-cell memory and viral antigen detection experiments.
Trials which did not include infected bovines were carried out at biosafety level 2 (BSL-2) facilities, while experiments with infected cattle were performed in BSL-4 OIE animal boxes, both located at the CICVyA–INTA. All experiments were completed by following biosecurity and animal welfare internal and federal regulations and according to protocols 05/2010, 25/2013, and 20/2014, approved by the Institutional Committee for Use and Care of Experimental Animals (CICUAE), CICVyA–INTA.
Virus, controlled aerosol infection, and clinical assessment in cattle.
Virulent FMDV strain O1/Campos/Brazil/58 (O1 Campos) was provided by the OIE FMD Reference Laboratory at SENASA, Argentina. Experimental infections through the oronasal route were performed with a jet nebulizer attached to an aerosol delivery system (107 50% tissue culture infective doses [TCID50] in a 2-ml volume per animal) as previously reported (20, 22). After infection, animals were monitored daily for clinical signs of FMD. Symptoms included vesiculation in the tongue, mouth, and feet, lameness, increased salivation, loss of appetite, and fever (rectal temperature above 39.5°C).
Vaccines, vaccinations, and inactivated FMDV antigens.
To assess local FMDV-specific ASC in the respiratory tract of vaccinated cattle before and after infection, 14 animals received one dose of a single-oil-emulsion monovalent vaccine containing 23.5 μg of inactivated FMDV O1/Campos strain per dose, kindly provided by Biogénesis-Bagó S.A. (Argentina). Two additional naive animals (326 and 370) as well as 3 multivaccinated bovines (177, 231, and 232), all utilized in the B-cell memory assays, were vaccinated with a commercial tetravalent FMD single-oil-emulsion vaccine, including the O1/Campos strain. In all cases, animals were injected with a 2-ml vaccine dose applied intramuscularly in the neck (left side).
Inactivated and concentrated suspensions of FMDV O1 Campos also were provided by Biogénesis-Bagó S.A., and 140S viral particles for in vitro experiments were purified by following a sucrose density gradient centrifugation method optimized in our laboratory as previously published (22).
Experimental design and sampling.
Fourteen animals receiving the monovalent O1/Campos strain FMD vaccine were used to assess the existence of local FMDV-specific ASC prior to (n = 4) and after (n = 10) aerosol infection with the homologous strain at 30 days postvaccination (dpv). Two vaccinated animals were euthanized and necropsied at times before infection (7 and 29 dpv) and then at 2, 3, 4, 5, and 6 days postinfection (dpi). Heparinized whole-blood and serum samples were collected from the jugular vein of all surviving animals at 0, 7, 21, and 29 dpv and daily between days 2 and 6. Also, at each time starting at 7 dpv, different lymphoid organs were obtained postmortem from each animal: left prescapular lymph node (PSL), mandibular lymph nodes (ML), medial and lateral retropharyngeal lymph nodes (MRL and LRL, respectively), pharyngeal tonsil (PhT), tracheobronchial lymph nodes (TBL), and spleen (SP). All lymphoid organs were collected aseptically and placed in ice-cold wash buffer (RPMI 1640, 10 mM HEPES, 106 U/ml penicillin G sodium, 700 mg/ml streptomycin, and 500 mg/ml gentamicin) until processing.
The remaining two naive animals were utilized in the memory B-cell experiments and sampled for heparinized blood and serum at 30 days after primary vaccination. Animals were euthanized and prescapular, mandibular, and tracheobronchial lymph nodes also were procured from these animals at that same time postvaccination. All three multivaccinated animals were sampled for heparinized blood 4 days after the last revaccination.
Purification of MNC.
Mononuclear cell (MNC) suspensions were obtained from lymphoid tissues as previously described (22). Peripheral blood MNC (PBMC) were isolated from heparinized whole-blood samples and centrifuged at 800 × g for 20 min at 4°C. Buffy coats were collected and seeded on top of Ficoll Histopaque solutions (1,083 g/ml; BD, New Jersey, USA) and centrifuged at 800 × g for 45 min at 20°C by following the manufacturer's instructions. Finally, cell suspensions were washed 2 times and pellets were resuspended in complete medium (wash buffer with 10% bovine fetal serum), and their viability was determined by trypan blue exclusion (>95%).
Serology assays.
Neutralizing antibodies were detected by a microplate virus neutralization assay modified in our laboratory (22). Neutralizing antibody titers were expressed as the TCID50 neutralized by the diluted serum samples (1:32) according to the Reed and Muench method (23). Isotype profiles of FMDV-specific bovine antibodies were determined by an indirect ELISA according to Capozzo et al. (24), except that sheep-anti-bovine IgG1 and IgG2 horseradish peroxidase (HRP)-conjugated antibodies were used (1:750) and sheep anti-bovine IgM HRP-conjugated antibody (1:5000) was used (BD-Serotec, Oxford, United Kingdom). Isotype antibody titers were expressed as the highest dilution of the serum reaching an optical density (OD) equal to the mean OD obtained from 25 negative sera ±2 standard deviations (SD).
FMDV-specific ASC detection.
Anti-FMDV ASC were detected by means of a virus-specific ASC-enzyme-linked immunosorbent spot (ELISPOT) assay as previously reported (22). Spots corresponding to ASC were visualized and enumerated manually under a stereomicroscope, and results were expressed as the mean numbers ± standard errors of the means (SEM) of FMDV-specific ASC per 5 × 105 total MNC in triplicate wells.
FMDV-specific memory B-cell detection.
To study the presence of FMDV-specific memory B cells in peripheral blood and lymph nodes, MNC suspensions from these tissues were incubated for 6 days under culture conditions promoting the polyclonal differentiation of memory B lymphocytes into ASC cells while leading to the depletion of preexisting ASC. This step was followed by the detection of FMDV-specific ASC by ELISPOT assay. Differentiation of memory B cells into plasma B cells was performed as described by Grant et al. (25), with minor modifications. Briefly, MNC suspensions were incubated in T25 flasks (1 × 106 cells/ml) for 6 days with complete medium enriched with 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 50 μM β-mercaptoethanol and containing a mixture of pokeweed mitogen (5 μg/ml; Sigma), recombinant human interleukin-2 (IL-2; 20 ng/ml; Meridian Life Science Inc.), recombinant bovine IL-10 (2,200 IU/ml; Kingfisher Biotech Inc.), and anti-bovine CD40 monoclonal antibody (MAb) (2 μg/ml; ILA-158), kindly provided by Brian Charleston. In parallel, cultures without the stimulating cocktail were run as negative controls. After 6 days, MNCs from both stimulated and nonstimulated cultures were harvested, washed, and seeded onto nitrocellulose plates in sextuplicate wells coated with purified inactivated FMDV O1/Campos (10 μg/well) diluted in phosphate-buffered saline (PBS) (pH 7.5) or with PBS alone. From this stage on, the FMDV-specific ASC detection was performed as described above, except that the assay was revealed only with mouse anti-bovine IgG1 MAb (BD-Serotec, Oxford, United Kingdom). Results for each animal were expressed as the mean number of ASC in wells with the purified FMDV, corrected by subtraction of the mean ASC counts from the corresponding wells without the viral antigen.
FMDV RNA quantification.
The presence of FMDV genomic RNA in serum samples taken daily from oronasally infected cattle was assessed by a real-time quantitative RT-PCR (qRT-PCR), performed as previously described (22). PCR primers and standard plasmid carrying the FMDV 3D target sequence were kindly provided by Guido König.
Statistical analysis.
Differences in mean FMDV-specific serum isotype titers at different time points and differences in the mean number of total ASC produced per isotype at different time points and with different organs were analyzed by two-way analysis of variance (ANOVA), and pair comparisons were carried out using a Bonferroni posttest. Analyses were performed using Graph Pad Prism 5.0 software (Graph Pad Software Inc.).
RESULTS
Progression of systemic adaptive antibody responses in FMDV-vaccinated cattle before and after experimental oronasal infection.
To analyze the progression of the systemic and local antibody response in FMDV-vaccinated cattle, 14 steers were intramuscularly immunized with a single-oil-emulsion vaccine comprised of 23.5 μg/dose of inactivated FMDV strain O1/Campos. Furthermore, 10 of them were infected with the homologous virus by the oronasal route at 30 dpv. All animals developed a solid protective immune response. None of the vaccinated and challenged animals showed symptoms of the disease between 2 and 6 dpi (n = 10), and accordingly, FMDV RNA could not be detected by qRT-PCR in any of the serum samples obtained between 1 and 6 dpi.
The development of systemic antibody responses in these animals was measured by different means. Circulating FMDV-neutralizing antibodies were measured before vaccination and at 7, 21, and 29 dpv in all surviving animals. Mean antibody titers reached 4.48 × 103 ± 1.91 × 103 TCID50 neutralized within 7 days after vaccination (n = 14) and then remained within titers of 103 to 104 TCID50 neutralized up to 29 dpv. Oronasal infection did not significantly modify neutralizing antibody titers, actually showing a slight decrease toward 6 dpi (Fig. 1).
FIG 1.
Time course of virus-neutralizing antibodies in FMD vaccinated cattle before and after oronasal FMDV infection. Naive cattle were immunized with a monovalent FMDV O1/Campos vaccine and further infected by aerosolized FMDV (107 TCID50 per animal) at 30 dpv (indicated by an arrow). Serum samples were obtained at 0, 7, 21, and 29 dpv and between 1 and 6 days postinfection. Systemic antibody responses are expressed as mean numbers of TCID50 neutralized by the diluted sera (1:32) from all surviving animals at each time ± standard errors of the means.
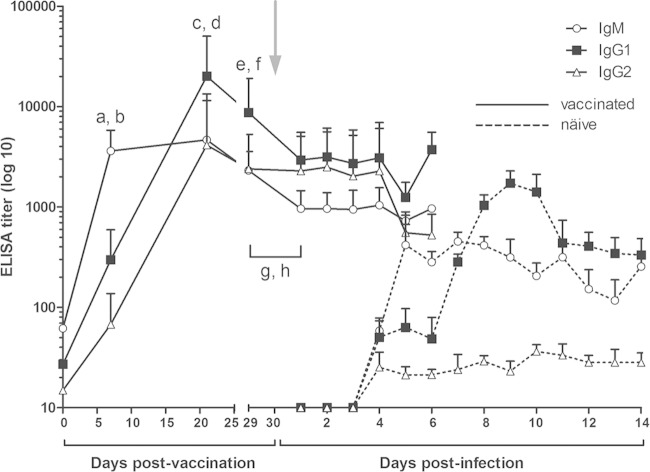
The maturation of the systemic antiviral antibody response also was assessed in terms of the immunoglobulin isotype composition. The isotype profiles of circulating antibodies before and after aerosol infection are shown in Fig. 2. One week after vaccination, the highest titers were obtained from the IgM subclass, followed by IgG1 and then IgG2 (P < 0.0001 in both cases). Three weeks after vaccination, all antibody titers increased and a switch in the isotype profile was evident with mean IgG1 titers significantly above those of IgM and IgG2 (P = 0.0004 and P = 0.0024, respectively).
FIG 2.
Isotypes of FMDV-specific serum antibodies in FMD-vaccinated cattle before and after oronasal FMDV infection. Each line corresponds to a particular isotype, and each point represents the average ELISA titer obtained for all animals assayed. Titers are expressed as the highest dilution of the serum reaching an OD value equal to 0.2 (mean value from 25 negative sera ± 2 SD). The time point for the oronasal infection is indicated by a gray arrow. For comparative purposes, isotype profiles obtained from naïve infected animals (previously published [22]) are shown as dotted lines. Significant differences in mean antibody titers are the following: a and b, IgM > IgG1 and IgM > IgG2 (P < 0.0001 in both cases) at 7 dpv, respectively; c and e, IgG1 > IgM at 21 (P = 0.0024) and 29 dpv (P = 0.0089), respectively; d and f, IgG1 > IgG2 at 21 (P = 0.0004) and 29 dpv (P = 0.0089), respectively; g, IgM at 29 dpv > IgM at 1 dpi (P = 0.0017); h, IgG1 at 29 dpv > IgG1 at 1 dpi (P = 0.026).
Just before oronasal infection, isotype profiles presented a very similar pattern, with mean IgG1 titers higher than those of IgM and IgG2 (P = 0.0089 in both cases). Interestingly, time course and isotype progression detected in FMD-vaccinated individuals resembled those already described by our group (22) for naive animals after oronasal infection with virulent FMDV (Fig. 2), except that IgG1 switch in infected animals could be verified earlier than that in vaccinated cattle, and IgG2 titers remained significantly below those for IgM and IgG1 for at least 2 weeks in naïve infected individuals. One day after oronasal infection, a significant decrease in mean antibody titers (which lasted at least until day 5 pi) was evident for IgG1 (P = 0.026) and IgM (P = 0.0017) but not for IgG2 (Fig. 2). These titers remained unaltered until day 4 postchallenge.
These results indicate that humoral responses elicited after systemic FMD vaccination shared similarities to those already reported for the oronasal FMDV infection (22) in terms of time course and progression of the isotype profiles. Subsequent oronasal infection of the vaccinated steers did not result in disease, but it was followed by a slight drop in neutralizing antibody titers, which was correlated with a transient decrease in IgM and IgG1 FMDV-specific antibodies.
Mucosal adaptive antibody responses in FMDV-vaccinated cattle before and after oronasal infection.
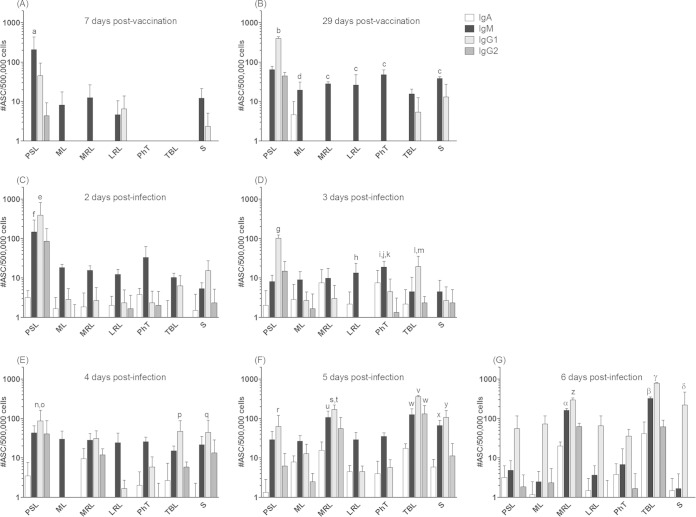
The assessment of a local FMDV-specific antibody response occurring along the respiratory tract of vaccinated cattle was accomplished by detecting the presence of mucosal antibody-producing B cells by ASC-ELISPOT assay. The assay was performed at early (7 dpv) and late (29 dpv) times postvaccination and after oronasal infection with the homologous virus (n = 2 at each time). As is shown in Fig. 3, systemic vaccination elicited a rapid and strong stimulation in the PSL ipsilateral to the immunization site (255 ± 155 ASC/5 × 105 total MNC), which increased almost two times in magnitude at 29 dpv (503 ± 17.5 ASC/5 × 105 total MNC). Interestingly, several lymphoid tissues draining the respiratory mucosa (ML, MRL, and LRL) revealed the presence of low but significant numbers of FMDV-specific ASC at 7 dpv. This phenomenon was somewhat enhanced in magnitude and expanded to all lymphoid tissues at 29 dpv, even to those such as TBL, which were far from the inoculation site. Therefore, parenteral immunization induced the early presence of FMDV-specific antibody responses in the respiratory tract that was sustained and extended at least up to 29 dpv.
FIG 3.
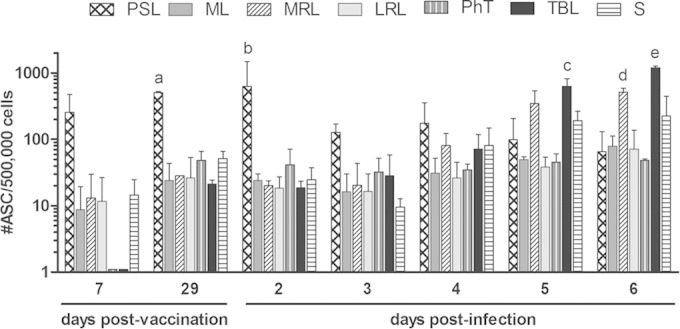
Detection of FMDV-specific antibody-secreting cells in lymphoid organs of the respiratory tract from vaccinated and further infected cattle. Prescapular (PSL) and mandibular lymph nodes (ML), pharyngeal tonsils (PhT), lateral (LRL) and medial retropharyngeal lymph nodes (MRL), tracheobronchial lymph nodes (TBL), and spleen (S) from FMD-vaccinated bovines were processed and assayed by the FMDV-ASC ELISPOT assay at 7 and 29 dpv and between 2 and 6 days postoronasal infection. Results are expressed as the number of FMDV-specific ASC per 5 × 105 extracted MNC, and each bar represents the average value for 2 individuals ± standard errors of the means. Significant differences in mean ASC numbers/5 × 105 total MNC were the following: a, PSL > ML, MRL, LRL, PhT, TBL, and S (P < 0.05) at 29 dpv; b, PSL > ML, MRL, LRL, PhT, TBL, and S (P < 0.01) at 2 dpi; c, TBL > PSL, ML, LRL, and PhT (P < 0.001) at 5 dpi; d, TBL > S (P < 0.01) at 5 dpi; e, MRL > PSL, ML, LRL, and PhT (P < 0.05) at 6 dpi; and f, TBL > PSL, ML, MRL, LRL, PhT, and S (P < 0.001) at 6 dpi.
The predominance of PSL also was observed soon after oronasal infection (2 dpi). Starting at 3 dpi, however, the mean number of FMDV-specific ASC in this tissue declined, while at the same time an increasing stimulation of some of the lymphoid tissues draining the respiratory system also was evident (Fig. 3). Total anti-FMDV ASC in TBL increased ∼42 times between 3 and 6 days after infection. Five days after infection, the mean number of FMDV-specific ASC in TBL (draining the lower respiratory tract) were significantly above those of PSL, ML, LRL, PhT, and SP (P < 0.01), and 1 day later, TBL ASC counts were higher than those of all the other tissues studied (P < 0.001) (Fig. 3). Similarly, mean anti-FMDV ASC counts in MRL (draining the upper respiratory tract) were raised ∼25 times from 3 to 6 dpi, and they were significantly higher than those in the remaining tissues studied, except for TBL, at 6 dpi (P < 0.05) (Fig. 3). The spleen also exhibited a rise in the total amount of FMDV-specific ASC from 3 to 6 dpi (an approximately 20-fold increase). The remaining lymphoid tissues from the respiratory tract (ML, LRL, and PhT) did not show significant variations in total ASC numbers after oronasal infection. Thus, the entrance of the challenge virus into the respiratory system stimulated the rapid growth of the preexisting vaccine-induced local antibody response, involving lymph nodes which drain early replication sites described for the virus entering through the aerogenous route (9).
Immunoglobulin isotype profiles of the local adaptive FMDV-specific antibody responses in cattle after FMD vaccination and further oronasal infection of vaccinated animals.
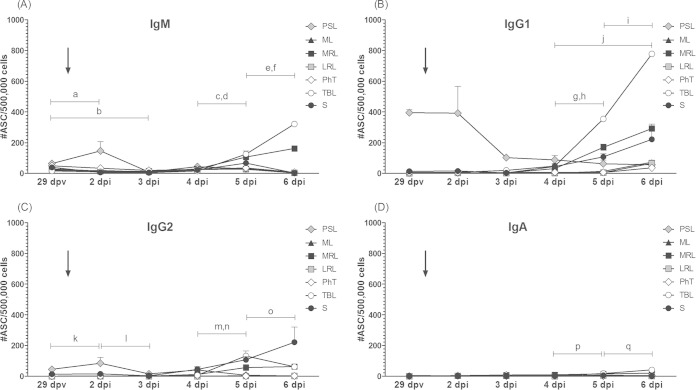
We next studied the isotype composition of anti-FMDV-specific antibody responses localized in lymphatic tissues using the ASC-ELISPOT assay. Seven days after vaccination, IgM was the dominant isotype among anti-FMDV ASC in PSL (205.3 ± 92.4 ASC/5 × 105 MNC), significantly above the level of IgG1 (P < 0.001) (Fig. 4A). FMDV-specific ASC from IgG2 or IgA isotypes could not be detected at this time. Similarly, the low number of FMDV-specific ASC observed in other lymphoid tissues at this time mostly corresponded to IgM-secreting cells.
FIG 4.
Isotype profiles of the anti-FMDV ASC developed in vaccinated cattle before and after FMDV aerosol exposure. Mononuclear cells were purified from the same lymphoid tissues as that described in the legend to Fig. 3 and assayed by the FMDV-ASC ELISPOT assay using monoclonal (IgG1 and IgG2) or polyclonal (IgM and IgA) antibodies against bovine immunoglobulin isotypes as probes. Each panel (A to G) represents a particular time point, and results are expressed as described in the legend to Fig. 3 for each particular isotype and organ. Lowercase letters above each bar indicate significant differences with other isotypes in the same tissue and time point (Table 1).
Just before oronasal infection (Fig. 4B), a class switch was clear in PSL, suggesting anti-FMDV IgG1 ASC counts (395.3 ± 18.9) were significantly greater than those of IgM and IgG2 (P < 0.001). Again, IgA ASC were undetectable. At the same time, most of the anti-FMDV ASC within the lymphoid organs related to the respiratory system consisted of IgM-producing B lymphocytes (Fig. 4B).
Soon after aerosol infection (2 dpi), isotype profiles displayed patterns similar to those prior to the challenge, except for lymphoid tissues draining the respiratory tract, which also showed low levels of IgG1 and IgA FMDV-specific ASC (Fig. 4C). Starting 3 days postinfection, the first changes were evident. Mean IgG1 levels in PSL at 3 dpi were significantly lower than those at 29 dpv (P < 0.001), and they remained below the ASC counts observed before vaccination (P < 0.001 for all times), with a slight but constant decrease up to 6 dpi (Fig. 4B).
Progression of anti-FMDV IgG1 ASC responses were the opposite in TBL. From 3 dpi, the average number of IgG1-producing cells in this tissue augmented daily (P < 0.001) to reach a mean value of 778 ± 17.9 ASC/5 × 105 MNC at 6 dpi, significantly above the rest of the organs and higher than that of the other isotypes in TBL at that time (P < 0.001) (Fig. 4D to G and 5B). A similar observation for IgG1, but starting after 4 dpi, also could be detected in MRL and spleen, reaching mean IgG1 ASC values of 292 ± 16.4 and 221 ± 98.8 ASC/5 × 105MNC, respectively (Fig. 4E to G and 5B). The other lymphoid tissues assayed (ML, LRL, and PhT) also seemed to experience a proliferation of IgG1-secreting cells but at a much lower magnitude and only after 5 dpi (Fig. 4G). FMDV-specific IgG2-secreting lymphocytes also increased their mean values from 4 to 5 dpi in TBL and MRL (P < 0.001 and P < 0.01, respectively) and from 5 to 6 dpi exclusively in TBL (P < 0.001). Significantly, IgM-secreting cells augmented their counts daily between 4 and 6 dpi exclusively in TBL and MRL (P < 0.001 and P < 0.01) (Fig. 5A). Consequently, IgM ASC in these two tissues were second in magnitude, above IgA and IgG2 ASC at 5 dpi and 6 dpi (Fig. 4F and G and Table 1). Once again, FMDV-specific IgA-secreting cells (Fig. 5D) did not demonstrate significant variations after aerosol infection, except for a slight increase in TBL from 4 to 5 dpi (P < 0.01) and from 5 to 6 dpi (P < 0.001). Table 1 comprises a full description of the significant differences found in the mean number of FMDV-specific ASC among isotypes for each organ and time point assayed.
FIG 5.
Effect of the oronasal infection on the time course of FMDV-specific ASC in different lymphoid tissues from FMD-vaccinated cattle. Each panel presents the time progression of the mean anti-FMDV ASC counts for each particular isotype. Tissues and results are expressed as described in the legend to Fig. 3. Arrows indicate the oronasal infection time point. Selected significant differences in mean FMDV-specific ASC numbers/5 × 105 total MNC are represented in each graph as letters in lowercase. (A) a, TBL and MRL levels at 4 dpi were lower than those at 5 dpi (P < 0.001); b, S at 4 dpi < 5 dpi (P < 0.05); c, TBL and S at 5 dpi < 6 dpi (P < 0.001); d, MRL at 5 dpi < 6 dpi (P < 0.01). (B) e, TBL at 4 dpi < 5 dpi (P < 0.001); f, MRL at 4 dpi < 5 dpi (P < 0.05); g, TBL at 5 dpi < 6 dpi (P < 0.001); h, S at 4 dpi < 6 dpi (P < 0.01). (C) i, TBL at 4 dpi < 5 dpi (P < 0.001); j, MRL at 4 dpi < 5 dpi (P < 0.01); k, TBL at 5 dpi < 6 dpi (P < 0.001). (D) l, TBL at 4 dpi < 5 dpi (P < 0.01); m, TBL at 5 dpi < 6 dpi (P < 0.001).
TABLE 1.
Significant differences in FMDV-specific ASC mean numbers among immunoglobulin isotypes for each time point and organ assayed
| Tissue | Significant differences by time postvaccination (dpv) or -infectiona (dpi) |
||||||
|---|---|---|---|---|---|---|---|
| 7 dpv | 29 dpv | 2 dpi | 3 dpi | 4 dpi | 5 dpi | 6 dpi | |
| PSL | IgM > IgG1 (P < 0.001) (a) | IgG1 > IgM and IgG2 (P < 0.001) (b) | IgG1 > IgM, IgG2, and IgA (P < 0.001) (e); (f) IgM > IgA (P < 0.05) | IgG1 > IgM, IgG2 and IgA (P < 0.001) (g) | IgG1 > IgM and IgG2 (P < 0.05) (n); IgG1 > IgA (P < 0.001) (o) | IgG1 > IgG2 and IgA (P < 0.01) (r) | |
| ML | IgM > IgG1 and IgG2 (P < 0.05) (d) | ||||||
| MRL | IgM > IgG1, IgG2, and IgA (P < 0.001) (c) | IgG1 > IgM (P < 0.01) (s); IgG1 > IgG2 and IgA (P < 0.001) (s); IgM > IgG2 and IgA (P < 0.01) (u) | IgG1 > IgM, IgG2, and IgA (P < 0.001) (z); IgM and IgG2 > IgA (P < 0.001) (α) | ||||
| LRL | IgM > IgG1, IgG2, and IgA (P < 0.001) (c) | IgM > IgG1, IgG2 and IgA (P < 0.05) (h) | |||||
| PhT | IgM > IgG1, IgG2 and IgA (P < 0.001) (c) | IgM > IgG1 (P < 0.01) (i); IgM > IgG2 (P < 0.001) (j); IgM > IgA (P < 0.05) (k) | |||||
| TBL | IgG1 > IgM (P < 0.01) (l); IgG1 > IgG2 and IgA (P < 0.001) (m) | (p) IgG1 > IgG2 and IgA (P < 0.01) | IgG1 > IgM, IgG2, and IgA (P < 0.001) (v); IgM and IgG2 > IgA (P < 0.001) (w) | IgG1 > IgM, IgG2, and IgA (P < 0.001) (γ); IgM and IgG2 > IgA (P < 0.001) (β) | |||
| S | IgM > IgG1, IgG2, and IgA (P < 0.001) (c) | IgG1 > IgA (P < 0.01) (q) | IgM > IgG2 and IgA (P < 0.05) (x); IgG1 > IgM, IgG2, and IgA (P < 0.001) (y) | IgG1 > IgM, IgG2, and IgA (P < 0.001) (δ) | |||
Lowercase letters in parentheses refer to the significant differences denoted in Fig. 4.
These experiments support an independent development of the anti-FMDV antibody responses occurring near the vaccination site, mostly coincident with the serum antibodies, and those taking place at distal lymphoid tissues, both before and after oronasal infection. Moreover, vaccine-induced immunity in distal lymph nodes consisted of active antibody-secreting B cells but also other resting B lymphocytes which were activated in large numbers to locally produce FMDV-specific ASC after aerosol infection.
Detection of FMDV-specific memory B cells in FMD-vaccinated cattle.
In an effort to identify the origin of the ASC locally detected in the respiratory tract after vaccination, the presence of FMDV-specific memory B lymphocytes was assayed in MNC from PSL, ML, or TBL or peripheral blood obtained from animals immunized with a commercial FMD vaccine. To this end, MNC suspensions from these tissues were incubated for 6 days under culture conditions promoting the depletion of ASC already present and the polyclonal differentiation of preexisting memory B cells into ASC, followed by the detection of FMDV-specific ASC by ELISPOT assay. Primo-vaccinated animals (n = 2) were tested at 30 dpv. Both animals had high antibody titers against FMDV O1/Campos by LPB-ELISA (data not shown). However, neither of these steers presented significant numbers of anti-FMDV ASC as a result of the differentiation from FMDV-specific memory B cells in any of the tissues assayed.
In order to maximize the chance of finding circulating FMDV-specific memory B cells, we repeated the experiment but used multivaccinated animals (n = 3) and performed the determination in peripheral blood 4 days after revaccination. In this case, all multivaccinated animals were consistently positive for FMDV-specific memory B cells in circulating blood but had low mean numbers: 8.4 ± 1.2 (animal 177), 14.6 ± 2.0 (animal 231), and 14.0 ± 4.5 (animal 232) per 5 × 105 total MNC. These results indicate that FMD vaccination effectively induces the presence of FMDV-specific memory B cells, although at a very low frequency.
DISCUSSION
Correlates of the immunity generated by FMD vaccines and protection against FMDV infections in cattle traditionally have been established based on the presence of specific serum antibody titers above defined thresholds. Such cutoff values are particular for each strain and were calculated from correlations involving large numbers of animals, combining results of in vivo challenge tests and in vitro serology assays (26, 27). This concept, however, diminishes the importance of other relevant aspects of immunity, such as the respiratory mucosal response that may play an important role during aerogenous FMDV infection in cattle.
In this work, systemic immunization with a high-payload monovalent FMD vaccine elicited neutralizing antibody kinetics and patterns of immunoglobulin isotypes resembling those observed after oronasal FMDV infection in naive individuals. Following a sharp increase during the first week postvaccination, neutralizing antibody titers reached a plateau that was maintained until 30 dpv. Accordingly, all animals exhibited a solid protection to the oronasal challenge performed at that time, showing neither symptoms of the disease nor detectable viremia. Interestingly, the slight reduction in mean neutralizing antibody titers observed after infection coincided with a significant decrease, 1 day after infection, in the mean titers of FMDV-specific IgM and IgG1, while IgG2 titers remained unchanged. On the one hand, this indicates that at least in some of the animals, circulating antibodies might have found virulent virus administered by the oronasal route. On the other hand, these observations are concurrent with previous findings by our group and others regarding the potential in vivo FMDV-neutralizing roles for the bovine IgM in naive cattle at early times postinfection (22) and the IgG1 isotype in vaccinated animals at later times postimmunization (24, 28).
Most significantly, we described for the first time that, besides the systemic and ASC responses in the draining lymph node, intramuscular (systemic) FMD vaccination in the neck also was capable of promoting local virus-specific ASC in respiratory lymphoid tissues in cattle. The detailed follow up of these coexisting adaptive responses, occurring in different sites but all initiated by the intramuscular vaccination, revealed differences between the local and systemic immunity and supported the idea of independent pathways in the antibody responses occurring near the vaccination site, mostly coincident with the serum antibodies, and those taking place at distal lymphoid tissues placed along the respiratory tract. The first of these differences was the important variation in the isotype composition observed from 7 to 29 dpv in the draining lymph node ASC and the systemic antibody responses compared to the rather invariant response, both in magnitude and isotype profile, registered in the distal lymph tissue ASC between 7 and 29 dpv.
One of the hypotheses to explain the existence of FMDV-specific ASC in lymphoid tissues distant from the vaccination spot is the local presence of vaccine viral antigen, alone or associated with antigen-presenting cells (29), and migration from the immunization site to stimulate the generation of ASC in situ. Our attempts to detect vaccine-derived antigens failed to demonstrate their existence in mandibular and tracheobronchial lymph nodes from vaccinated bovines at 30 dpv. Crude extracts from these organs rendered negative results (data not shown) for genome detection by PCR, capsid protein detection by immunoassays, and immunization experiments. The inoculation of tissue extracts in mice after waning of the primary immune response was induced by 50 ng of inactivated FMDV O1/Campos in PBS to detect secondary anti-FMDV responses by an indirect ELISA (29). While we cannot rule out the occurrence of virus antigen below the detection thresholds, the modest increase in magnitude and lack of significant changes in the isotype composition of the FMDV-specific ASC in these lymph nodes between 7 and 29 dpv were compatible with the absence of vaccine-derived viral antigens in these tissues. In contrast, ASC responses of the PSL at the vaccination spot, exposed to persisting antigen stimulation, underwent a significant isotype switch mainly toward IgG1 antibodies at 29 dpv.
Interestingly, oronasal infection in the vaccinated animals revealed additional differences between both types of responses. Further aerogenous infection, the early changes in isotype composition, and rapid increases in magnitude of the antibody responses triggered in the local respiratory lymph nodes, especially in TBL and MRL, contrasted with the lack of major modifications and the slow waning of the ASC responses in the draining PSL. Hence, it was evident that vaccine-induced immunity in distal lymph nodes not only consisted of active antibody-secreting B cells but also included other resting B lymphocytes which were activated in secondary lymphoid tissues to locally produce FMDV-specific ASC upon aerosol infection. Such immune reactions started around 3 to 4 days postinfection and followed closely the early tissue replication pattern already described for aerogenous FMDV infections in naive cattle (9). Also notably, these B-cell memory responses lead to a marked predominance of IgG1 ASC from 4 dpi but also to a significant growth in the IgM ASC counts in these organs, being the second isotype in magnitude from 4 to 6 dpi. These IgM-producing cells also could have resulted from the activation of new clones by the virulent FMDV entering the respiratory tract, as in naive animals, although we do not have direct evidence to support this hypothesis.
Our results do not allow us to determine the exact origin of the FMDV-specific B cells in distal lymph nodes. Nevertheless, the detection of circulating memory B cells in multivaccinated cattle, albeit at very low frequencies, suggests that these cells originated at the site of inoculation. Antigen-specific memory B cells can be retained in draining lymphoid sites together with follicular Th cells after subcutaneous protein immunization (30), and recirculation of antigen-specific B lymphocytes has been demonstrated after primo-vaccination with other immunogens in mice and humans. Conjugate polysaccharide-protein vaccine antigens carrying T-cell-dependent and -independent antigenic determinants, as described for whole FMDV particles (31, 32), are able to generate antibody responses and transient memory B cells in nasopharyngeal mucosa after systemic immunization in humans (33, 34). Other reports also showed that circulating antigen-specific B cells, some of which were memory B cells expressing surface markers enabling their recirculation to secondary lymphoid organs, also could be detected within 7 to 10 days following systemic immunization in mice (35, 36). It also is possible that memory B lymphocytes generated after local immunization can be recalled with a local challenge at a distal site in the presence of an inflammatory stimulus, permitting their entry into resting lymph nodes, from where they usually are excluded (37). Even when we cannot confirm any of these possibilities, the previous literature indicates that such mechanisms actually exist in other animal models, sharing similarities with our present observations.
These results provide new evidence and a more comprehensive understanding of the immune responses elicited in cattle by the existing inactivated FMD vaccines, offering new insights and posing questions about the actual mechanisms put in place in vaccinated bovines to prevent the development of the disease after aerogenous infection. It is worth noting that each time point determination for FMDV-specific ASC was performed on a different pair of bovines, and despite this potential source of variation, time course progressions for each isotype and organ proved to remain consistent, overcoming potential physiological and genetic differences among individuals. As a whole, our results indicate that FMD vaccination in cattle result in the circulation of virus-specific B lymphocytes, including memory B cells that differentiate to ASC soon after contact with the infective virus in secondary lymphoid tissues. Among others, an interesting aspect of this study is to investigate whether the magnitude of these local memory B-cell responses can be used as a correlate of the protective efficacy for new vaccines under development.
ACKNOWLEDGMENTS
This work was funded by the Agricultural Research Service Collaborative Agreement no. 58-1940-2-107F and the Instituto Nacional de Tecnología Agropecuaria FMD-Project, PNSA 1115052.
We thank Osvaldo Zábal for his invaluable work in the BSL-4 OIE facilities at the CICVyA–INTA and Daniel Romero, José Vallejos, and Ramón Escobar for their help in handling and caring for experimental animals. We also thank Andrea Ayude for her excellent technical and administrative assistance in our laboratory, Guido König for providing material and helpful guidance in qRT-PCR, and Claudia Perez-Beascoechea for preparing and providing the infectious FMDV. Special thanks also to Melanie Prarat for critical reading and editing of the manuscript. Finally, we express our gratitude to Cyril Gay for his continuous support for and interest in this collaborative effort.
REFERENCES
- 1.Alexandersen S, Mowat N. 2005. Foot-and-mouth disease: host range and pathogenesis. Curr Top Microbiol Immunol 288:9–42. [DOI] [PubMed] [Google Scholar]
- 2.Pinto AA. 2004. Foot-and-mouth disease in tropical wildlife. Ann N Y Acad Sci 1026:65–72. doi: 10.1196/annals.1307.008. [DOI] [PubMed] [Google Scholar]
- 3.Alexandersen S, Zhang Z, Donaldson AI, Garland AJ. 2003. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol 129:1–36. doi: 10.1016/S0021-9975(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 4.Thompson D, Muriel P, Russell D, Osborne P, Bromley A, Rowland M, Creigh-Tyte S, Brown C. 2002. Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Rev Sci Tech 21:675–687. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter TE, O'Brien JM, Hagerman AD, McCarl BA. 2011. Epidemic and economic impacts of delayed detection of foot-and-mouth disease: a case study of a simulated outbreak in California. J Vet Diagn Investig 23:26–33. doi: 10.1177/104063871102300104. [DOI] [PubMed] [Google Scholar]
- 6.Perry BD, Rich KM. 2007. Poverty impacts of foot-and-mouth disease and the poverty reduction implications of its control. Vet Rec 160:238–241. doi: 10.1136/vr.160.7.238. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZD, Kitching RP. 2001. The localization of persistent foot and mouth disease virus in the epithelial cells of the soft palate and pharynx. J Comp Pathol 124:89–94. doi: 10.1053/jcpa.2000.0431. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Alexandersen S. 2004. Quantitative analysis of foot-and-mouth disease virus RNA loads in bovine tissues: implications for the site of viral persistence. J Gen Virol 85:2567–2575. doi: 10.1099/vir.0.80011-0. [DOI] [PubMed] [Google Scholar]
- 9.Arzt J, Pacheco JM, Rodriguez LL. 2010. The early pathogenesis of foot-and-mouth disease in cattle after aerosol inoculation: identification of the nasopharynx as the primary site of infection. Vet Pathol 47:1048–1063. doi: 10.1177/0300985810372509. [DOI] [PubMed] [Google Scholar]
- 10.Mackay D, Parida S, Paton D, Anderson J. 2004. Making a vaccinate-to-live policy a reality in foot-and-mouth disease. Dev Biol 119:261–266. [PubMed] [Google Scholar]
- 11.Poulin MC, Christianson WT. 2006. On-farm eradication of foot-and-mouth disease as an alternative to mass culling. Vet Rec 158:467–472. doi: 10.1136/vr.158.14.467. [DOI] [PubMed] [Google Scholar]
- 12.Sumption K, Domenech J, Ferrari G. 2012. Progressive control of FMD on a global scale. Vet Rec 170:637–639. doi: 10.1136/vr.e4180. [DOI] [PubMed] [Google Scholar]
- 13.Doel TR. 2003. FMD vaccines. Virus Res 91:81–99. doi: 10.1016/S0168-1702(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 14.Anderson EC, Doughty WJ, Anderson J. 1974. The effect of repeated vaccination in an enzootic foot-and-mouth disease area on the incidence of virus carrier cattle. J Hyg 73:229–235. doi: 10.1017/S0022172400024062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orsel K, Dekker A, Bouma A, Stegeman JA, de Jong MC. 2005. Vaccination against foot and mouth disease reduces virus transmission in groups of calves. Vaccine 23:4887–4894. doi: 10.1016/j.vaccine.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Cox SJ, Voyce C, Parida S, Reid SM, Hamblin PA, Hutchings G, Paton DJ, Barnett PV. 2006. Effect of emergency FMD vaccine antigen payload on protection, sub-clinical infection and persistence following direct contact challenge of cattle. Vaccine 24:3184–3190. doi: 10.1016/j.vaccine.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Pay TW, Hingley PJ. 1987. Correlation of 140S antigen dose with the serum neutralizing antibody response and the level of protection induced in cattle by foot-and-mouth disease vaccines. Vaccine 5:60–64. doi: 10.1016/0264-410X(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 18.Kapil S, Ahuja KL, Prasad S. 1987. Immunoglobulin profiles in nasal and buccal secretions from normal crossbred calves after vaccination with inactivated virus and/or experimental exposure to foot-and-mouth disease virus type Asia I. Adv Exp Med Biol 216B:1831–1838. [PubMed] [Google Scholar]
- 19.Francis MJ, Ouldridge EJ, Black L. 1983. Antibody response in bovine pharyngeal fluid following foot-and-mouth disease vaccination and, or, exposure to live virus. Res Vet Sci 35:206–210. [PubMed] [Google Scholar]
- 20.Pega J, Bucafusco D, Di Giacomo S, Schammas JM, Malacari D, Capozzo AV, Arzt J, Perez-Beascoechea C, Maradei E, Rodriguez LL, Borca MV, Perez-Filgueira M. 2013. Early adaptive immune responses in the respiratory tract of foot-and-mouth disease virus-infected cattle. J Virol 87:2489–2495. doi: 10.1128/JVI.02879-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Periolo OH, Seki C, Grigera PR, Robiolo B, Fernandez G, Maradei E, D'Aloia R, La Torre JL. 1993. Large-scale use of liquid-phase blocking sandwich ELISA for the evaluation of protective immunity against aphthovirus in cattle vaccinated with oil-adjuvanted vaccines in Argentina. Vaccine 11:754–760. doi: 10.1016/0264-410X(93)90261-U. [DOI] [PubMed] [Google Scholar]
- 22.Pacheco JM, Arzt J, Rodriguez LL. 2010. Early events in the pathogenesis of foot-and-mouth disease in cattle after controlled aerosol exposure. Vet J 183:46–53. doi: 10.1016/j.tvjl.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Reed LJ, Muench H. 1938. A simple method to estimate fifty percent end points. Am J Hyg 27:493–497. [Google Scholar]
- 24.Capozzo AV, Periolo OH, Robiolo B, Seki C, La Torre JL, Grigera PR. 1997. Total and isotype humoral responses in cattle vaccinated with foot and mouth disease virus (FMDV) immunogen produced either in bovine tongue tissue or in BHK-21 cell suspension cultures. Vaccine 15:624–630. doi: 10.1016/S0264-410X(96)00284-8. [DOI] [PubMed] [Google Scholar]
- 25.Grant CF, Lefevre EA, Carr BV, Prentice H, Gubbins S, Pollard AJ, Charreyre C, Charleston B. 2012. Assessment of T-dependent and T-independent immune responses in cattle using a B cell ELISPOT assay. Vet Res 43:68. doi: 10.1186/1297-9716-43-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maradei E, La Torre J, Robiolo B, Esteves J, Seki C, Pedemonte A, Iglesias M, D'Aloia R, Mattion N. 2008. Updating of the correlation between lpELISA titers and protection from virus challenge for the assessment of the potency of polyvalent aphtovirus vaccines in Argentina. Vaccine 26:6577–6586. doi: 10.1016/j.vaccine.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Robiolo B, Grigera PR, Periolo OH, Seki C, Bianchi T, Maradei E, La Torre JL. 1995. Assessment of foot and mouth disease vaccine potency by liquid-phase blocking ELISA: a proposal for an alternative to the challenge procedure in Argentina. Vaccine 13:1346–1352. doi: 10.1016/0264-410X(94)00084-Z. [DOI] [PubMed] [Google Scholar]
- 28.Mulcahy G, Gale C, Robertson P, Iyisan S, DiMarchi RD, Doel TR. 1990. Isotype responses of infected, virus-vaccinated and peptide-vaccinated cattle to foot-and-mouth disease virus. Vaccine 8:249–256. doi: 10.1016/0264-410X(90)90054-P. [DOI] [PubMed] [Google Scholar]
- 29.Wigdorovitz A, Zamorano P, Fernandez FM, Lopez O, Prato-Murphy M, Carrillo C, Sadir AM, Borca MV. 1997. Duration of the foot-and-mouth disease virus antibody response in mice is closely related to the presence of antigen-specific presenting cells. J Gen Virol 78(Part 5):1025–1032. [DOI] [PubMed] [Google Scholar]
- 30.Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. 2007. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol 8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 31.Carr BV, Lefevre EA, Windsor MA, Inghese C, Gubbins S, Prentice H, Juleff ND, Charleston B. 2013. CD4+ T-cell responses to foot-and-mouth disease virus in vaccinated cattle. J Gen Virol 94:97–107. doi: 10.1099/vir.0.045732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juleff N, Windsor M, Lefevre EA, Gubbins S, Hamblin P, Reid E, McLaughlin K, Beverley PC, Morrison IW, Charleston B. 2009. Foot-and-mouth disease virus can induce a specific and rapid CD4+ T-cell-independent neutralizing and isotype class-switched antibody response in naive cattle. J Virol 83:3626–3636. doi: 10.1128/JVI.02613-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke ET, Williams NA, Dull PM, Findlow J, Borrow R, Finn A, Heyderman RS. 2013. Polysaccharide-protein conjugate vaccination induces antibody production but not sustained B-cell memory in the human nasopharyngeal mucosa. Mucosal Immunol 6:288–296. doi: 10.1038/mi.2012.70. [DOI] [PubMed] [Google Scholar]
- 34.Clarke ET, Williams NA, Findlow J, Borrow R, Heyderman RS, Finn A. 2013. Polysaccharide-specific memory B cells generated by conjugate vaccines in humans conform to the CD27+IgG+ isotype-switched memory B Cell phenotype and require contact-dependent signals from bystander T cells activated by bacterial proteins to differentiate into plasma cells. J Immunol 191:6071–6083. doi: 10.4049/jimmunol.1203254. [DOI] [PubMed] [Google Scholar]
- 35.Inamine A, Takahashi Y, Baba N, Miyake K, Tokuhisa T, Takemori T, Abe R. 2005. Two waves of memory B-cell generation in the primary immune response. Int Immunol 17:581–589. doi: 10.1093/intimm/dxh241. [DOI] [PubMed] [Google Scholar]
- 36.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. 2005. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med 201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shenoy GN, Chatterjee P, Kaw S, Mukherjee S, Rathore DK, Bal V, Rath S, George A. 2012. Recruitment of memory B cells to lymph nodes remote from the site of immunization requires an inflammatory stimulus. J Immunol 189:521–528. doi: 10.4049/jimmunol.1102814. [DOI] [PubMed] [Google Scholar]