Abstract
Thunbergia laurifolia Lindl (TL) has been traditionally used as an antidote, anti-inflammatory, and anti-drug addiction. This study investigated the burn wound healing activity of TL leaf extract (TLL) from supercritical CO2 extraction in rats. The extract was prepared to 2.5%, 5%, and 10% gel (TLL gel). Rats were induced to second-degree burn wounds. They were randomly divided into six groups (six rats/group), which five groups were topically applied gel base, 1% silver sulfadiazine gel, 2.5%, 5%, and 10% TLL gel, respectively, for 14 days. Six untreated burn rats were used as the control group. The rats in each group were evaluated for wound healing rate, histological parameters, and wound collagen content. Rats treated with 10% TLL gel had a higher wound healing rate than rats in the control and untreated groups. An increase in collagen content, which indicates good regeneration of wound skin, was observed in the TLL treated rats from a pathological study by Masson's trichrome and collagen content assay. The results from this study suggest that T. laurifolia leaf extract obtained by supercritical CO2 extraction promotes the recovery of wound skin by shortening the inflammation phase, increasing collagen content, and stimulating fibroblasts proliferation and migration in wound healing.
Keywords: Supercritical CO2 extract, Thunbergia laurifolia, wound healing
INTRODUCTION
A burn is an injured skin or tissues. The severity of burn depends on the depth of skin damage.[1] Wound healing process is divided into inflammatory phase, proliferative phase, and remodeling phase.[2,3,4,5,6] Accelerating wound healing is a challenge in the management of burn wounds. Some medicinal plants such as Aloe vera and Centella asiatica have been used topically as alternative medicines for burn wound care.[7,8,9]
Thunbergia laurifolia has been traditionally used as an antidote.[10,11] It is used to treat dysmenorrheal, deafness, and as a poultice for cuts and boil.[12] Reported activities of T. laurifolia leaves include antioxidant, anti-inflammatory, anti-drug addiction, antidote, hepatoprotective, and anti-diabetic activities.[13,14,15,16,17,18,19] We intended to explore the wound healing effect of these leaves.
MATERIALS AND METHODS
Plant material
T. laurifolia leaves were collected from Sra Kaew Province, Thailand in July 2012. The leaves were authenticated by Dr. Chanai Noysang (Thai Traditional Medicine College, Rajamangala University of Technology Thanyaburi, Pathumtani, Thailand) and deposited at the Royal Forestry Department, Bangkok, Thailand (BKF.No. 183890). They were selected, washed, dried, and extracted by supercritical CO2 extraction apparatus (24 L-SFE, China). The obtained yield was 2.38%.
Preparation of gel
Thunbergia laurifolia Lindl leaf (TLL) gel and 1% silver sulfadiazine (SSD) gel were prepared in a gel base. The gel base (100 g) contained carbopol® 940 0.5 g, propylene glycol 35 g, methyl paraben 0.15 g, propyl paraben 0.3 g, EDTA 0.1 g, distilled water 63.95 ml, and triethanolamine was added to 100 g. TLL gel concentrations of 2.5%, 5.0%, and 10% w/w in gel base were used in this study.
Animals
This study was approved by the Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University. One hundred and fifty male Wistar rats weighing 200–250 g were obtained from the National Laboratory Animal Center, Mahidol University, Salaya Campus, Thailand. The rats were housed under the standard condition at 25°C and a 12 h light/dark cycle. They were acclimatized for 1-week before experimentation. The rats were induced to second-degree burn and randomly divided into six groups (six rats/group) as follows: Burns without treatment, burns treated with gel base, burns treated with 1% SSD gel, and burns treated with 2.5%, 5% and 10% TLL gel groups. The treated groups received their respective topical application on wound areas daily for 14 days.
Induction of second-degree burn
The method of burn wound induction was modified from Thakur, et al.[20] The rats were anesthetized with 60 mg/kg BW pentobarbital sodium. The backs of the rats were shaved and depilated between the lower parts of the scapula. A second-degree burn was induced by applying a 90°C hot plate (2 cm diameter) for 10 s.
Percent of wound contraction and epithelialization time
Wound lesions were photographed every 3 days from day 1 to day 28.[21] Wound contraction area was estimated by Image Pro v. 6 software (Media Cybernetics, Inc., Rockville, MD, USA) for determining epithelialization time and percentage of wound contraction compared to wound on initial day.
Histopathology examination
Wound tissues were collected on days 3rd, 7th, and 14th. The tissues were fixed in 10% formalin, paraffinized, and sectioned on slides. The sections were deparaffinized in xylene, hydrated, and stained with hemotoxylin and eosin (H and E). These sections were observed under a light microscope.
Masson's trichrome examination
Wound tissue sections were deparaffinized in xylene, hydrated, and stained in Weigert's iron hematoxylin solution for 10 min. They were washed and stained in acid fuchsin solution for 2 min, rinsed in distilled water, treated with phosphomolybdic-phosphotugstic acid solution for 10 min, immediately submerged into aniline blue solution for 5 min, rinsed in distilled water, treated with acetic solution for 5 min, dehydrated in 95% alcohol, cleared twice in xylene, mounted with a cover slip, and observed under a light microscope.
Determination of collagen content
Wound total collagen content was determined using Sircol Collagen Assay Kit (Biocolor, Northern Ireland, UK). Frozen punched wound sections (4 mm diameter) were homogenized with 100 mg pepsin/gram wet tissue in 0.5 ml of 0.5 M acetic acid. Each homogenate was mixed with 1 ml Sircol dye reagent for 30 min and centrifuged at 12,000 × g for 10 min. The pellets were dissolved in 1 ml alkali reagent for 10 min and measured at 540 nm. Collagen content in the samples was calculated from the standard curve.
Statistical analysis
The data were presented as mean ± standard deviation. They were analyzed by one-way analysis of variance, followed by Turkey's pos hoc. SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. P values < 0.05 and < 0.01 were considered as statistically significant.
RESULTS
Effect of Thunbergia laurifolia Lindl leaf gel on wound contraction and epithelialization time
Thunbergia laurifolia Lindl leaf gel treated groups had higher wound healing acceleration than the untreated group. TLL gel concentrations of 5% and 10% significantly accelerated wound closure as early as day 4, postoperation [Figure 1]. The epithelialization times of the TLL gel treated groups were also significantly shorter than in the untreated group [Figure 2]. Times were 26.83 ± 3.1, 26.0 ± 0.37, and 23.83 ± 0.65 days in 2.5%, 5% and 10% TLL gel treated groups, when compared to 30.17 ± 0.48 days in the untreated group. The gel based and 1% SSD treated groups did not show significant differences in wound healing rate and epithelialization time, when compared to the untreated group.
Figure 1.
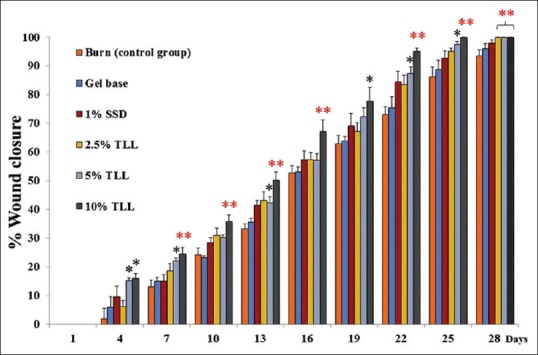
Effect of Thunbergia laurifolia Lindl leaf gel on burn wound. Data are expressed as mean ± standard deviation; *P < 0.05, **P < 0.01 compared with the untreated control (n = 6)
Figure 2.
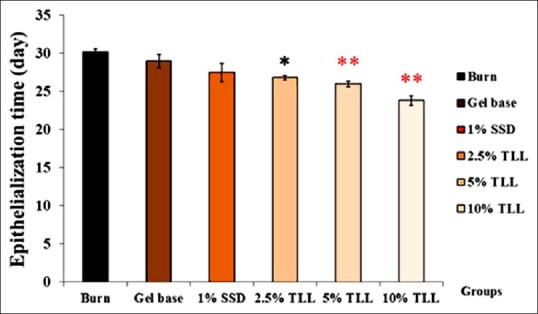
Effect of Thunbergia laurifolia Lindl leaf gel on epithelialization time. Data are expressed as mean ± standard deviation; *P < 0.05, **P < 0.01 compared with the untreated control (n = 6)
Histopathology evaluation
On day 3 after the burn operation, all groups showed damage of the dermis of the burn wound with epidermal detachment, scabs formation of necrotic tissue remnants, hyperemic vessels, no hair follicles or sebaceous glands, and phagocytic cell infiltration [Figure 3]. The wounds of all TLL gel treated groups had no edema or congestion. On day 7, epithelialization, fibroblast infiltration, and angiogenesis were found in all TLL gel treated groups, more than in the untreated control group. Scabbing still persisted in all groups, but scabs were thinner in all TLL gel groups. All groups had damaged epidermis and dermis with.
Figure 3.
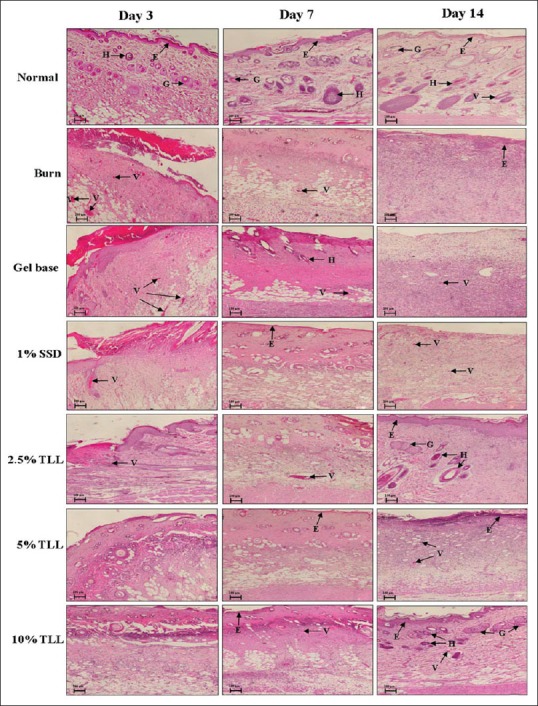
Histological observation of wound skin sections on days 3, 7, and 14, postburn operation, stained with H and E ×4, Bar = 100 μm, E = Epidermis; G = Sebaceous gland; H = Hair follicle; V = Blood vessel (n = 6)
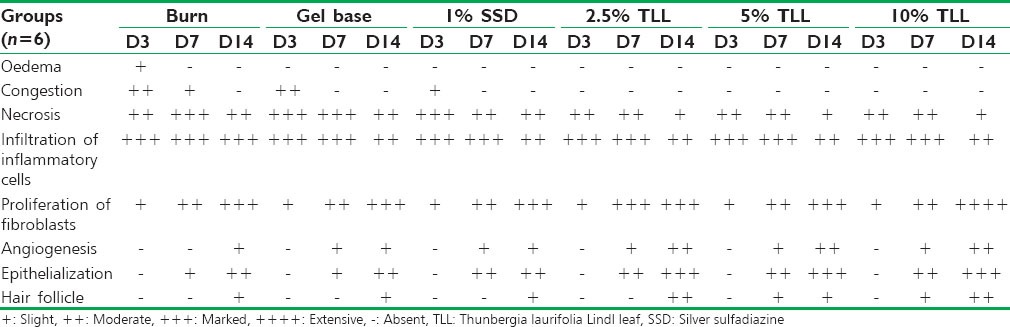
Epithelialization and fibroblast infiltration. Mononuclear cell infiltration was found in all groups [Table 1]. On day 14, all TLL gel treated groups showed clearly developed epithelialization, angiogenesis, and hair follicles, more than the other groups. Extensive fibroblast proliferation was observed in the 10% TLL group.
Table 1.
Histopathological scores on day 3, day 7, and day 14 in burn wound rats after topical application of TLL gel and control groups topical applications
Masson's trichrome examination
Results from Masson's trichrome staining can clearly differentiate important morphological keys for wound healing assessment. Keratin, hemoglobin, and muscle fiber are stained red color. Cytoplasm and adipose cells are stained light red or pink. Cell nuclei show dark brown to black, and collagen fiber is stained blue.
On day 3, all groups demonstrated acute inflammation of wound with clot formation, scarring, and minimal content of collagen [Figure 4]. On day 7, epithelialization was accelerated, and epithelial migration was noted. All TLL gel treated groups, and the 1% SSD group showed a decrease in inflammatory cells, whereas the untreated burn group still had inflammatory cells infiltration [Figure 4]. On day 14, all TLL gel treated groups, most notably the 10% TLL group, showed tissue healing with higher levels of re-epithelialization, fibroblast proliferation, and collagen fiber, as compared to the other groups.
Figure 4.
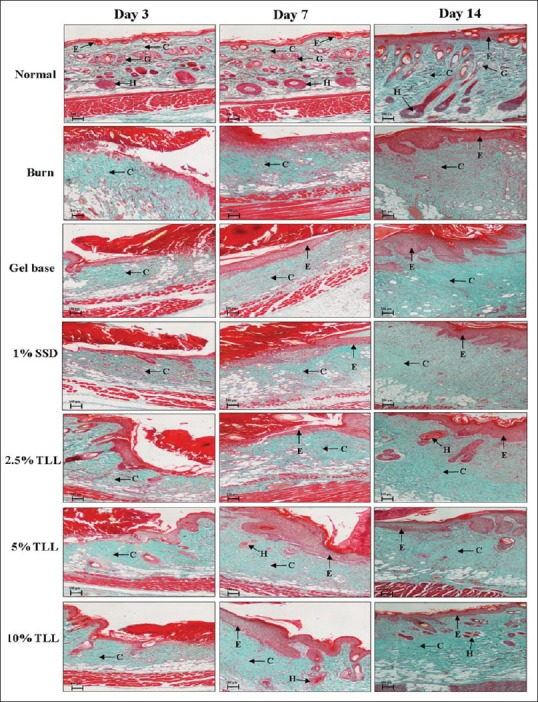
Histological observation of the wound skin sections on days 3, 7, and 14, postburn operation, stained with Masson's trichrome ×4, Bar = 100 μm, E = Epidermis; G = Sebaceous gland; H = Hair follicle; C = Collagen (n = 6)
Determination of collagen content
Wound collagen was significantly increased in the 10% TLL gel treated group on day 7 and day 14, when compared to the untreated burn group [Figure 5].
Figure 5.
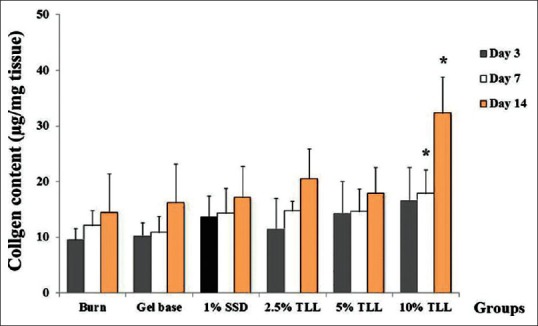
Effect of Thunbergia laurifolia Lindl leaf gel on collagen content in wound tissue. Data are presented as mean ± standard deviation; *P < 0.01 compared to the untreated group (n = 6)
DISCUSSION
Burn wound healing is a tissue repair process involving four phases; coagulation and hemostasis, inflammatory, proliferative, and remodeling.[22] It has been accepted that the acceleration of wound repair is the best treatment for burn wounds.[23] This study clearly demonstrated the accelerating activity of 10% TLL gel. The gel significantly increased the percentage of wound closure as early as day 4, postoperation. The epithelialization period was also shortened to 23.83 ± 0.65 days in the 10% TLL gel group, when compared to 30.17 ± 0.48 days in the untreated burn group. An increase in epithelialization rate in the 10% TLL group was confirmed by the histological study.
The inflammatory phase of burn wounds is initiated by neutrophils and followed by macrophages to remove microorganisms and cell debris at the tissue injury site. These cells eliminate pathogens and dead cells and produce several inflammatory mediators.[24] These mediators cause pain, fever, vasodilation and an increase in vascular permeability.[25] It has been reported that T. laurifolia leaf extract inhibited the production of inflammatory mediators in LPS-activated macrophage RAW 264.1 cells.[26] The extract also demonstrated anti-inflammatory.[13,16,18,19] There was no edema or congestion on day 3, postoperation in all TLL gel treated groups but these signs of inflammation were observed in the untreated burn group. Congestion was observed until day 7 in the untreated group. A reduction in the signs of inflammation may indicate anti-inflammatory effects, which may be a factor in the wound acceleration properties of TLL gel. Infiltrated inflammatory cells in wound tissues observed on day 3 and day 7 were reduced more quickly in all TLL gel treated groups, as compared to the untreated burn group. TLL gel may shorten the inflammatory phase of the wound healing process.
In the proliferative phase, fibroblasts and their matrix proteins, especially collagens, are essential for tissue repair and remodeling. Fibroblasts migrate into the wound area and extensively proliferate. They produce the matrix proteins, hyaluronan, fibronectin, proteoglycan, and collagens for wound repair.[27] In this study, migrated fibroblasts were found in all burn groups on day 3, postoperation. The increase in these cells on day 7 and day 14 was higher in the TLL gel treated groups, as compared to the untreated group. The fibroblast proliferation was correlated to collagen content in wound tissue. Collagen content in wound tissue of the 10% TLL gel treated group was higher than in the untreated group. TLL gel may play a role in fibroblast proliferation. Increases in both fibroblasts and collagen content in wound tissue correlated well with the shortened period of epithelialization of wound skin in the 10% TLL gel treated group.
CONCLUSION
The results from this study suggest that a supercritical CO2 extract of T. laurifolia leaf may accelerate burn wound healing by shortening the inflammation phase and promoting the proliferation and remodeling phase. These results provide scientific information for the use of T. laurifolia leaves for the acceleration of wound healing. Nevertheless, toxicity test should be performed to further validate the therapeutic effects of T. laurifolia supercritical CO2 extraction.
ACKNOWLEDGMENT
This study was supported by a grant from the 90th Anniversary of Chulalongkorn University Fund. We are very thankful to Dr. Chanai Noysang for gel preparation and to Assistant Professor Waraphan Toniti, Faculty of Veterinary Science, Mahidol University, Thailand for histopathology analysis.
Footnotes
Source of Support: A grant from the 90th Anniversary of Chulalongkorn University Fund
Conflict of Interest: Nil.
REFERENCES
- 1.Cinat ME, Smith MM. Acute burn management. In: Achauer BM, editor. Achauer and Sood's Burn Surgery Reconstruction and Rehabilitation. Philadelphia: Elsevier Inc; 2006. pp. 50–7. [Google Scholar]
- 2.Porth CM, Matfin G. 8th ed. Philadelphia: Lippincott Williams and Wilkin; 2009. Pathophysiology. Concepts of Altered Health States; pp. 392–6. [Google Scholar]
- 3.Ahuja V, Efron DE. Wound healing. In: Brunicardi FC, editor. Schwartz's Principles of Surgery. 8th ed. New York: McGraw-Hill; 2007. pp. 139–52. [Google Scholar]
- 4.Falanga V, Iwamoto S. Wound repair: Mechanisms and practical considerations. In: Wolff K, editor. Fitzpatrick's Dermatology in General Medicine. 7th ed. New York: McGraw-Hill; 2008. pp. 2342–9. [Google Scholar]
- 5.Singer AJ, Simon M. Wound healing and skin substitutes. In: Battler A, Leor J, editors. Stem Cell and Gene-Based Therapy. London: Springer-Verlag; 2006. pp. 375–93. [Google Scholar]
- 6.Sephel GC, Davidson JM. Repair, regeneration and fibrosis. In: Rubin R, Strayer DS, editors. Rubin's Pathology Clinicopathologic Foundations of Medicine. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2012. pp. 83–103. [Google Scholar]
- 7.Kelly AP, Taylor SC. China: McGraw-Hill; 2009. Dermatology for Skin of Color; pp. 42–3. [Google Scholar]
- 8.Krishnan P. The scientific study of herbal wound healing therapies: Current state of play. Curr Anaesth Crit Care. 2006;17:21–7. [Google Scholar]
- 9.Liu M, Dai Y, Li Y, Luo Y, Huang F, Gong Z, et al. Madecassoside isolated from Centella asiatica herbs facilitates burn wound healing in mice. Planta Med. 2008;74:809–15. doi: 10.1055/s-2008-1074533. [DOI] [PubMed] [Google Scholar]
- 10.Tejsen P, Thongtharb S. Use of Thunbergia laurifolia L. against the toxicity of insecticide. Chiang Mai Bull. 1980;19:105–14. [Google Scholar]
- 11.Utogapachn C. Bangkok: Phrapittaya; 1976. Medicinal Plants and Tropical Diseases. [Google Scholar]
- 12.Burkill IH. II. Kuala Lumpur: (I-Z) Ministry of Agriculture and Cooperatives; 1966. A Dictionary of the Economic Products of the Malay Peninsula. [Google Scholar]
- 13.Wonkchalee O, Boonmars T, Aromdee C, Laummaunwai P, Khunkitti W, Vaeteewoottacharn K, et al. Anti-inflammatory, antioxidant and hepatoprotective effects of Thunbergia laurifolia Linn. on experimental opisthorchiasis. Parasitol Res. 2012;111:353–9. doi: 10.1007/s00436-012-2846-5. [DOI] [PubMed] [Google Scholar]
- 14.Thongsaard W, Marsden CA. A herbal medicine used in the treatment of addiction mimics the action of amphetamine on in vitro rat striatal dopamine release. Neurosci Lett. 2002;329:129–32. doi: 10.1016/s0304-3940(02)00658-4. [DOI] [PubMed] [Google Scholar]
- 15.Aritajat S, Wutteerapol S, Saenphet K. Anti-diabetic effect of Thunbergia laurifolia Linn. aqueous extract. Southeast Asian J Trop Med Public Health. 2004;35(Suppl 2):53–8. [PubMed] [Google Scholar]
- 16.Khobjai W, Jaihan U, Watcharasamphankul W, Somsak V. Protective effect of Thunbergia laurifolia extract on hemolysis during Plasmodium berghei infection. Parasitol Res. 2014;113:1843–6. doi: 10.1007/s00436-014-3831-y. [DOI] [PubMed] [Google Scholar]
- 17.Phyu MP, Tangpong J. Protective effect of Thunbergia laurifolia (Linn.) on lead induced acetylcholinesterase dysfunction and cognitive impairment in mice. Biomed Res Int. 2013;2013:186098. doi: 10.1155/2013/186098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inta A, Trisonthi P, Trisonthi C. Analysis of traditional knowledge in medicinal plants used by Yuan in Thailand. J Ethnopharmacol. 2013;149:344–51. doi: 10.1016/j.jep.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 19.Chan EW, Eng SY, Tan YP, Wong ZC. Phytochemistry and pharmacological properties of Thunbergia laurifolia: A review. Pharmacogn J. 2011;3:1–6. [Google Scholar]
- 20.Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid Based Complement Alternat Med. 2011;2011:438056. doi: 10.1155/2011/438056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh RR, Sagar VS, Shalini A. Effect of Punica Granatum peel extract on burn wound healing in albino Wistar rats. Int J Appl Biol Pharm Technol. 2011;2:353–7. [Google Scholar]
- 22.Velnar T, Bailey T, Smrkolj V. The wound healing process: An overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 23.Hartford CE. Care of outpatient burns. In: Harndon DN, editor. Total Burn Care. 4th ed. Edinburgh: Elsevier Inc; 2012. p. 81. [Google Scholar]
- 24.Sinno H, Prakash S. Complements and the wound healing cascade: An updated review. Plast Surg Int. 2013;2013:146764. doi: 10.1155/2013/146764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goljan EF. 3rd ed. United States: Mosby Inc., Affiliate of Elsevier Inc; 2010. Rapid Review Pathology; p. 25. [Google Scholar]
- 26.Mahakunakorn P, Sripanidkulchai B, Suwannaroj N, Topark-ngarm A. Study on the Anti-Inflammatory Mechanisms of Thunbergia laurifolia Leaf Extracts. Project Code: DIGS180028. Faculty of Pharmaceutical Sciences, Khon Kaen University. 2010 [Google Scholar]
- 27.Teller P, White TK. The physiology of wound healing: Injury through maturation. Surg Clin North Am. 2009;89:599–610. doi: 10.1016/j.suc.2009.03.006. [DOI] [PubMed] [Google Scholar]


