Abstract
There is growing recognition of the impact of Clostridum difficile infection (CDI) on patients with inflammatory bowel disease. Clostridium difficile infection causes greater morbidity and mortality. This study aimed to evaluate the impact of C. difficile on surgical risk among ulcerative colitis (UC) patients. We searched the following databases: MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, ACP Journal Club, DARE, CMR, and HTA. Studies were included if fulfilled the following criteria: (1) Cohort or case–control studies, which involved a comparison group that lacked CDI, (2) Patients were given a primary diagnosis of UC, (3) Comorbidity of CDI was evaluated by enzyme immunoassay of stool for C. difficile toxin A and B or C. difficile stool culture, (4) Studies evaluated surgical rate, and (5) Studies reported an estimate of odds ratio, accompanied by a corresponding measure of uncertainty. Five studies with 2380 patients fulfilled the inclusion criteria. Overall, meta-analysis showed that UC with CDI patients had a significant higher surgical rate than patients with UC alone. (OR=1.76, 95% CI=1.36–2.28). C. difficile infection increased the surgical rate in UC patients. However, results should be interpreted with caution, given the limitations of this stud.
Keywords: C. difficile infection, surgical risk, ulcerative colitis
There is a growing recognition of the impact of Clostridum difficile infection (CDI) on patients with inflammatory bowel disease.[1] There has been a 2 to 3 fold increase in the proportion of ulcerative colitis (UC) and Crohn's disease (CD) hospitalizations complicated by C. difficile.[2] Clostridium difficile infection causes greater morbidity and mortality.[3] Although a growing body of knowledge on the epidemiology, pathogenesis, risk factors, and management of CDI has been obtained over the last decade, the increased incidence and severity of CDI continue to pose challenges to the medical community.[4,5] The expected health care costs due to CDI alone are estimated to be about 3.2 billion dollars per year in the United States.[6]
The past decade has seen a change in the epidemiology of C. difficile infection. Some of the original risk factors such as antibiotic use or health care exposure are no longer considered essential to entertain the diagnosis of C. difficile.[1] There is a greater recognition of community-acquired C. difficile infection, where a significant proportion has a positive test result within 2 days of hospitalization.[7]
Recently, debate in the literature has centered on whether infection with C. difficile is related to the adverse clinical outcomes among IBD patients; some studies reported that IBD patients with C. difficile had worse clinical outcomes than those without C. difficile infection.[8,9,10] However, other studies found that CDI was not associated with any adverse clinical outcomes.[11,12] Understanding this potential risk for IBD is important. as it will allow clinicians to evaluate the cost-effectiveness of medical versus non-medical interventions in management strategies and plan health care resource utilization. Patients with UC appeared to be at a higher risk for CDI than CD.[7] Therefore, we conducted a meta-analysis and systemic review to know whether CDI is related to increased colectomy among UC patients.
MATERIALS AND METHODS
Search strategy
This review was performed according to the standard guidelines for meta-analyses and systematic reviews of observational studies.[13] To find relevant articles for this review, we searched the following databases (from inception to December 2013): MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, ACP Journal Club, DARE, CMR, and HTA. The search strategy used free-text words and MeSH terms to increase the sensitivity of the search. The following search terms were used: Inflammatory bowel disease, UC, C. difficile, clinical outcome, colectomy, and surgery. Boolean operators (AND, OR, NOT) were used to narrow and widen the search results. A comprehensive search of reference lists of all review articles and original studies retrieved by this method was performed to identify additional reports. Furthermore, we hand searched abstracts of major gastroenterological meetings, such as the Digestive Disease Week of the American Gastroenterological Association and the World Congress of Gastroenterology. No language restrictions were made. Authors of some identified trials were asked whether they knew of additional studies, including unpublished randomized ones.
Inclusion and exclusion criteria
For inclusion in the systematic review, a study had to meet the following criteria established by the study team: (1) Cohort or case–control studies involved a comparison group that lacked CDI, (2) patients were given a primary diagnosis of UC, (3) diagnosis of CDI was evaluated by enzyme immunoassay of stool for C. difficile toxin A and B or C. difficile stool culture, (4) studies evaluated surgical rate, and (5) studies reported an estimate of relative risk or odds ratio, accompanied by a corresponding measure of uncertainty [ie, 95% confidence interval (CI), standard error, variance, or P value].
Studies were excluded if (1) Age was younger than 18 years, (2) patients had no known history of IBD, (3) outcome of interest was not reported, or (4) incomplete data.
Data extraction
To reduce reporting bias and error in data collection, all papers were examined independently for eligibility by two reviewers (Peng and Shen). Disagreement was resolved by consulting a third reviewer (Ran). Standardized data extraction form created by the study team was used. This form included the authors, location, year of publication, study design, number of CDI-UC patients, number of non-CDI-UC patients, outcome, and the Newcastle–Ottawa Scale.
Statistical analysis
The primary outcome of this analysis was the odds ratio (OR) of surgical rate in CDI-UC versus controls. We calculated the OR with a 95% confidence interval (CI) based on a fixed-effects model using the methods of DerSimonian and Laird.[14] Heterogeneity between the studies was assessed by Chi-square test and the I2-statistic.[15] P values <0.05 were considered statistically significant. If significant heterogeneity exists, it would be inappropriate to combine the data for further analysis using a fixed-effects model, whereas the random model was used for calculations. Publication bias was assessed by constructing a funnel plot. Any heterogeneity identified would prompt subgroup analysis in an attempt to explain these findings. Statistical analysis was performed with the software REVMAN X6 from the Cochrane Collaboration.
RESULTS
Characteristics and description of the studies
Our initial search strategy yielded 124 potential articles for inclusion. After detailed analysis of selected articles, 24 articles were reviewed in detail. Subsequently, 19 articles[5,8,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] did not meet the inclusion criteria. The reasons for exclusion included the following: five articles[16,20,24,25,26] were review, four studies[17,22,29,31] focused on children, six studies[18,21,23,27,28,30] did not report the outcome of interest, one article[19] was case report, and three studies[5,32,33] did not give complete data. Therefore, five studies[9,10,11,12,36] with 2380 patients fulfilled the inclusion criteria [Figure 1].
Figure 1.
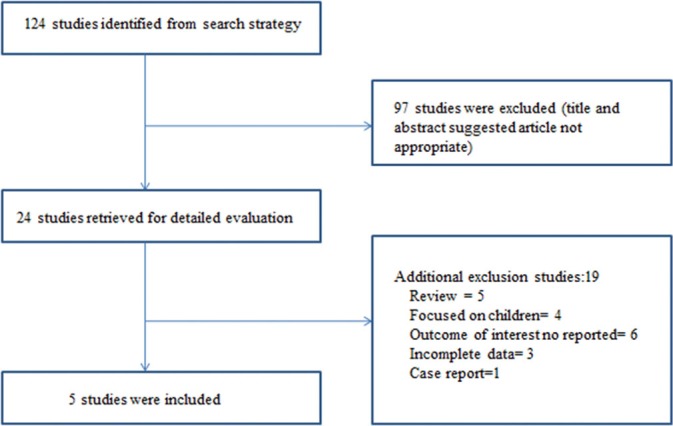
Flow diagram of studies identified in the systemic review
The characteristics of the included studies are listed in Table 1. Three studies were performed in the United States, and the other two were from Japan and Canada. The patients in the study group were diagnosed as CDI and UC, whereas the patients in the control group were diagnosed as UC alone. The diagnosis of CDI in most studies was according to the following criteria: enzyme immunoassay of stool for C. difficile toxin A and B, C. difficile stool culture, pathological evidence of C. difficile. Two studies[9,36] measured 1-year risks of colectomy between CDI-UC patients and UC patients. One study[10] measured a 5-year outcome of CDI-UC patients compared with UC patients. One study[11] performed a 3-month impact of C. difficile on UC patients. The other one[12] focused on the 3-year impact of C. difficile on acute UC. Three studies[10,36,37] reported increased surgical risks among CDI-UC patients, whereas another two[11,12] showed that there was no significant difference between CDI-UC and UC patients.
Table 1.
The characteristics of the included studies
Methodological quality of included studies was evaluated using the Newcastle–Ottawa scale. It uses a “star” rating system to judge quality on the basis of three aspects of the study: Selection of study groups, comparability of study groups, and assessment of the exposure. This scale awards a maximum of 9 stars to each study: up to 4 for selection of participants, 2 for comparability of participants on the basis of the design or analysis, and 3 for ascertainment of exposure. We assigned scores of 0–3, 4–6, and 7–9 for low, moderate and high quality studies, respectively [Table 1]. All included studies had scores ≥7, which were considered as high quality.
Impact of C. difficile on surgical rate among patients with IBD
Overall, a total of 41% of CDI-UC patients and 31.6% of UC patients had colectomy. The OR of surgical rate in UC-CDI patients compared with controls was 1.76 (95% CI = 1.36–2.28). There was no significant heterogeneity among the examined studies, (P = 0.09) [Figure 2] so a fixed effect model was used for assessment of the pooled OR. The result showed that C. difficile infection was associated with increased risks of colectomy among UC patients.
Figure 2.
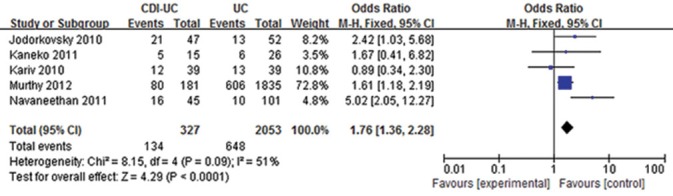
Risk of surgery between ulcerative colitis (UC)-Clostridium difficile infection patients and UC patients
Kariv et al.[11] also reported that subjects who did not use 5-ASA were 3.3 times more likely to have UC-related surgery within 3-months of C. difficile testing than those treated with 5-ASA, (95% CI 1.2–9.4, P = 0.03), which to some extent explained the role of immunosuppressants on clinical outcome in patients who were infected with C. difficile. Jodorkovsky et al.[36] found that UC patients with C. difficile infection had significantly higher rates of colectomy in the long-term compared with those not infected, regardless of age and gender, which suggested an association between C. difficile infection and a need for eventual colectomy.
Publication bias
Figure 3 shows a funnel plot of studies included in this meta-analysis. On visual inspection, the funnel plot was symmetrical in distribution. So, there was no significant publication bias in this analysis.
Figure 3.
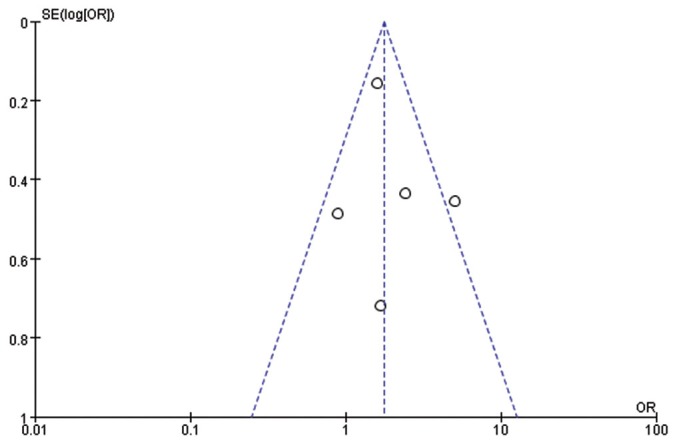
Flunnel plot analysis of the included studies
DISCUSSION
CDI has become the leading identifiable infective cause of antibiotic-associated diarrhea in general population.[38] Studies have shown that there was increased risk of CDI with use of antibiotics disrupting the bacterial flora, intensive care or prolonged hospital stay and more recently IBD.[5,33] Multiple studies from tertiary-care institutions as well as large nationwide inpatient databases have demonstrated an increase in the incidence and severity of CDI in patients with IBD when compared with general population. In a study of risk factors for CDI in IBD patients, maintenance immunomodulator use and colonic involvement were independently associated with the risk of CDI. In addition, 61% of IBD patients who developed CDI had antibiotic exposure up to 2 months prior to development of CDI.[5] A subsequent study also reported that increasing age and colonic involvement were independently associated with CDI in IBD.[7] One study reported that the use of antibiotics up to 30 days prior to C. difficile testing, was independently associated with the development of CDI.[11]
Pathogenicity of C. difficile infection begins with colonization of this Gram-positive anaerobic bacterium. Disruption of the normal intestinal flora allowing C. difficile to flourish is usually associated with antibiotic use and hospitalization. Inflammation may be an additional risk factor that disrupts the normal flora. C. difficile toxins were speculated to complicate IBD and contributed to relapse of IBD in some patients.[39] With increasing incidence of CDI, superimposed CDI in patients with IBD has been increasingly reported. CDI can even present with enteritis,[37] particularly in patients with IBD who had undergone bowel-diverting surgery or colectomy or as chronic antibiotic refractory pouchitis in patients with ileal pouch anal anastomosis for underlying IBD.[40]
In our study, we evaluated the association between CDI and surgical rate among UC patients. We found UC-CDI patients had statistically significant higher risks of surgery when compared with UC patients (OR = 1.76, 95% CI = 1.36–2.28). There was no statistical significance of heterogeneity among the included studies (P = 0.09).
The explanation could be that UC patients were more susceptible to CDI. In recent years, the reported incidence of CDI has increased, and the OR of acquiring CDI in UC patients was found to be four times as many as in non-IBD patients.[7,33] The exact reason for this phenomenon was unclear. The universal colonic involvement in UC compared with less frequent involvement in CD may contribute to a higher susceptibility to C. difficile infection.[41] Second, C. difficile spores and vegetative organisms in some patients could theoretically alter the natural history of UC by activating abnormal mucosal immune response, or CDI might be a sign reflecting an aggressive form of UC course, which resulted in more ER visits, more hospitalization, escalation of medical treatment and ultimately resulting in colectomy.[9,10] Use of immunosuppressive therapy, in addition to antibiotics, to treat C. difficile in the setting of UC may have also increased the duration or severity of colitis in many individuals.[42] Besides, sicker patients might get CDI. This might play a major role in colectomy because, on endoscopy, it was found that these patients had a significantly active or severe disease when compared with patients without CDI.[9]
There were also some limitations to this analysis. First, the number of included studies was small. Second, the unadjusted differences in severity of underlying IBD, comorbidity burden, or antibiotic use between infected and uninfected patients may influence the observed association in this analysis. Possibly, patients with severe manifestations of IBD and more comorbidities were at a greater risk of both acquiring C. difficile infection and worse clinical outcome. However, such information was not available in most of the included studies. Although one study[11] evaluated the role of immunosuppressants on the clinical outcome of UC-CDI patients, more studies are needed to classify whether the use of biologics and immunosuppressants could affect clinical outcome in patients infected with C. difficile. Furthermore, C. difficile exposure status may have been misclassified in a proportion of patients, as a result of both misreporting on hospital discharge abstracts and inadequate stool testing for C. difficile. No studies have evaluated the diagnostic accuracy of CDI among IBD patients. Last but not the least, most of the studies were at an academic center with multiple primary physicians, treatment strategy was subjective and unlikely to be uniform. Specifically, decisions about inpatient therapy and need for surgery is highly individualized. These factors may affect the role of C. difficile in UC patients.
In summary, our analysis suggested an association between C. difficile infection and surgical risks among UC patients. Although no significant heterogeneity was found among the included studies, the number of included studies was small. Therefore, the result should be interpreted with caution and further clinical studies investigating the effect of C. difficile infection on UC patients are warranted. In our analysis, we found CDI increased the surgical rate in UC patients. If it is found that C. difficile does indeed exacerbate UC, this will have profound influence not only on the way we approach C. difficile testing, but also on the way we approach the treatment of UC patients complicated with C. difficile.
Footnotes
Source of Support: This work was supported by the National Natural Science Foundation of China (No. 81000161 and No.81170362).
Conflict of Interest: None declared.
REFERENCES
- 1.Ananthakrishnan AN, Issa M, Binion DG. Clostridium difficile and inflammatory bowel disease. Gastroenterol Clin North Am. 2009;38:711–28. doi: 10.1016/j.gtc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Ricciardi R, Ogilvie JW, Jr, Roberts PL, Marcello PW, Concannon TW, Baxter NN. Epidemiology of Clostridium difficile colitis in hospitalized patients with inflammatory bowel diseases. Dis Colon Rectum. 2009;52:40–5. doi: 10.1007/DCR.0b013e31819733fd. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–4. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 4.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–50. doi: 10.1111/j.1572-0241.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: Clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–27. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 7.Rodemann JF, Dubberke ER, Reske KA, Seo da H, Stone CD. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:339–44. doi: 10.1016/j.cgh.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Ananthakrishnan AN, McGinley EL, Saeian K, Binion DG. Temporal trends in disease outcomes related to Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:976–83. doi: 10.1002/ibd.21457. [DOI] [PubMed] [Google Scholar]
- 9.Navaneethan U, Mukewar S, Venkatesh PG, Lopez R, Shen B. Clostridium difficile infection is associated with worse long term outcome in patients with ulcerative colitis. J Crohns Colitis. 2012;6:330–6. doi: 10.1016/j.crohns.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Daneman N, Nguyen GC. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther. 2012;36:1032–9. doi: 10.1111/apt.12073. [DOI] [PubMed] [Google Scholar]
- 11.Kariv R, Navaneethan U, Venkatesh PG, Lopez R, Shen B. Impact of Clostridium difficile infection in patients with ulcerative colitis. J Crohns Colitis. 2011;5:34–40. doi: 10.1016/j.crohns.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko T, Matsuda R, Taguri M, Inamori M, Ogura A, Miyajima E, et al. Clostridium difficile infection in patients with ulcerative colitis: Investigations of risk factors and efficacy of antibiotics for steroid refractory patients. Clin Res Hepatol Gastroenterol. 2011;35:315–20. doi: 10.1016/j.clinre.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Reddy SS, Brandt LJ. Clostridium difficile Infection and Inflammatory Bowel Disease. J Clin Gastroenterol. 2013;47:666–71. doi: 10.1097/MCG.0b013e31828b288a. [DOI] [PubMed] [Google Scholar]
- 17.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile Infection in children. JAMA Pediatr. 2013;167:567–73. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 18.Campbell R, Dean B, Nathanson B, Haidar T, Strauss M, Thomas S. Length of stay and hospital costs among high-risk patients with hospital-origin Clostridium difficile-associated diarrhea. J Med Econ. 2013;16:440–8. doi: 10.3111/13696998.2013.770749. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CH, Jeng YM, Ni YH. Clostridium difficile infection in a patient with Crohn disease. J Formos Med Assoc. 2012;111:347–9. doi: 10.1016/j.jfma.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis. 2013;19:194–204. doi: 10.1002/ibd.22964. [DOI] [PubMed] [Google Scholar]
- 21.Ananthakrishnan AN, Guzman-Perez R, Gainer V, Cai T, Churchill S, Kohane I, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:789–95. doi: 10.1111/j.1365-2036.2012.05022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezoff E, Mann EA, Hart KW, Lindsell CJ, Cohen MB. Clostridium difficile infection and treatment in the pediatric inflammatory bowel disease population. J Pediatr Gastroenterol Nutr. 2011;52:437–41. doi: 10.1097/MPG.0b013e3181f97209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ott C, Girlich C, Klebl F, Plentz A, Iesalnieks I, Scholmerich J, et al. Low risk of Clostridium difficile infections in hospitalized patients with inflammatory bowel disease in a German tertiary referral center. Digestion. 2011;84:187–92. doi: 10.1159/000324617. [DOI] [PubMed] [Google Scholar]
- 24.Sinh P, Barrett TA, Yun L. Clostridium difficile Infection and Inflammatory Bowel Disease: A Review. Gastroenterol Res Pract. 2011;2011:136064. doi: 10.1155/2011/136064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananthakrishnan AN, Issa M, Binion DG. Clostridium difficile and inflammatory bowel disease. Med Clin North Am. 2010;94:135–53. doi: 10.1016/j.mcna.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Musa S, Thomson S, Cowan M, Rahman T. Clostridium difficile infection and inflammatory bowel disease. Scand J Gastroenterol. 2010;45:261–72. doi: 10.3109/00365520903497098. [DOI] [PubMed] [Google Scholar]
- 27.Navaneethan U, Venkatesh PG, Shen B. Clostridium difficile infection and inflammatory bowel disease: Understanding the evolving relationship. World J Gastroenterol. 2010;16:4892–904. doi: 10.3748/wjg.v16.i39.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnenberg A. Similar geographic variations of mortality and hospitalization associated with IBD and Clostridium difficile colitis. Inflamm Bowel Dis. 2010;16:487–93. doi: 10.1002/ibd.21054. [DOI] [PubMed] [Google Scholar]
- 29.Wultanska D, Banaszkiewicz A, Radzikowski A, Obuch-Woszczatynski P, Mlynarczyk G, Brazier JS, et al. Clostridium difficile infection in Polish pediatric outpatients with inflammatory bowel disease. Eur J Clin Microbiol Infect Dis. 2010;29:1265–70. doi: 10.1007/s10096-010-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ. 2008;179:767–72. doi: 10.1503/cmaj.071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascarella F, Martinelli M, Miele E, Del Pezzo M, Roscetto E, Staiano A. Impact of Clostridium difficile infection on pediatric inflammatory bowel disease. J Pediatr. 2009;154:854–8. doi: 10.1016/j.jpeds.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis. 2013;7:107–12. doi: 10.1016/j.crohns.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Issa M, Vijayapal A, Graham MB, Beaulieu DB, Otterson MF, Lundeen S, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:345–51. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Jen MH, Saxena S, Bottle A, Aylin P, Pollok RC. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:1322–31. doi: 10.1111/j.1365-2036.2011.04661.x. [DOI] [PubMed] [Google Scholar]
- 35.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–10. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 36.Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig Dis Sci. 2010;55:415–20. doi: 10.1007/s10620-009-0749-9. [DOI] [PubMed] [Google Scholar]
- 37.Navaneethan U, Giannella RA. Thinking beyond the colon-small bowel involvement in Clostridium difficile infection. Gut Pathog. 2009;1:7. doi: 10.1186/1757-4749-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asha NJ, Tompkins D, Wilcox MH. Comparative analysis of prevalence, risk factors, and molecular epidemiology of antibiotic-associated diarrhea due to Clostridium difficile, Clostridium perfringens, and Staphylococcus aureus. J Clin Microbiol. 2006;44:2785–91. doi: 10.1128/JCM.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaMont JT, Trnka YM. Therapeutic implications of Clostridium difficile toxin during relapse of chronic inflammatory bowel disease. Lancet. 1980;1:381–3. doi: 10.1016/s0140-6736(80)90939-3. [DOI] [PubMed] [Google Scholar]
- 40.Shen BO, Jiang ZD, Fazio VW, Remzi FH, Rodriguez L, Bennett AE, et al. Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:782–8. doi: 10.1016/j.cgh.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Lundeen SJ, Otterson MF, Binion DG, Carman ET, Peppard WJ. Clostridium difficile enteritis: An early postoperative complication in inflammatory bowel disease patients after colectomy. J Gastrointest Surg. 2007;11:138–42. doi: 10.1007/s11605-006-0022-x. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Horin S, Margalit M, Bossuyt P, Maul J, Shapira Y, Bojic D, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridium difficile infection. Clin Gastroenterol Hepatol. 2009;7:981–7. doi: 10.1016/j.cgh.2009.05.031. [DOI] [PubMed] [Google Scholar]


