Abstract
Inhibition of T-cell responses in tumor microenvironments by myeloid-derived suppressor cells (MDSCs) is widely accepted. We demonstrated augmentation of monocytic MDSCs whose suppression of not only T-cell, but also B-cell, responsiveness paralleled the immunodeficiency during LP-BM5 retrovirus infection. MDSCs inhibited T cells by inducible nitric oxide synthase (iNOS)/nitric oxide (NO), but uniquely, inhibition of B cells was ∼50% dependent each on iNOS/NO and the MDSC-expressed negative-checkpoint regulator VISTA. Blockade with a combination of iNOS/NO and VISTA caused additive or synergistic abrogation of MDSC-mediated suppression of B-cell responsiveness.
TEXT
Myeloid-derived suppressor cells (MDSCs) inhibit the generation and/or effector activities of antitumor T-cell responses (1, 2). Limited reports indicate MDSC regulation of autoimmunity (3) and selected viral infections (4–7), including only recently, retroviral infections and murine and human AIDS (8–11). Murine MDSCs are relatively immature and heterogeneous, but all express Gr-1 and CD11b. Two subsets, monocytic Ly6G+/−/lo Ly6C+/hi and granulocytic/polymorphonuclear-leukocyte-like Ly6G+/hi Ly6C+/−/lo, use differential suppressive mechanisms to inhibit T cells (12, 13). MDSC inhibition of B-cell responses is studied rarely, if at all.
Retroviruses are adept at co-opting immunoregulatory mechanisms. Human immunodeficiency virus type 1/simian immunodeficiency virus induction of PD-1 downregulates T effector cells (14, 15), and murine Friend retrovirus infection-induced PD-1 and Tim-3 affect pathogenesis and retroviral loads (16, 17), sometimes with “functionless” T cells occurring (14, 15). Viral infections can also induce CD4+ FoxP3+ regulatory T (Treg) cells (18), including in LP-BM5 murine retroviral pathogenesis (19–21). By 5 weeks postinfection (wpi), LP-BM5 causes profound immunodeficiency, with increased susceptibility to “opportunistic” infections and B-cell lymphomas (22, 23). Immunodeficiency requires “pathogenic” CD4+ T-effector cell expression of CD154 and ligation of CD40 (22, 24, 25), and PD-1/PD-L1 and IL-10 downregulate effector T-cell activity (21, 26).
A CD11b+ FcRγIII/II+ myeloid cell subset expands during LP-BM5 pathogenesis (26, 27). We recently defined these monocytic MDSCs as Gr-1+ Ly6C+/hi Ly6G+/−/low CD11b+ with strong ex vivo inhibition of T- and B-cell responses used to measure LP-BM5-induced immunodeficiency (10). This robust direct MDSC-induced inhibition of B-cell responsiveness is novel for murine retrovirus-induced immunosuppression, if not generally. Also, a new negative-checkpoint regulatory ligand, VISTA (V-domain Ig suppressor of T-cell activation) (28–30), also designated PD-1H (31), with homology to PD-L1 has been defined. VISTA can be highly upregulated on myeloid-derived cells and can inhibit T-cell responses in autoimmunity and antitumor immunity in a nonredundant manner with PD-L1 (28).
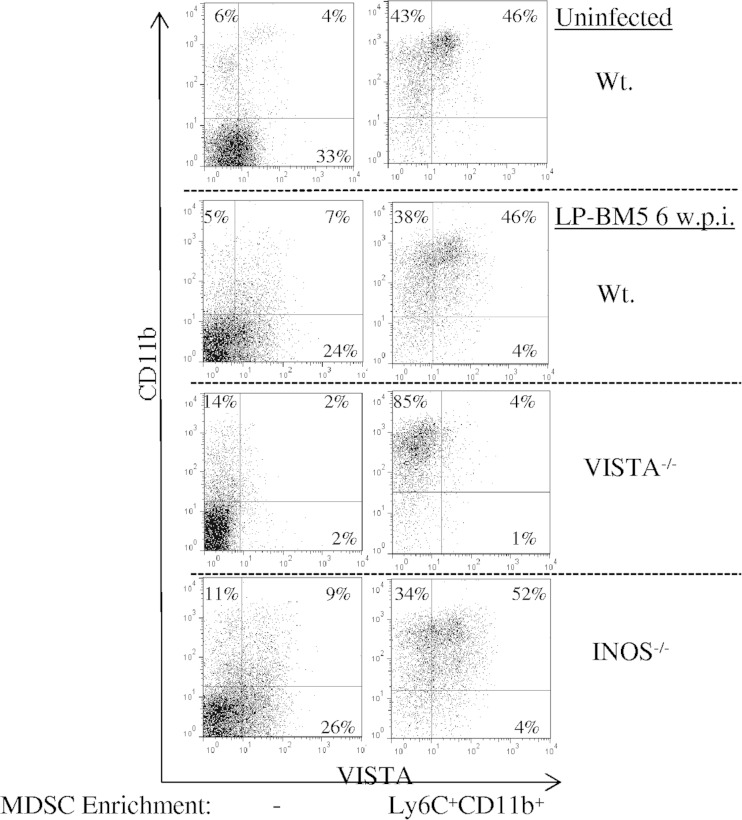
At 5 wpi with LP-BM5, regarding cell surface VISTA expression, the percentage of VISTA+ spleen cells had not expanded but VISTA mean fluorescence intensity (MFI) increased and the shape of the positive peak changed, consistent with the dominance of CD4 T-cell-expressed VISTA in uninfected B6 mice (28) and with CD11b+ VISTA+ cell expansion. Comparison of cells from wild-type (WT), iNOS−/−, and VISTA−/− B6 mice (32) at 5 wpi confirmed VISTA and CD11b coexpression by the highly enriched monocytic Ly6C+ MDSC population we have previously described (10), as depicted in the representative experiment in Fig. 1 (consistent with the average MFI and percent positivity over three experiments [legend to Fig. 1]). Of note, there was minimal contamination with other cells, particularly CD4+ Treg cells (legend to Fig. 1). Interestingly, similar monocytic MDSCs could be isolated from the spleens of uninfected mice. These MDSCs expressed levels of VISTA approaching (and, over three repeat experiments, not significantly statistically significantly different from) that of their counterparts from infected mice—with respect to both the percent positive and the MFI (legend to Fig. 1). However, such MDSCs from uninfected mice were much less frequent in total cell numbers per spleen and, even compared on a per-cell basis, displayed substantially less suppressive activity—resulting in about 12-fold less MDSC suppressive function than MDSCs from infected mice (legend to Fig. 1).
FIG 1.
Surface expression of VISTA on unfractionated and Ly6C+ CD11b+ enriched spleen cells from B6 background strains of mice uninfected or infected for 5 weeks with LP-BM5 virus (5 × 104 ecotropic PFU) (33). Contamination with residual CD4+ FoxP3+ Treg cells is ∼1.1% of the enriched infected WT and INOS−/− MDSC preparations, with only ∼0.2% of the Ly6C+/hi CD11b+ MDSCs which provide the majority of the suppressive activity (not shown). The percentages shown are the proportions of monocytic MDSCs singly or doubly positive for VISTA (13F3) and CD11b (M1/70) expression. The MFI values for VISTA expression on the cells shown here in the upper right quadrants were as follows: WT uninfected/unfractionated, 33; WT uninfected/enriched, 36; WT infected/unfractionated, 30; WT infected/enriched, 56; INOS−/− infected/unfractionated, 32; INOS−/− infected/enriched, 56. The mean percentages for enriched MDSCs (MFIs) for VISTA expression, based on three independent experiments for each mouse strain or condition, were as follows: WT uninfected, 39% ± 8% (41 ± 9); WT infected, 42% ± 4% (60 ± 3); INOS−/− infected, 45% ± 8% (63 ± 6). In four independent experiments, an average of 2.75-fold fewer enriched, monocytic MDSCs were obtained from the spleens of uninfected (versus infected) mice, and on a per-cell basis, the MDSCs from uninfected mice were sufficiently less suppressive (4.3-fold) than those from LP-BM5-infected mice, so that there was approximately 12-fold less total suppressive activity.
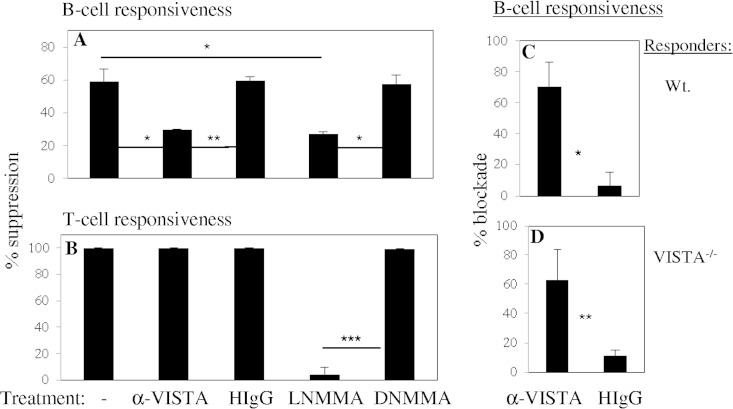
The possible mechanistic involvement of VISTA was compared to the known differential role of inducible nitric oxide synthase (iNOS)/nitric oxide (NO) in MDSC-mediated suppression (Fig. 2A and B). However, MDSC-mediated suppression of uninfected WT T cells was essentially completely dependent on iNOS/NO, as shown with the iNOS inhibitor NG-monomethyl l-arginine (l-NMMA). For B-cell responsiveness, l-NMMA blocked MDSC-mediated suppression by ∼50% (range, 40 to 65%), as previously shown (10), but an anti-VISTA monoclonal antibody (MAb) blocked WT MDSC-mediated suppression of only B-cell (by ∼50%), and not T-cell, responsiveness (Fig. 2A and B). We found the range of anti-VISTA MAb blocking centered around 65%, but a delta of ∼55%, by subtracting control hamster immunoglobulin effects (Fig. 2C), a level consistent with the reduced suppression observed with VISTA−/− MDSCs (see Fig. 4). Thus, VISTA appeared to serve differentially, relative to iNOS/NO, for MDSC-mediated suppression of T-cell versus B-cell responses. With VISTA−/− responder cells (Fig. 2D), the anti-VISTA MAb also showed highly significant (P = 0.003) but partial specific blocking, confirming that VISTA blocking was directed to the MDSCs; for clarity, follow-up experiments employed VISTA−/− responders. In experiments not shown (three of three), the anti-VISTA MAb also blocked the suppression of B-cell responses by iNOS−/− MDSCs—and, as expected, the blockade was greater than that obtained with WT MDSCs (see below).
FIG 2.
Distinct mechanistic requirements, involving iNOS/NO versus VISTA, differentiate LP-BM5-augmented Ly6C+ CD11b+ MDSC suppression of B-cell versus T-cell proliferation. Three-day cultures terminating with 6-h [3H]thymidine pulses were set up as previously reported (10). WT responder spleen cells stimulated with 50 μg/ml anti-CD40 antibody plus 10 ng/ml IL-4 (A) or 0.75 μg/ml concanavalin A (B) were cocultured with Ly6C+ CD11b+ MDSCs (10) left untreated or treated with anti-VISTA MAb 13F3 (80 μg/ml, the optimal concentration determined by dose-response experiments), 0.8 mM l-NMMA (AG Scientific, San Diego, CA), or analogous controls. The pattern of results presented is representative of two additional experiments for each stimulation. (C, D) Cocultures of WT (C) and VISTA−/− (D) responders with stimulation by anti-CD40 antibody plus IL-4, depicting the average percent blockade of suppression by anti-VISTA MAb-treated MDSCs. Each panel represents the mean values of three independent experiments. Error bars represent the standard deviations. Statistical significance levels: *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student t test, with the Holm-Bonferroni post hoc test when there are multiple comparisons).
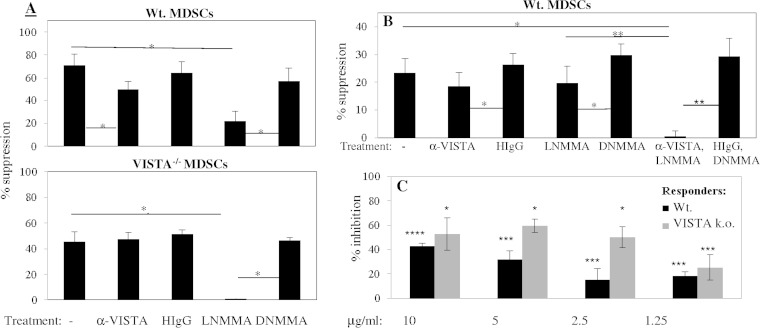
FIG 4.
Blockade of LP-BM5-augmented Ly6C+ CD11b+ MDSCs with iNOS/NO and anti-VISTA MAb combined decreases their suppression of ex vivo proliferation of B cells stimulated with anti-CD40 antibody and IL-4. Naive VISTA knockout responder cells were cultured with anti-VISTA MAb- or l-NMMA-treated Ly6C+ CD11b+ MDSCs (responder/suppressor [R/S] ratio, 4:1) of either WT (A, top) or VISTA−/− origin (A, bottom) or WT Ly6C+ CD11b+ MDSCs (R/S ratio, 6:1) treated with the anti-VISTA MAb, l-NMMA, or both (B). The pattern of results of additive/synergistic effects is representative of one additional experiment for both panels A and B. (C) Plate-bound VISTA-Ig-effected percent inhibition of splenic B-cell proliferation in response to LPS stimulation (compared to control mIgG2a). The pattern of results presented is representative of repeat experimentation with responder B cells stimulated with LPS, two additional experiments for WT mice, and one additional experiment for VISTA−/− mice or anti-CD40 antibody plus IL-4, two additional experiments for WT mice, and two additional experiments for VISTA−/− mice. All data were derived from 3-day cultures with terminal 6-h [3H]thymidine incorporation assessments. Error bars represent the standard deviations. Statistical significance levels: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (Student t test with the Holm-Bonferroni post hoc test).
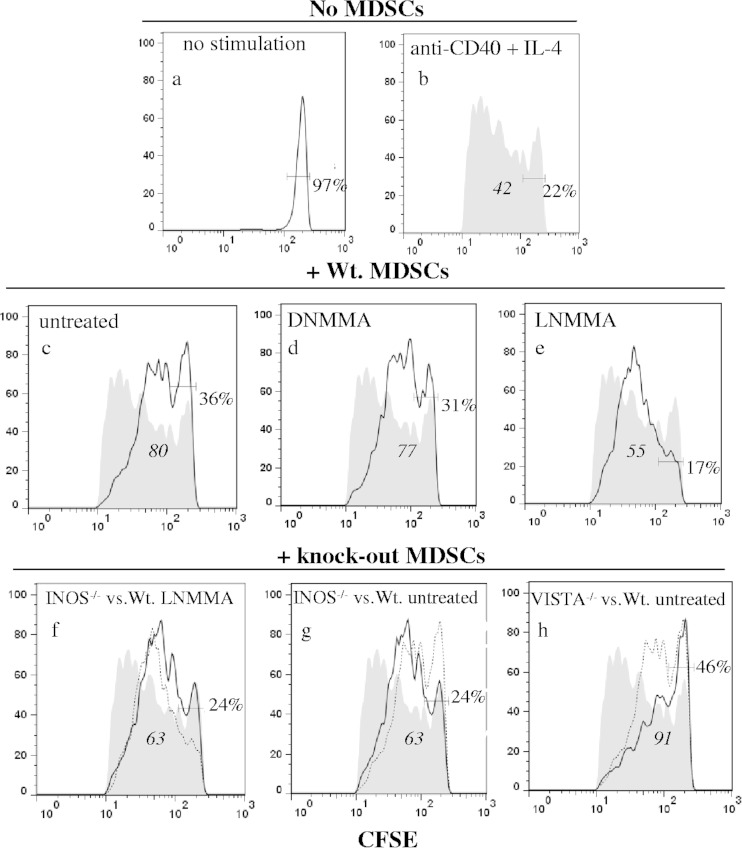
To directly compare the iNOS/NO and VISTA-dependent mechanisms, carboxyfluorescein succinimidyl ester (CFSE)-preloaded responder cells were stimulated in coculture with LP-BM5-augmented monocytic MDSCs (WT versus various knockout origin cells, Fig. 3). By flow cytometry focusing on CD19+ CFSE+ responder B cells, WT MDSC-mediated suppression of stimulated B cells was clear, as revealed by both increased percentages of undivided cells and CFSE geometric means (panels a and b versus panel c). By inclusion of the iNOS inhibitor l-NMMA, the proliferation curve was partially restored toward B cells stimulated in the absence of MDSCs (panel e versus panel b). Alternative use of iNOS−/− MDSCs led to a very similar CFSE profile, as shown by the overlaid proliferation curves of iNOS−/− MDSCs and WT MDSCs treated with l-NMMA (panel f). Full control B-cell responsiveness was not achieved by either inclusion of l-NMMA with WT MDSCs (panel e) or the use of iNOS−/− MDSCs (panel g), consistent with a second, VISTA-dependent suppressive pathway. Indeed, by using the MFI values and defining suppression as the difference between increased MFI for the WT MDSCs (panel c) and the control B-cell responsiveness in the absence of MDSCs (panel b), the proportion of the MDSC suppression due to iNOS/NO could be calculated as ∼45% (legend to Fig. 3). When VISTA−/− MDSCs were employed, (i) there was still strong, if not enhanced, MDSC-mediated suppression compared to that obtained with WT MDSCs (panel h versus panel b) and (ii) the blocking profiles of the proliferation curves resulting from genetic removal of MDSC VISTA versus inhibitor or knockout blockade of iNOS/NO were very different (panel h versus panels e to g). These findings confirmed the distinguishable nature of the VISTA versus the iNOS/NO-dependent components of MDSC-mediated suppression, with the results from the VISTA−/− MDSCs also compatible with a possible additional minor mechanism(s).
FIG 3.
Ly6C+ CD11b+ MDSCs from WT, INOS−/−, and VISTA−/− mice infected for 5 weeks with LP-BM5, each uniquely suppress B-cell proliferation of responder spleen cells. CFSE-labeled responder B cells (identified as CD19+) were cultured for 4 days with no stimulation (a) or anti-CD40 antibody plus IL-4 only (b) or with WT MDSCs that were left untreated (c) or treated with d-NMMA (d) or l-NMMA (e). The fully shaded control response in panel b is repeated throughout panels c to h for comparison. For direct comparisons, overlay panels were created for responders with INOS−/− MDSCs (dark line) versus l-NMMA-treated WT MDSCs (dotted line) (f), iNOS−/− MDSCs (dark line) versus WT untreated MDSCs (dotted line) (g), and VISTA−/− MDSCs (dark line) versus WT untreated MDSCs (dotted line) (h). Italicized values are the geometric MFIs of the CFSE+ cells under the dark line in panels b to h, and the nonitalicized values to the right within each panel are the percentages of undivided cells. Calculation for quantitative assessment of responder B-cell suppression: MFI 80 (WT MDSCs) − MFI 42 (control proliferation) = ΔMFI 38 (suppression due to WT MDSCs). As an example, to assess the effect of the INOS knockout on suppression (panel g), MFI 80 (WT MDSCs) − MFI 63 (INOS−/− MDSCs) = ΔMFI 17, or 17 fewer suppression units than for WT MDSCs (Δ38). Thus, Δ17/D38 = 45% (iNOS-associated suppression by WT MDSCs). The pattern of results presented is representative of one additional experiment.
Combined treatment experiments were conducted. We first employed MDSCs of WT versus VISTA−/− origin in the presence of the iNOS/NO inhibitor l-NMMA. As expected, suppression of B-cell responsiveness by WT MDSCs was partially blocked by either the anti-VISTA MAb or l-NMMA (Fig. 4A, top). But in the same experiment, MDSCs of VISTA−/− origin suppressed B-cell responsiveness in a manner that was blocked only by l-NMMA (Fig. 4A, bottom). And the extent of blocking was then essentially 100%, suggesting that the retained iNOS/NO pathway was the sole major suppressive mechanism. As a follow-up approach, WT MDSCs were treated with the anti-VISTA MAb, l-NMMA, or both reagents (Fig. 4B). Only when the involvement of both VISTA and iNOS/NO was interfered with was there an essentially complete blockade of suppression (Fig. 4B). Considering also repeat experimentation, additive, if not synergistic, blocking by simultaneously interfering with iNOS/NO and VISTA corroborated these two primary independent mechanisms of MDSC-mediated suppression of B-cell responses.
To independently assess the ability of VISTA to inhibit B-cell responsiveness, we employed a VISTA-Ig chimeric fusion protein (28) (legend to Fig. 4). As shown in the representative results in Fig. 4C, VISTA-Ig inhibited polyclonal B-cell responsiveness by 40 to 59%, in a titratable manner, relative to control mIg2a—for both WT and VISTA−/− B-cell responders. Considering repeat experimentation (legend to Fig. 4), such inhibition was observed upon stimulation either with anti-CD40 antibody plus interleukin-4 (IL-4) or with lipopolysaccharide (LPS), complete titration could be achieved, and there was no consistent difference in susceptibility between WT and VISTA−/− responder B cells to blocking by VISTA-Ig.
In conclusion, we describe here for the first time, to our knowledge, the involvement of the novel negative-checkpoint regulator VISTA in MDSC-mediated suppression—in particular, of B-cell responsiveness. Our recent finding (10) that LP-BM5-augmented MDSC-mediated suppression depends on neither PD-L1 nor PD-1 further underscores the uniqueness of VISTA-related function versus its closest relative by sequence homology, PD-L1 (28, 30). The differential involvement of VISTA in the suppression of B-cell versus T-cell responsiveness by the same population of monocytic MDSCs raises the possibility of functional/phenotypic MDSC subpopulations. In addition, the inability to block MDSC inhibition of T-cell polyclonal responses by anti-VISTA MAb treatment here is to be contrasted with previous results demonstrating substantial effects on T-cell function by anti-VISTA MAb blockade in in vitro and in vivo assays (28, 29). Whether this dichotomy relates to the different myeloid compartment cells studied (e.g., MDSCs here versus myeloid DCs), the differential strength of the stimulatory signal to the responder T cells, and/or differences in the experimental microenvironments from which the myeloid cells were derived is at present unclear but likely insightful.
ACKNOWLEDGMENTS
We thank Brent Berwin, Edward Usherwood, James Cripps, Megan O'Connor, Jessica Rastad, Whitney Fu, Cynthia Stevens, and Jiannan Li for many helpful discussions and technical assistance.
This work was supported by U.S. Public Health Service grant CA50157, a pilot grant from P30 GM10345 (both to W.R.G.), grant CA164225 (LW), and grant A1048667 (RJN); a Hitchcock Foundation Research grant (L.W.); the Wellcome Trust (R.J.N.); and the Medical Research Council Centre for Transplantation and Biomedical Research Center at King's College London (R.J.N.). Flow cytometry was performed at the Geisel School of Medicine at Dartmouth in the DartLab Flow Cytometry core facility, which was established by equipment grants from the Fannie E. Rippel Foundation, the NIH Shared Instrument Program, and the Geisel School of Medicine and is supported in part by a core grant (CA23108) from the National Cancer Institute to the Norris Cotton Cancer Center and NIH/NCRR COBRE P20/P30 grant GM10345 on Molecular, Cellular, and Translational Immunological Research (W.R.G.).
R.J.N. and L.W. are involved with ImmuNext Inc. and receive financial support for the anti-VISTA MAb development for immunotherapy.
REFERENCES
- 1.Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S. 2010. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen JL, Olson JK. 2009. Innate immune CD11b+ Gr-1+ cells, suppressor cells, affect the immune response during Theiler's virus-induced demyelinating disease. J Immunol 183:6971–6980. doi: 10.4049/jimmunol.0902193. [DOI] [PubMed] [Google Scholar]
- 4.Jeisy-Scott V, Davis WG, Patel JR, Bowzard JB, Shieh W, Zaki SR, Katz JM, Sambhara S. 2011. Increased accumulation and Th2 biased response to influenza A virus infection in the absence of TLR7 in mice. PLoS One 6:e25242. doi: 10.1371/journal.pone.0025242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S, Akbar SMF, Abe M, Hiasa Y, Onji M. 2011. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 166:134–142. doi: 10.1111/j.1365-2249.2011.04445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willmon C, Diaz RM, Wongthida P, Galivo F, Kottke T, Thompson J, Albelda S, Harrington K, Melcher A, Vile R. 2011. Vesicular stomatitis virus-induced immune suppressor cells generate antagonism between intratumoral oncolytic virus and cyclophosphamide. Mol Ther 19:140–149. doi: 10.1038/mt.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker JD, Sehgal I, Kousoulas KG. 2011. Oncolytic herpes simplex virus 1 encoding 15-prostaglandin dehydrogenase mitigates immune suppression and reduces ectopic primary and metastatic breast cancer in mice. J Virol 85:7363–7371. doi: 10.1128/JVI.00098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R. 2012. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS 26:F31–F37. doi: 10.1097/QAD.0b013e328354b43f. [DOI] [PubMed] [Google Scholar]
- 9.Macatangay BJC, Landay AL, Rinaldo CR. 2012. MDSC: a new player in HIV immunopathogenesis. AIDS 26:1567–1569. doi: 10.1097/QAD.0b013e328355e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green KA, Cook WJ, Green WR. 2013. Myeloid-derived suppressor cells in murine retrovirus-induced AIDS inhibit T- and B-cell responses in vitro that are used to define the immunodeficiency. J Virol 87:2058–2071. doi: 10.1128/JVI.01547-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, Chen X, Chen L, Zhang H, Tang X, Zhou J. 2013. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol 87:1477–1490. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peranzoni E, Zilo S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. 2010. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Talmadge JE, Gabrilovich DI. 2013. History of myeloid-derived suppressor cells. Nat Rev Cancer 13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJR, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 15.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takamura S, Tsuji-Kawahara S, Yagita H, Akiba H, Sakamoto M, Chikaishi T, Kato M, Miyazawa M. 2010. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J Immunol 184:4696–4707. doi: 10.4049/jimmunol.0903478. [DOI] [PubMed] [Google Scholar]
- 17.Zelinskyy G, Myers L, Dietze KK, Gibbert K, Roggendorf M, Liu J, Lu M, Kraft AR, Teichgraber V, Hasenkrug KJ, Dittmer U. 2011. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute Friend retrovirus infection but are highly cytotoxic and control virus replication. J Immunol 187:3730–3737. doi: 10.4049/jimmunol.1101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccirillo CA, Shevach EM. 2004. Naturally-occurring CD4+ CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol 16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Beilharz MW, Sammels LM, Paun A, Shaw K, van Eeden P, Watson MW, Ashdown ML. 2004. Timed ablation of regulatory CD4+ T cells can prevent murine AIDS progression. J Immunol 172:4917–4925. doi: 10.4049/jimmunol.172.8.4917. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Green WR. 2006. The role of CD4+ T cells in the pathogenesis of murine AIDS. J Virol 80:5777–5789. doi: 10.1128/JVI.02711-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Green WR. 2011. Immunotherapy of murine retrovirus-induced acquired immunodeficiency by CD4+ T regulatory cell depletion and PD-1 blockade. J Virol 85:13342–13353. doi: 10.1128/JVI.00120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yetter RA, Buller RML, Lee JS, Elkins KL, Mosier DE, Fredrickson TN, Morse HC III. 1988. CD4+ T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS). J Exp Med 168:623–635. doi: 10.1084/jem.168.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simard C, Klein SJ, Mak T, Jolicoeur P. 1997. Studies of the susceptibility of nude, CD4 knockout, and SCID mutant mice to the disease induced by the murine AIDS defective virus. J Virol 71:3013–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green KA, Crassi KM, Laman JD, Schoneveld A, Strawbridge RR, Foy TM, Noelle RJ, Green WR. 1996. Antibody to the ligand for CD40 (gp39) inhibits murine AIDS-associated splenomegaly, hypergammaglobulinemia, and immunodeficiency in disease-susceptible C57BL/6 mice. J Virol 70:2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green KA, Noelle RJ, Green WR. 1998. Evidence for a continued requirement for CD40/CD40ligand (CD154) interactions in the progression of LP-BM5 retrovirus-induced murine AIDS. Virology 241:260–268. doi: 10.1006/viro.1997.8970. [DOI] [PubMed] [Google Scholar]
- 26.Green KA, Okazaki T, Honjo T, Cook WJ, Green WR. 2008. The programmed death-1 and interleukin-10 pathways play a down-modulatory role in LP-BM5 retrovirus-induced murine immunodeficiency syndrome. J Virol 82:2456–2469. doi: 10.1128/JVI.01665-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoetig SM, Torrey TA, Naghashfar Z, McCarty T, Morse HC III. 2002. CD19 signaling pathways play a major role for murine AIDS induction and progression. J Immunol 169:5607–5614. doi: 10.4049/jimmunol.169.10.5607. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu L, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ. 2011. VISTA, a novel super family ligand that negatively regulates T cell responses. J Exp Med 208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Mercier L, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L. 2014. VISTA regulates the development of protective antitumor immunity. Cancer Res 74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS, Noelle RJ. 2014. VISTA is an immune checkpoint molecule for human T cells. Cancer Res 74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flies D, Wang S, Xu H, Chen L. 2011. Cutting edge: a monoclonal antibody specific for the programmed-death-1 homolog prevents graft-versus host disease in mouse models. J Immunol 187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, Forrest W, Ghilardi N, Oravecz T, Platt KA, Rice DS, Hansen GM, Abuin A, Eberhart DE, Godowski P, Holt KH, Peterson A, Zambrowicz BP, de Sauvage FJ. 2010. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol 28:749–755. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 33.Rowe WP, Pugh WE, Hartley JW. 1970. Plaque assay techniques for murine leukemia viruses. Virology 42:1136. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]