Abstract
Background/Aims:
Treatment success for Helicobacter pylori infection in Saudi Arabia is relatively unexplored. This prospective study compared the efficacy of sequential versus standard triple therapy in curing H. pylori infections.
Patients and Methods
Eligible patients underwent upper endoscopy at a single center in Saudi Arabia from October 2011 to February 2014. Patients who tested positive for H. pylori infection were randomly assigned to sequential therapy or standard triple therapy. Sequential treatment: Esomeprazole (20 mg bid for 10 days), amoxicillin (1000 mg for 5 days), then clarithromycin 500 mg and tinidazole 500 mg; both bid for 5 days. Standard triple treatment: Esomeprazole 20 mg, clarithromycin 500 mg, and amoxicillin 1000 mg each bid for 14 days. After 6 weeks of treatment, patients were tested for cure using a validated urea breath test. Application of the E-test determined susceptibility of H. pylori to different antibiotics.
Results:
Of the 115 patients who received sequential therapy, 93 completed treatment. In the triple-therapy arm, 103 of 117 patients completed treatment. The eradication rate was 58/93 (62.3%) with sequential therapy and 69/102 (67.6%) with standard triple therapy, P = 0.44. Risk ratio was 0.92 (95% CI; 0.75–1.13), and number needed to treat was 19. Overall primary resistance: Metronidazole (48.5%), clarithromycin (23.3%), amoxicillin (14.8%), levofloxacin (11.1%), and tetracycline (2.3%). Mild adverse events occurred in 35 and 17 patients in the sequential and standard therapy groups, respectively.
Conclusion:
Sequential and standard triple therapies were similarly effective at eradicating H. pylori in two-thirds of Saudi patients. Metronidazole and clarithromycin resistance to H. pylori strains was common.
Keywords: Helicobacter pylori, Saudi Arabia, sequential therapy, triple therapy
Helicobacter pylori is a slow-growing, spiral-shaped, highly motile, gram-negative bacteria. Approximately half of the world's population has H. pylori infection[1] with an estimated prevalence of 51%–78% in Saudi Arabia.[2,3,4,5] Consequences of infection include chronic gastritis, duodenal ulcer, gastric ulcer, gastric cancer, or primary gastric MALT lymphoma.[6] Curing H. pylori infection might cure dyspepsia, peptic ulcer, and MALT lymphoma and may prevent the development of gastric cancer as well.[7]
Recommended first-line treatment for H. pylori infection consists of a triple-therapy regimen containing a proton pump inhibitor (PPI), clarithromycin (CLR), and either amoxicillin (AML) or metronidazole (MTZ) given for 7–14 days.[8] Recent data suggest that the success rate of classic triple therapy has decreased worldwide with increased rates of resistance to CLR and/or MTZ suspected as the underlying cause.[9] A previous study from Saudi Arabia reported H. pylori resistance to MTZ in 80%, CLR in 4%, AML in 1.3%, and tetracycline (TE) in 0.4% of infected people.[10] Assessment of H. pylori clinical isolates from 368 Saudi patients found a similar pattern of antibiotic resistance. The highest resistance was reported for MTZ (48.2%) followed by CLR (27.7%), AML (14.6%), and TE (9.5%).[11]
There are no new drugs in development for treating this infection. Therefore, researchers have looked at combinations of antibiotics and determined sequential therapy as a successful alternative.[9] A 10-day sequential therapy consists of 5 days of treatment with a PPI plus one antibiotic (usually AML 1 g bid) followed by a 5-day treatment with a PPI plus two other antibiotics (usually CLR and tinidazole/MTZ). A recent meta-analysis concluded that a 10-day sequential regimen is superior to standard triple therapy given for 7 or 10 days. The crude rates of H. pylori eradication in 10 randomized controlled trials (RCTs) involving 2747 patients were 93.4% for sequential therapy (n = 1363) and 76.9% for standard triple therapy (n = 1384).[12] Prolonging duration of PPI-based triple therapy to an optimal 14 days also appears to increase the cure rate.[13]
Data from the Middle East comparing the efficacy of sequential therapy with standard first-line therapy in naïve, adult, H. pylori–infected patients are lacking. Therefore, we conducted a prospective open-label RCT to compare the success rate of sequential therapy versus standard triple therapy for eradication of H. pylori infection. As a second objective, we investigated primary resistance rates of H. pylori organisms to different antibiotics.
PATIENTS AND METHODS
Study design and patients
The prospective open-label RCT enrolled patients undergoing gastroscopy for upper-gastrointestinal symptoms at a tertiary referral teaching hospital in Saudi Arabia. The enrollment lasted from October 2011 to February 2014. Patients eligible for randomization had H. pylori infection, previously untreated with H. pylori eradication treatment (naïve), and were older than 18 years. The study excluded pregnant or lactating patients, those allergic to AML, CLR, or tinidazole, and patients with severe cardiac, renal, pulmonary or hepatic disease, or malignancy. Patients provided written-informed consent prior to undergoing esophago-gastro-duodenoscopy (EGD). The study was approved by the institute's ethics committee and the research promotion group (RAC #2111019).
Procedures
All patients underwent EGD. During endoscopy, five biopsies were retrieved; two from the antrum, one from the incisura, and two from gastric body. Tissue from the antrum was used for the rapid urea test (RUT). H. pylori culture and sensitivity testing involved antrum and gastric body tissue and histology assessment evaluated specimens from the incisura and gastric body. A patient was considered H. pylori positive if the RUT (HP Fast. GI supply™, PA, USA) detected a H. pylori organism, demonstrating H. pylori organism in the histology or the culture was positive for H. pylori.
Patients were randomly allocated to sequential therapy or standard therapy by computer-generated assignment. A research assistant managed the randomization and distributed the medication. Two pathologists and the nuclear medicine staff remained blinded to the treatment allocation. The information on treatment allocation was kept hidden from the physician until the medications were prescribed.
Patients randomized to sequential treatment received esomeprazole 20 mg twice daily for 10 days, AML 1000 mg bid for the first 5 days, followed by 5 days of CLR 500 mg and tinidazole 500 mg both given twice daily. The standard triple treatment consisted of esomeprazole 20 mg, CLR 500 mg, and AML 1000 mg (each drug administered twice daily) for 14 days. Patient interviews assessed compliance to treatment by asking for details about the number of prescribed pills consumed.
The primary endpoint was a negative urea breath test (UBT) after 6 weeks of eradication treatment. The Nuclear Medicine Department took care of administering the UBT, using Carbon-14 bound urea (3 microcurie of C-14) as the source of radiolabeled carbon dioxide. Patients abstained from PPIs and bismuth-containing agents for 2 weeks and antibiotics for at least 4 weeks prior to the test.
Culture and antibiotic susceptibility testing of H. pylori
Urease-positive gastric biopsy specimens were cultured in a microaerophilic environment (CO2 at 37°C for 72 h). Blood agar, chocolate agar, and selective Campylobacter agar and Thayer–Martin agars were utilized as culture media. The organism was identified as H. pylori with its predefined properties. Once the colonies were identified as H. pylori in the primary isolation, a subculture was performed for the purpose of acquiring a secondary isolation, which was used for antibiotic sensitivity testing.
Minimum inhibitory concentrations (MICs) were determined with the E-test method (Epsilometer test; AB Biodisk Solna, Sweden; Biomerieux, France). Antibiotics evaluated with E-test strips included AML, MTZ, CLR, TE, rifampin, and levofloxacin. The strips were aseptically placed onto the dried surface of inoculated agar plates, which were then incubated under microaerophilic conditions at 35 ± 2°C. MIC was read as the intersection of the elliptical zone of growth inhibition using the MIC scale on the E-test strip after 48–72 h of incubation. The following breakpoints were used: (AML; MIC ≤0.12 sensitive and >0.12 μg/mL resistant), (CLR; MIC ≤0.25 sensitive, 0.5 intermediate, and ≥1 μg/mL resistant), (levofloxacin; MIC <1 sensitive and >1 μg/mL resistant), (TE; MIC ≤1 sensitive and >1 μg/mL resistant), (MTZ; MIC ≤8 sensitive and ≥8 μg/mL resistant). All MICs were interpreted according to the European Committee on Antimicrobial Susceptibility Testing clinical breakpoints criteria except for CLR, which followed the Clinical Laboratory Standard Institute interpretations.
Statistics
Sample size was calculated using a two-tailed alpha test (significance level of 0.05); 80% powered to detect a 75% therapeutic response to standard triple therapy and 90% therapeutic response to sequential treatment (a difference in the eradication rate of 15%). The total number of cases required to prove the hypothesis was 230 patients (115 patients in each arm).
Numerical data were shown as mean, median, and range and qualitative data expressed as a ratio. Statistical tools such as the Chi-square test and t-test were used at appropriate places and statistical significance was calculated with a two-tailed test. A P< 0.05 was considered as statistically significant. The risk ratio and number needed to treat was also calculated. The SPSS 20 statistics software (IBM, Amonk, NY, USA) was used for the statistical analyses.
Eradication rate by intention-to-treat (ITT) analysis involved finding the percentage of eradicated patients over the total number of patients recruited. The per-protocol analysis focused on the percentage of patients who completed treatment divided by the total number of patients recruited to respective treatment arms.
RESULTS
A total of 652 patients were screened for H. pylori infection during EGD. The most common indication for performing EGD was dyspepsia in 292 (44.8%). The H. pylori infection was confirmed by histology and staining in 214 (32.8%) patients, by RUT in 193 (29.6%) patients, and by culture in 85 (13%) patients. Of the 652 patients screened, 232 (35.5%) enrolled into the study and were randomized, of whom 195 completed the study (93 from the sequential therapy group and 102 from the standard therapy group) and 37 dropped out [Figure 1]. Failure to confirm eradication of infection by UBT was the main reason for incomplete study results.
Figure 1.
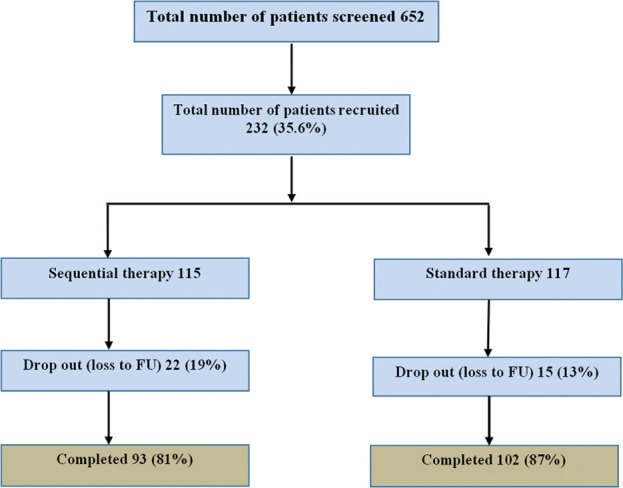
Flow diagram of patient's recruitment
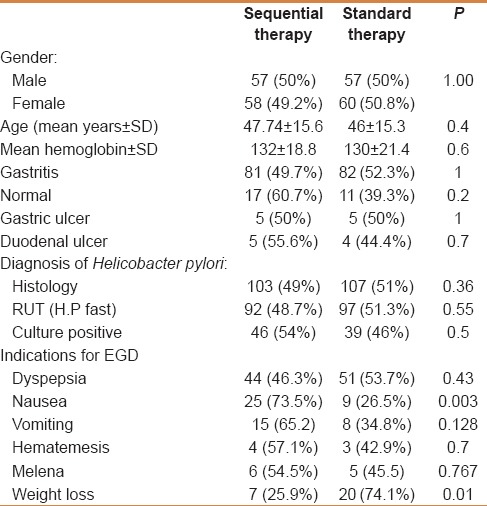
Table 1 summarizes the baseline characteristics of the 232 patients. The gender, mean age, endoscopy findings, diagnosis of H. pylori and indications for EGD were similar between treatment groups, except nausea, which occurred more frequently in the sequential therapy group.
Table 1.
Baseline characteristics of 232 randomized patients
The per protocol analysis confirmed eradication in 58/93 (62.4%) patients in the sequential therapy group and 69/102 (67.6%) patients in the triple-therapy group (P = 0.44) [Figure 2]. The ITT analysis showed a lower rate of eradication for both groups; 58/115 (50.4%) and 69/117 (59%), respectively, for sequential therapy and standard therapy [Figure 2]. The risk ratio for the cure was 0.92 (95% confidence interval, 0.75–1.13) and the number needed to treat was 19.
Figure 2.
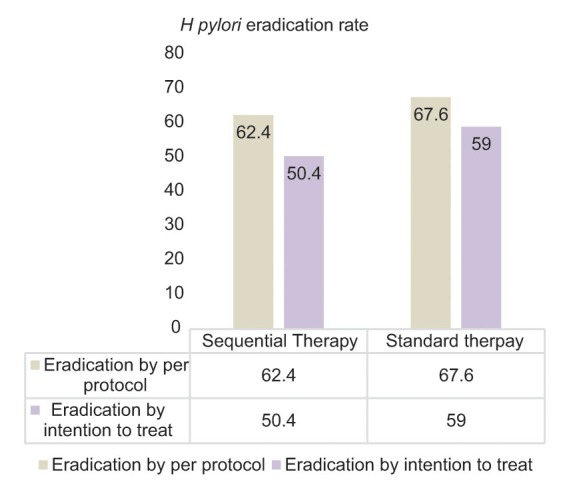
Results of H. pylori eradication treatment with sequential and standard therapy, ITT and PP analysis
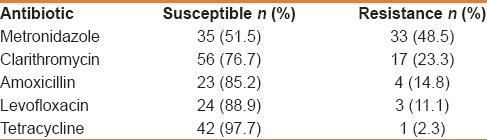
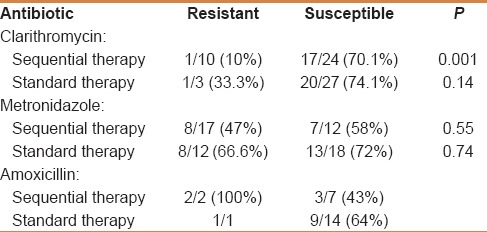
The H. pylori culture was successful in 85/232 (36.6%) patients. Table 2 lists the results of overall antibiotic sensitivity from secondary cultures as determined with the E-test. H. pylori resistance was highest for MTZ (in 48.5% of isolates), followed by CLR (in 23.3% of isolates), AML (in 14.8% of isolates), then levofloxacin (11.1). Resistance to TE was observed in one strain.
Table 2.
Antibiotic sensitivity and resistance of recovered Helicobacter pylori strains (n=85)
Patients treated with sequential therapy experienced a significantly lower cure rate if they harbored a CLR-resistant strain (P = 0.001). The cure rate was only 10% in these patients. Resistance to either AML or MTZ did not adversely affect the efficacy of either treatment. H. pylori eradication rates stratified by resistance or susceptibility to different antibiotics are presented in Table 3.
Table 3.
Helicobacter pylori eradication rate based on the susceptibility to different antibiotics (univariate analysis)
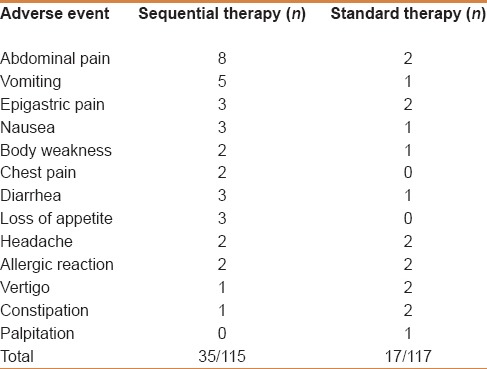
Patients self-reported adverse events. In the sequential therapy group, 35 patients reported adverse events, and 17 patients on standard treatment complained of side effects. The most common adverse events were abdominal pain, vomiting, and nausea. There were no serious adverse events. Table 4 details the distribution of adverse events for all subjects.
Table 4.
Adverse events as reported by the patients
DISCUSSION
Our study demonstrated that sequential therapy and standard triple therapy offer a similar treatment benefit for H. pylori infection although suboptimal. The eradication rate was 50.4% with sequential therapy and 59% with standard therapy as per ITT analysis. These data suggest that we need to develop a better therapy against H. pylori.
The rate of resistance to both AML (14.8%) and CLR (23.3%) in this study mirrors data published by Momenah et al., but is higher than earlier reported values.[10,11] Increasing resistance to these antibiotics may be a consequence of their frequent usage for treating bacterial infections such as upper respiratory tract infections and gastroenteritis. Saudi Arabian studies have also consistently reported a high incidence of H. pylori resistance to MTZ. A study from Jeddah (Western region, Saudi Arabia) showed H. pylori resistance to MTZ in 80% of isolates.[10] We evaluated H. pylori antibiotic susceptibility using the E-test method and determined MTZ resistance in 48.5% of patients. The Momenah study, which examined susceptibility using the disk diffusion method, identified a similar MTZ resistance (48.2%).
Several studies have investigated the role of sequential therapy in the treatment of naïve H. pylori, the bulk of which were carried out in European countries and North America.[14,15] The meta-analysis by Jafri et al. suggested a superiority of sequential therapy over standard therapy for H. pylori eradication. In that particular meta-analysis, the crude eradication rate with sequential therapy was 93.4% versus 76.9% with standard therapy.[12] However, most studies included in this meta-analysis took place in Italy, and only one was double- blinded.[12]
To the best of our knowledge, this is the first report addressing the use of sequential therapy for H. pylori in adults from the Middle East. A study on pediatric patients from Jeddah (Western region, Saudi Arabia) that compared sequential and standard therapy showed no difference in treatment effects.[16] A multicenter study involving a center from Egypt and two centers from Saudi Arabia compared the role of levofloxacin-based firstline therapy versus standard therapy consisting of AML, CLR, and esomeprazole; both regimens were administered for 7 days. In this trial, the eradication rate was 90.6% using the levofloxacin-based regimen and 78.6% with standard therapy.[17]
In our study, eradication of H. pylori occurred more frequently with standard therapy, which may relate to CLR resistance. The efficacy of CLR decreases if the bacteria can develop efflux channels, by which they can transfer the drug out of the cell. AML given in the first 5 days weakens the cell walls and thereby inhibits the production of efflux channels, ultimately increasing the availability of CLR intracellularly.[14] However, this advantage in sequential therapy may not work if the organism is already resistant to CLR. Hence, the success of treatment involving CLR depends highly on bacterial susceptibility. We noted CLR resistance as a predictive factor for failure of sequential therapy possibly due to its diminished availability within the cell. It is also possible that the higher eradication with standard therapy relates to 14 days of treatment in this group.
That 30%–40% of our patients failed to eradicate the H. pylori infection seems rather high. In addition to the above-mentioned reasons, poor adherence to medications and other factors such as age, smoking, intragastric bacterial load, and genetic polymorphisms might have played a role.[18]
We attempted to culture gastric tissue from each patient and detect the resistance pattern. Culturing the H. pylori organism was difficult because of its innocuous nature. We succeeded in culturing the organism in 85 of 232 (36.6%) patients. Identifying antibiotic susceptibility of H. pylori was another difficult task, as many centers still do not have E-test kits for performing sensitivity tests. The resistance pattern of H. pylori to different antibiotics established in our study should be helpful to researchers in their future work.
Determining the antibiotic susceptibility through molecular-based tests could improve the success rate of eradicating H. pylori.[8] PCR-based tests such as restriction fragment length polymorphism represents one such method. Another method known as the rRNA-based whole cell hybridization method detects Helicobacter species in situ within gastric tissue and identifies CLR-resistance genotype.[19]
Future trials incorporating TE will be justified in view of the very low resistance to this antibiotic. Switching to bismuth- based quadruple therapy or adding newer molecules specifically targeting H. pylori to current regimens represents a second option for increasing the H. pylori cure rate. Developing diagnostic agents for detecting antibiotic susceptibility and making them available freely in the market is another important step in the eradication of the organism.
The strength of the current study is its methodology; it was conducted strictly following all the rules for an RCT. Adequate numbers of patients were recruited and followed up in both arms. The study had some notable weaknesses. Patients self-reported adverse events and adherence to medication was from the patient's history. For unknown reasons, the study determined lower overall eradication rates than expected for both the groups.
CONCLUSION
The current randomized controlled study conducted in Saudi Arabia showed that both sequential therapy and standard therapy were equal in efficacy to eradicate H. pylori. The efficacy rate was low for both treatments. Metronidazole and clarithromycin resistance was common in this population.
ACKNOWLEDGMENTS
We are grateful to Dr. Sahar Althawadi, Microbiology Department, King Faisal Specialist Hospital and Research Centre for her assistance in processing H. pylori cultures and sensitivities. We also thank Prof. Sander Veldhuyzen van Zanten, Edmonton, Alberta, Canada, for reviewing this article and providing his invaluable feedback.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Go MF. Review article: Natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16(Suppl 1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- 2.Khan MA, Ghazi HO. Helicobacter pylori infection in asymptomatic subjects in Makkah, Saudi Arabia. J Pak Med Assoc. 2007;57:114–7. [PubMed] [Google Scholar]
- 3.Marie MA. Seroprevalence of Helicobacter pylori Infection in Large Series of Patients in an Urban Area of Saudi Arabia. Korean J Gastroenterol. 2008;52:226–9. [PubMed] [Google Scholar]
- 4.Akbar DH, Eltahawy AT. Helicobacter pylori infection at a university hospital in Saudi Arabia: Prevalence, comparison of diagnostic modalities and endoscopic findings. Indian J Pathol Microbiol. 2005;48:181–5. [PubMed] [Google Scholar]
- 5.Ayoola AE, Ageely HM, Gadour MO, Pathak VP. Prevalence of Helicobacter pylori infection among patients with dyspepsia in South-Western Saudi Arabia. Saudi Med J. 2004;25:1433–8. [PubMed] [Google Scholar]
- 6.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 7.Bayerdorffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591–4. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection: The Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 9.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 10.Eltahawy AT. Prevalence of primary Helicobacter pylori resistance to several antimicrobials in a Saudi Teaching Hospital. Med Princ Pract. 2002;11:65–8. doi: 10.1159/000058009. [DOI] [PubMed] [Google Scholar]
- 11.Momenah AM, Asghar AH. Prevalence and antibiotic resistance among Helicobacter pylori clinical isolates from main hospitals in the western region of Saudi Arabia. Pak J Med Sci. 2008;24:100–3. [Google Scholar]
- 12.Jafri NS, Hornung CA, Howden CW. Meta-analysis: Sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Y, Ford AC, Khan KJ, Gisbert JP, Forman D, Leontiadis GI, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337. doi: 10.1002/14651858.CD008337.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A randomized trial. Ann Intern Med. 2007;146:556–63. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 15.Gatta L, Vak, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: Systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–79. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 16.Ali Habib HS, Murad HA, Amir EM, Halawa TF. Effect of sequential versus standard Helicobacter pylori eradication therapy on the associated iron deficiency anemia in children. Indian J Pharmacol. 2013;45:470–3. doi: 10.4103/0253-7613.117757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assem M, El Azab G, Rasheed MA, Abdelfatah M, Shastery M. Efficacy and safety of Levofloxacin, Clarithromycin and Esomeprazol as first line triple therapy for Helicobacter pylori eradication in Middle East. Prospective, randomized, blind, comparative, multicenter study. Eur J Intern Med. 2010;21:310–4. doi: 10.1016/j.ejim.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Perri F, Villani MR, Festa V, Quitadamo M, Andriulli A. Predictors of failure of Helicobacter pylori eradication with the standard ‘Maastricht triple therapy’. Aliment Pharmacol Ther. 2001;15:1023–9. doi: 10.1046/j.1365-2036.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- 19.Trebesius K, Panthel K, Strobel S, Vogt K, Faller G, Kirchner T, et al. Rapid and specific detection of Helicobacter pylori macrolide resistance in gastric tissue by fluorescent in situ hybridisation. Gut. 2000;46:608–14. doi: 10.1136/gut.46.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]


