Abstract
Much research has been carried out with the aim to discover the therapeutic values of chalcone derivatives. Chalcones possess wide range of pharmacological activity such as antibacterial, antimalarial, antiprotozoal, antitubercular, anticancer, and antifungal agents etc. The presence of reactive α,β-unsaturated keto group in chalcones is found to be responsible for their biological activity. The rapid developments of resistance to antifungal agents, led to design, and synthesize the new antifungal agents. The derivatives of chalcones were prepared using Claisen–Schmidt condensation scheme with appropriate tetralone and aldehyde derivatives. Ten derivatives were synthesized and were biologically screened for antifungal activity. The newly synthesized derivatives of chalcone showed antifungal activity against fungal species, Microsporum gypseum. The results so obtained were superior or comparable to ketoconazole. It was observed that none of the compounds tested showed positive results for fungi Candida albicans nor against fungi Aspergillus niger. Chalcone derivatives showed inhibitory effect against M. gypseum species of fungus. It was found that among the chalcone derivatives so synthesized, two of them, that is, 4-chloro derivative, and unsubstituted derivative of chalcone showed antifungal activity superior to ketoconazole. Thus, these can be the potential new molecule as antifungal agent.
Keywords: Antifungal activity, Claisen–Schmidt reaction, dermatophytes, Microsporum gypseum, tetralone
INTRODUCTION
Fungal infections have emerged as a growing threat to human health. There are many reasons behind this, but two important reasons for this are the increasing number of HIV infected patients and the number of patients being treated with cancer chemotherapy drugs.[1] This has led to search for novel molecular targets for new antifungal drugs. There have been many new antifungal drugs that have come up in the last 5 years, but still some patients remain difficult to treat.[2] There are many reasons behind and main reason for this includes intrinsic or acquired antifungal resistance. Furthermore, many of the newly developed antifungal agents lead to serious side effects. Hence, there is a continuous need for new antifungal drugs, which may selectively attack the fungi without inhibiting any biochemical system of the host.[1,3]
Chalcones, or 1,3-diaryl-2-propen-1-ones, which are polyhydroxylated in the aryl rings[4,5] are said to possess many biological properties,[6] including antiinflammatory,[7,8] antimicrobial,[7,8] antifungal,[7,8] antioxidant,[7,8] and antitumor activities.[7,8] The antimicrobial property of chalcones is due to the presence of a reactive unsaturated keto function in the molecule[9] while antifungal properties are present in some phenolic synthetic chalcones.[4,10,11] Chalcones inhibit β(1,3)-glucan and chitin synthases, enzymes, that catalyze the biosynthesis of β(1,3)-glucan and chitin polymers of the fungal cell wall, respectively.[12,13]
Hence, chalcones and their derivatives may be the potential candidate to investigate as a safe antifungal agent, as these may not affect the host. A series of chalcone derivatives were synthesized and evaluated for antifungal activity against fungal species: Aspergillus niger, Candida albicans, and Microsporum gypseum.
MATERIALS AND METHODS
Reagents, starting materials and solvents were purchased from common commercial suppliers. The melting point of synthesized compounds was determined by open capillary method on a Veego digital melting point apparatus. Mass spectral analysis was carried out using Applied Biosystem Qtrap 3200 MS/MS system in ESI mode. The infrared spectra of the synthesized compounds were recorded on Fourier Transformer Infrared Spectrophotometer Model Schimadzu 8400S using potassium bromide pellets. 1H NMR spectra were recorded on the Bruker NMR using DMSO-d6, tetramethylsilane as an internal standard.
Experimental
General procedure for synthesis of 2-arylidene-3, 4-dihydronaphthalen-1(2H)-ones (3a-3j)
A mixture of NaOH (2.2 g, 0.055 mol), water (20 ml), ethanol (12.25 ml), 1-tetralone (0.043 mol), and the appropriate aldehyde derivative (0.043 mol) was stirred at 15–30°C for 48 h and kept in the refrigerator overnight. It was then filtered, washed with water, then washed with the least amount of ethanol, dried, refluxed with glacial acetic acid (15 ml) for 3 h. The crystals separated after cooling were filtered and washed with water. It was then recrystallized from absolute ethanol.
2-benzylidene-3,4-dihydronaphthalen-1(2H)-one (3a)
Seventy-four percent yield – MP = 94–96°C, IR (KBr) ν/cm: 1661 (C = O), 1605 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.68 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 7.30–7.14 (m, 5H, [Ar1-H]), 2.39 (m, 4H, [CH2]), MS-API: [M + H] + 234.00 (calculated 234.10) Anal. Calculated for C17H14O: C, 87.15; H, 6.02; O, 6.83; Found: C, 87.40; H, 6.20; O, 6.70.
2-(2-chlorobenzylidene)-3,4-dihydronaphthalen-1(2H)-one (3b)
Seventy-nine percent yield – MP = 212–214°C, IR (KBr) ν/cm: 1663 (C = O),1605 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.62 (s, 1H, [CH]), 7.76–7.25 (m, 4H, [Ar-H]), 7.21–7.07 (m, 4H, [Ar1-H]), 2.45 (m, 4H, [CH2]), MS-API: [M + H] + 268.10 (calculated 268.07) Anal. Calculated for C17H13ClO: C, 75.98; H, 4.88; Cl, 13.19; O, 5.95; Found: C, 76.40; H, 5.10; Cl, 13.10; O, 6.50.
2-(3-chlorobenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3c)
Eight-two percent yield – MP = 209–211°C, IR (KBr) ν/cm: 1664 (C = O), 1603 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.67(s, 1H, [CH]), 7.76–7.27 (m, 4H, [Ar-H]), 7.31–7.14 (m, 4H, [Ar1-H]), 2.39 (m, 4H, [CH2]), MS-API: [M + H] + 268.00 (calculated 268.07) Anal. Calculated for C17H13ClO: C, 75.98; H, 4.88; Cl, 13.19; O, 5.95; Found: C, 76.10; H, 5.30; Cl, 13.00; O, 6.20.
2-(4-chlorobenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3d)
Seventy-two percent yield – MP = 210–212°C, IR (KBr) ν/cm: 1668 (C = O), 1593 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.63 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 7.25–7.21 (m, 4H, [Ar1-H]), 2.59 (m, 4H, [CH2]) MS-API: [M + H] + 268.00 (calculated 268.07) Anal. Calculated for C17H13ClO: C, 75.98; H, 4.88; Cl, 13.19; O, 5.95; Found: C, 76.00; H, 5.00; Cl, 13.00; O, 6.00.
2-(4-bromobenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3e)
Seventy-five percent yield – MP = 242–244°C, IR (KBr) ν/cm: 1666 (C = O), 1593 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.64 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 7.35–7.18 (m, 4H, [Ar1-H]), 2.39 (m, 4H, [CH2]). MS-API: [M + H] + 313.00 (calculated 312.01) Anal. Calculated for C17H13BrO: C, 65.19; H, 4.18; Br, 25.51; O, 5.11; Found: C, 65.40; H, 4.30; Br, 26.00; O, 5.10.
2-(4-fluorobenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3f)
Seventy-three percent yield – MP = 183–185°C, IR (KBr) ν/cm: 1663 (C = O), 1605 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.62 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 7.27–6.93 (m, 4H, [Ar1-H]), 2.29 (m, 4H, [CH2]). MS-API: [M + H] + 252.00 (calculated 252.10) Anal. Calculated for C17H13FO: C, 80.93; H, 5.19; F, 7.53; O, 6.34; Found: C, 81.00; H, 5.30; F, 7.00; O, 6.10.
2-(4-methoxybenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3 g)
Seventy-two percent yield – MP = 215–217°C, IR (KBr) ν/cm: 1666 (C = O), 1601 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ,7.62 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 7.2–6.93 (m, 4H, [Ar1-H]), 2.29 (m, 4H, [CH2]), 3.75 (s, 3H, [CH3]). MS-API: [M + H] + 264.10 (calculated 264.12) Anal. Calculated for C18H16O2: C, 81.79; H, 6.10; O, 12.11; Found: C, 81.40; H, 6.30; O, 12.50.
2-(2,4-dichlorobenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3 h)
Seventy-eight percent yield – MP = 254–256°C, IR (KBr) ν/cm: 1672 (C = O), 1614 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.82 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 7.23–7.10 (m, 3H, [Ar1-H]), 2.29 (m, 4H, [CH2]). MS-API: [M + H] + 303.00 (calculated 302.03) Anal. Calculated for C17H12Cl2O: C, 67.35; H, 3.99; Cl, 23.39; O, 5.28; Found: C, 67.40; H, 4.10; Cl, 23.50; O, 5.70.
2-(3,4-dimethoxybenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3i)
Seventy-seven percent yield – MP = 261263°C, IR (KBr) ν/cm: 1672 (C = O), 1614 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.62 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 6.71–6.63 (m, 3H, [Ar1-H]), 2.29 (m, 4H, [CH2]), 3.72 (m, 3H, CH3), 3.73 (m, 3H, CH3). MS-API: [M + H] + 294.10 (calculated 294.13) Anal. Calculated for C19H18O3: C, 77.53; H, 6.16; O, 16.31; Found: C, 77.40; H, 6.20; O, 16.70.
2-(3,4,5-trimethoxybenzylidene)-3,4-dihydronaphthalen-1 (2H)-one (3j)
Sixty-eight percent yield – MP = 306–308°C, IR (KBr) ν/cm: 1660 (C = O), 1589 (C = C). 1H NMR (DMSO-d6, 400 MHz): δ7.62 (s, 1H, [CH]), 7.76–7.29 (m, 4H, [Ar-H]), 6.28 (m, 2H, [Ar1-H]), 2.29 (m, 4H, [CH2]), 3.72 (m, 3H, CH3), 3.73 (m, 3H, CH3), 3.75 (m, 3H, CH3). MS-API: [M + H] + 324.20 (calculated 324.14) Anal. Calculated for C20H20O4: C, 74.06; H, 6.21; O, 19.73; Found: C, 74.11; H, 6.24; O, 19.78.
Antifungal activity
The antifungal activity of chalcones was evaluated by the cup-plate method[4] against 3 fungal species: C. albicans ATCC 10231, A. niger ATCC 1015, and M. gypseum C 115 2000, dermatophyte fungal species.[4] Stock solutions of synthesized compounds were prepared in DMSO. Aliquots of the stock solution were used to prepare series of subsequent concentration. The lowest concentration that produces no visible fungal growth after the incubation time is termed as minimum inhibitory concentration (MIC).[4] Control experiments were performed under similar conditions without the synthesized compounds. Ketoconazole was used as a standard for antifungal activity.
RESULTS AND DISCUSSION
Chemistry
The synthesis of 2-arylidene -3,4-dihydronaphthalen-1 (2H)-ones (3a-3j) was carried out by Claisen–Schmidt condensation of the appropriate aldehyde derivatives and tetralone in the presence of a base (NaOH), according to the reported procedure [Scheme 1].[4]
Scheme 1.
Synthesis of 2-arylidene-3,4-dihydronaphthalen-1(2H)-ones
The purity of the compounds was checked by TLC-using Silicagel-G (Merck). Their structures were established with IR, NMR and mass spectrometry analysis.
Antifungal activity
Antifungal activity was carried out by using cup-plate method.[4] The synthesized compounds have no activity against C. albicans and A. niger. Significant antifungal activity was shown by the synthesized compounds against M. gypseum, a dermatophyte. Compounds 3a and 3d showed strong antifungal activities and were superior to ketoconazole [Table 1 and Figure 1].
Table 1.
Antifungal activity of chalcones
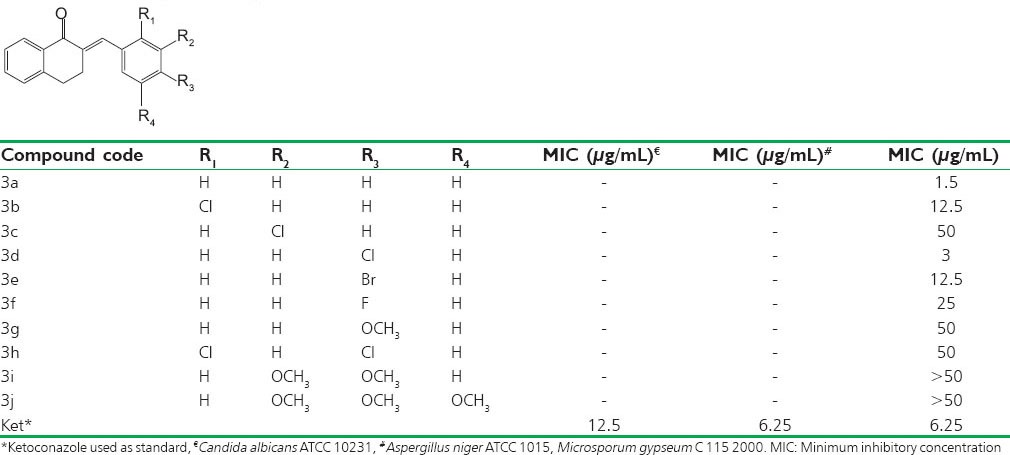
Figure 1.
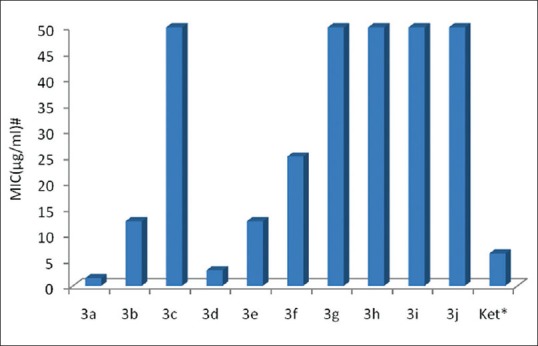
Antifungal activity of chalcone derivatives. Ket*: Ketoconazole used as standard, MIC: Minimum inhibitory concentration in (μg/ml)
Dermatophytes are a group of fungi, which normally infect the keratinized areas of the body and causes Dermatomycoses, which is difficult to eradicate. The synthesized chalcone derivatives showed activity against dermatophytes, and thus are a potential candidate to treat dermatomycoses.
Structure activity relationship
On studying the effect of the substituents on the activity, an interesting structure-activity relationship can be seen. An electron withdrawing group, that is, Cl group when placed in the para position as in the synthesized compound 3d, showed MIC better than ketoconazole indicating better potency than ketoconazole. The presence of Cl group at ortho position as in the synthesized compound 3b has comparable potency as the compound with Br group at the para position as in the synthesized compound 3e as their MIC were found to be comparable. The presence of OCH3 group as in the synthesized compound 3i and 3j showed a decrease in potency. On considering the relationship of the antifungal activity of substituted chalcone derivatives with the planarity of their molecules, it was observed that as substituent increased, that is, it turned into a bulky group activity of the compound was observed to be lower as compared with the nonsubstituted chalcone.
This shows that the steric hindrance may reduce the activity.
CONCLUSION
In conclusion, a novel series of 2-arylidene-3, 4-dihydronaphthalen-1(2H)-ones was successfully synthesized and tested for antifungal activity against three fungal strains. The results of the biological studies revealed that among the three fungal strains, M. gypseum was found to be more sensitive to the studied 2-arylidene-3,4-dihydronaphthalen-1(2H)-ones. Infact, two (3a, 3d) among the 10 compounds tested were more effective than the clinical candidate ketoconazole. M. gypseum is a type of fungi which causes dermatomycoses, a type of infection difficult to treat, hence, the studied compounds, specifically, 3a, 3d could be promising lead molecules for development of more potent and safer antifungal drugs for the treatment of dermatomycoses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to express thanks to Dr. Rekha Gupta, Head of the Department, Biotechnology, Modern College Pune, for cooperation in biological screening of the synthesized compounds. They are also grateful to Shri Subhash Gupta, Oasis Test House Ltd., Jaipur, for screening of compounds for IR studies, and to the authorities at Punjab University, Chandigarh, for their kind cooperation for NMR studies.
REFERENCES
- 1.Georgopapadakou NH, Walsh TJ. Antifungal agents: Chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–91. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. [Last accessed on 2009 Feb 08]. Available from: http://www.researchandmarkets.com/reports/220279/
- 3.Pasaqualotto AC, Denning DW. New and emerging treatment for fungal infections. J Antimicrob Chemother. 2008;61:19–30. doi: 10.1093/jac/dkm428. [DOI] [PubMed] [Google Scholar]
- 4.López SN, Castelli MV, Zacchino SA, Domínguez JN, Lobo G, Charris-Charris J, et al. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg Med Chem. 2001;9:1999–2013. doi: 10.1016/s0968-0896(01)00116-x. [DOI] [PubMed] [Google Scholar]
- 5.Dhar DN. New York: John Wiley; 1981. The Chemistry of Chalcones and Related Compounds. [Google Scholar]
- 6.Yathirajan HS. Acta Crystallograpica. In Section E Structure Reports Online. 2007 [Google Scholar]
- 7.Dimmock JR, Elias DW, Beazely MA, Kandepu NM. Bioactivities of chalcones. Curr Med Chem. 1999;6:1125–49. [PubMed] [Google Scholar]
- 8.Go ML, Wu X, Liu XL. Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem. 2005;12:483–99. doi: 10.2174/0929867053363153. [DOI] [PubMed] [Google Scholar]
- 9.Rajendra Prasad Y. Synthesis and antimicrobial activity of some Chalcone derivatives. E J Chem. 2008:151. [Google Scholar]
- 10.Tsuchiya H, Sato M, Akagiri M, Takagi N, Tanaka T, Iinuma M. Anti-Candida activity of synthetic hydroxychalcones. Pharmazie. 1994;49:756–8. [PubMed] [Google Scholar]
- 11.Sato M, Tsuchiya H, Akagiri M, Fujiwara S, Fujii T, Takagi N, et al. Growth inhibitory properties of chalcones to candida. Lett Appl Microbiol. 1994;18:53. [Google Scholar]
- 12.Reddy NP, Aparoy P, Reddy TC, Achari C, Sridhar PR, Reddanna P. Design, synthesis, and biological evaluation of prenylated chalcones as 5-LOX inhibitors. Bioorg Med Chem. 2010;18:5807–15. doi: 10.1016/j.bmc.2010.06.107. [DOI] [PubMed] [Google Scholar]
- 13.Lahtchev KL, Batovska DI, Parushev SP, Ubiyvovk VM, Sibirny AA. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur J Med Chem. 2008;43:2220–8. doi: 10.1016/j.ejmech.2007.12.027. [DOI] [PubMed] [Google Scholar]


