Abstract
Ischemia-Reperfusion (IR) injury is known to contribute significantly to the morbidity and mortality associated with ischemic strokes. Ischemic cerebrovascular accidents account for 80% of all strokes. A common cause of IR injury is the rapid inflow of fluids following an acute/chronic occlusion of blood, nutrients, oxygen to the tissue triggering the formation of free radicals.
Ischemic stroke is followed by blood-brain barrier (BBB) dysfunction and vasogenic brain edema. Structurally, tight junctions (TJs) between the endothelial cells play an important role in maintaining the integrity of the blood-brain barrier (BBB). IR injury is an early secondary injury leading to a non-specific, inflammatory response. Oxidative and metabolic stress following inflammation triggers secondary brain damage including BBB permeability and disruption of tight junction (TJ) integrity.
Our protocol presents an in vitro example of oxygen-glucose deprivation and reoxygenation (OGD-R) on rat brain endothelial cell TJ integrity and stress fiber formation. Currently, several experimental in vivo models are used to study the effects of IR injury; however they have several limitations, such as the technical challenges in performing surgeries, gene dependent molecular influences and difficulty in studying mechanistic relationships. However, in vitro models may aid in overcoming many of those limitations. The presented protocol can be used to study the various molecular mechanisms and mechanistic relationships to provide potential therapeutic strategies. However, the results of in vitro studies may differ from standard in vivo studies and should be interpreted with caution.
Keywords: Medicine, Issue 99, Oxygen-glucose deprivation and reoxygenation, ischemia-reperfusion injury, blood-brain barrier, brain endothelial cells, tight junctions, immunofluorescence, f-actin staining
Introduction
Ischemia-Reperfusion (IR) injury is found to be the frequent cause of various debilitating complications and deaths associated with stroke, myocardial infarction, trauma, peripheral vascular disease and traumatic brain injury1,2. IR injury in cerebral vessels is an early secondary injury leading to inflammation and edema3. One of the serious complications that occurs as a result of oxidative and metabolic stress following inflammation is loss of homeostatic balance leading to free radical formation, alterations in the blood-brain barrier (BBB) tight junctions (TJs) and microvascular permeability4,5.
Currently, in vivo models used to study the effects of IR injury on the BBB include middle cerebral artery occlusion (MCAO), microembolism, and transgenic or knockout animals. However, each has its drawbacks and limitations as discussed by Hossmann6. MCAO model is used to study the effects of redox stress, changes in junctional communications of the BBB and the interactions between brain and immune cells. However, they present various technical challenges such as the need for precise microsurgical procedures and the difficulties therein. Microembolism instantaneously breaks down the BBB while use of transgenic or knockout animals to study cerebral ischemia may have challenges like gene-dependent molecular influences on infarct formation, changes in vascular anatomy and varying body weights6. Hence, in vitro models of ischemia have found increasing interest in recent times mainly due to their applicability in performing mechanistic studies for drugs. However, the results of in vitro studies may not fully represent an in vivo study and must be interpreted with caution6.
Counteractive effect of low oxygen concentrations on endothelial cell monolayers and microvascular permeability have been studied by Ogawa7. Rat brain microvascular endothelial cells (RBMECs) were used to develop the in vitro BBB. The oxygen-glucose deprivation and reoxygenation (OGD-R) technique presented in this protocol has been adapted from studies by Zulueta et al and Zhu et al8,9. We exposed brain endothelial cells to OGD-R by placing them in a hypoxia/anoxia chamber containing 0% O2, 5% CO2 and 95% N2. Cells were later assessed for alterations in TJ integrity and stress fiber formation using immunofluorescence localization and rhodamine phalloidin labeling respectively. Immunofluorescence staining for zonula occludens-1 (ZO-1) is performed to determine TJ integrity, as ZO-1 is an important scaffolding membrane bound TJ protein. Rhodamine Phalloidin labeling determines the filamentous actin (f-actin) in the cell cytoskeleton and is a clear indication of actin stress fiber formation in endothelial cells.
The goal of this method is to provide insight into developing OGD-R as an in vitro IR model for studying BBB endothelial cell TJ integrity and f-actin stress fiber formation. The results will provide information on the fate of TJ protein, ZO-1 and stress fiber formation following OGD-R. Understanding these relationships will provide an opportunity to determine the underlying molecular mechanisms that are triggered following OGD-R and develop potential therapeutic strategies to enhance the BBB disruption following OGD-R treatment.
Protocol
1. Seeding of Endothelial Cells
Obtain primary cultures of RBMEC’s from adult Sprague Dawley rats (or obtain them commercially).
Cultivate RBMECs in 100 cm fibronectin (50 µg/ml) coated petri dishes using the rat brain endothelial cell growth medium. Change the medium every two days, until confluency is reached.
On reaching 80-90% confluency, gently wash the cells in 5 ml phosphate buffered saline (PBS) by swirling. The cells are then detached by exposing them to 1 ml of warm 0.25% trypsin- ethylenediaminetetraacetic acid (EDTA) solution, equilibrated to 37 °C.
Incubate the cells at 37 °C for 2-5 min until the cells are detached and dispersed. NOTE: Tap the culture dish to detach the cells. View cells under the microscope to confirm complete detachment of cells from the surface of the dish.
Add 5 ml complete media to the petri dish in order to neutralize trypsin. Pipette out the medium containing detached cells and collect it into a 15 ml centrifuge tube. .
Centrifuge the media containing endothelial cells at 220 x g for 5 min.
Aspirate the supernatant and preserve the pellet containing cells.Suspend the pellet into 3-5 ml fresh rat brain endothelial cell growth medium by gently mixing it up and down with pipette Cells will be then be counted using automated cell counter or a hemocytometer.
Transfer the cell suspension into fibronectin (50 μg/ml) precoated 8 well sterile chamber slide system, 0.7 cm2/well with a seeding density ranging between 10,000-15,000 cells per well. Grow the cells at 37 °C until confluence is achieved NOTE: For performing western blots or other experiments,cells can be grown in 10 cm cell culture dishes or special dishes as required by the experiment.
2. Oxygen and Glucose Deprivation-Reoxygenation In Vitro Model
NOTE: The following protocol has been adapted from Zulueta et al. 1997; Zhu H et al. 20128,9.
Use the hypoxia cell culture system to study the effects of of OGD-R on RBMECs in (see Figure 1). Setup and calibrate the hypoxia cell culture system before beginning the experiment, according to manufacturer’s instructions.
Remove the confluent chamber slides (step 1.8) from the 37 °C incubator. Replace the complete medium in the chamber slide with deoxygenated, no glucose, Dulbecco's Modified Eagle's Medium (DMEM) and placed in hypoxia chamber with 95%, N2 and 5% CO2 for 2 h at 37 °C, to represent OGD condition.
Move the cells back to the incubator with 95% O2, 5% CO2 at 37 °C and provided with fresh rat brain endothelial cell complete medium and incubate for another 1 hr at 37 °C. NOTE: This step represents a reoxygenation situation.
Use the chamber slides for immunofluorescence localization and rhodamine phalloidin labeling (see section 3).
3. Immunofluorescence Localization of ZO-1 and f-actin Labeling Using Rhodamine Phalloidin
Expose the chamber slides containing RBMEC monolayers to 100 μl of opti-MEM/reduced serum media/well for 1 hr. Wash the chamber slides 3 times in 100 μl of phosphate-buffered saline (PBS, pH 7.0-7.2).
Fix the cells using 100 μl of 4% paraformaldehyde in PBS (pH 7.0-7.2) for 15 min and wash the chamber slides for 3 more times in PBS (pH 7.0-7.2).
- Permeabilize the cells using 100 μl of 0.5% Triton X-100 in PBS, (pH 7.0-7.2) for another 15 min. Block with 100 μl of 2% bovine serum albumin (BSA) in PBS for an hour. After this step either stain/label cells either for ZO-1 or f-actin.
- For immunofluorescence staining incubate the cells with an anti-rabbit primary antibody against ZO-1 in 1:150, prepared in 2% BSA-PBS for O/N at 4 °C. Wash the cells 3 times in PBS,(pH 7.0-7.2). Incubate with 100 μl of Fluorescein isothiocyanate (FITC)-tagged anti-rabbit secondary antibody for 1 hr at RT.
- For Rhodamine Phalloidin labeling, following blocking, expose the cells to 100 μl of rhodamine phalloidin in 1:50 dilution, prepared in 2% BSA-PBS, for 20 min.
- Wash the cells from immunofluorescence staining and rhodamine phalloidin labeling in PBS, (pH 7.0-7.2). Mount the chambers using mounting media containing anti-fade reagent with DAPI.
Visualize the cells using a 60 X water immersion lens and cells are scanned in a single optical plane under a confocal microscope.
Representative Results
Cells cultured on fibronectin precoated Nunc II chamber slides were subjected to OGD-R by placing in a Biospherix ProOx model 110 chamber. After subjecting cells to OGD-R, they were processed for ZO-1 junctional staining using immunofluorescence technique as shown in Figure 2 and cytoskeletal assembly indicating F-actin stress fiber formation using rhodamine phalloidin stain label as shown in Figure 3. Control cells that were not subjected to OGD-R showed continual junctional integrity while endothelial cells subjected to OGD-R showed discontinuous junctions, indicating loss of TJ integrity Figure 2. Control cells that were not subjected to OGD-R treatment showed no or minimal f-actin stress fiber formation while endothelial cells subjected to OGD-R demonstrated increased f-actin stress fiber formation indicating the changes in the actin cytoskeletal assembly Figure 3; Reprint with permission from Reference #13
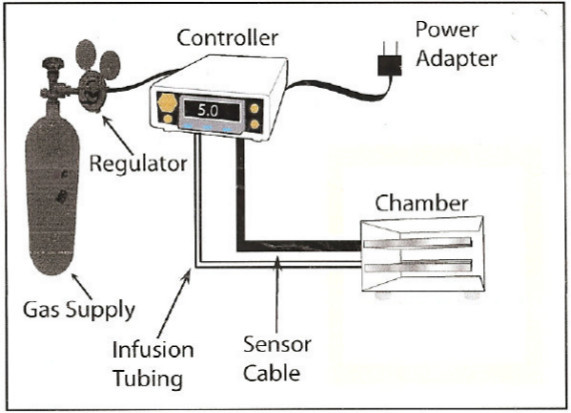 Figure 1. Typical Configuration of the Hypoxia System ProOx Model 110. Please click here to view a larger version of this figure.
Figure 1. Typical Configuration of the Hypoxia System ProOx Model 110. Please click here to view a larger version of this figure.
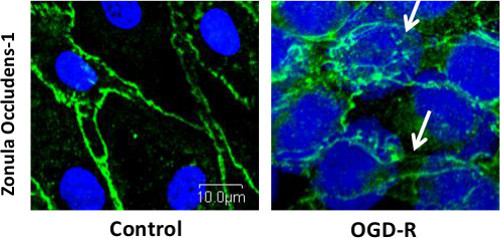 Figure 2. Immunofluorescence Localization of Tight Junction Protein (TJP) ZO-1 Demonstrating Disruption of the Tight Junctions following OGD-R in RBMECs, as Shown by White Arrows. Scale bar = 10 μm. Please click here to view a larger version of this figure.
Figure 2. Immunofluorescence Localization of Tight Junction Protein (TJP) ZO-1 Demonstrating Disruption of the Tight Junctions following OGD-R in RBMECs, as Shown by White Arrows. Scale bar = 10 μm. Please click here to view a larger version of this figure.
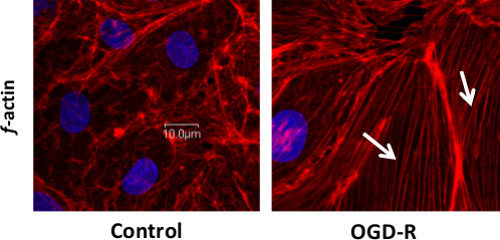 Figure 3. Rhodamine Phalloidin Labeling for f-actin Stress Fiber Formation Demonstrating Changes in Cytoskeletal Assembly Following OGD-R in RBMECs, as Shown by White Arrows. Scale bar = 10 μm. Please click here to view a larger version of this figure.
Figure 3. Rhodamine Phalloidin Labeling for f-actin Stress Fiber Formation Demonstrating Changes in Cytoskeletal Assembly Following OGD-R in RBMECs, as Shown by White Arrows. Scale bar = 10 μm. Please click here to view a larger version of this figure.
Discussion
OGD-R as an in vitro model for ischemia-reperfusion injury has been well established for studying neurons10,11. There are also studies showing the effect of OGD on brain endothelial cells and alterations in permeability and TJ integrity9. However, our study shows the effect of OGD as well as reoxygenation, which is a closer representation of ischemic reperfusion injury in in vivo conditions that occur following ischemic stroke.
Hypoxic-ischemic conditions are known to induce inflammation in the central nervous system, leading to BBB disruption by increasing paracellular permeability and vasogenic edema4. Reperfusion injury is a very complex and dynamic process, in which there is excessive reactive oxygen species production, ATP depletion, rise in extracellular potassium, release of excitatory neurotransmitters, endothelial and neuronal swelling, immune cell activation. This, in turn causes activation of various inflammatory pathways, leading to cytokine production, induction of nucleases and proteases, leading to edema, all of which are shown to activate various caspases, leading to disruption of the BBB integrity5,12. Endothelial cells are particularly prone to ischemic injury, due to the presence of large numbers of mitochondria. The neighboring endothelial cells are linked to each other by tight junctions maintained by tight junction proteins.
ZO-1 is shown to play an important role in maintaining the TJ integrity by its interaction with the other tight junction proteins and the actin cytoskeletal assembly. Also, precise regulation of actin cytoskeleton is essential for many developmental and physiological processes including cell-cell adhesions. In endothelial cells, actin stress fiber formation is found to be associated with barrier dysfunction and hyperpermeability13,14. Rhodamine phalloidin labeling technique used in the present study to observe f-actin stress fiber formation is an end point study. However, dynamic and live imaging of the stress fiber formation can also be performed as shown by Doggett and Breslin15.
The current study majorly emphasizes the contribution of ZO-1 towards the BBB tight junction integrity. However, other tight junction molecules like claudin-5, occludin, junctional adhesion molecules (JAM) etc can also be used as markers to study the BBB tight junction integrity following OGD-R. In this study, we employed immunofluorescence localization and f-actin labeling as a qualitative technique to determine the tight junction integrity. However, we can also study the other important characteristics of the BBB like quantitative measurement of permeability using fluorescent markers and transendothelial electrical resistance (TEER).
The OGD-R technique presented in this study using the Biospherix system can only be used for hypoxia/anoxia studies at a fixed concentration of oxygen; however, we cannot employ this system for studying the effect of varying concentrations of oxygen on endothelial cells. For studying the effects of varying concentrations of oxygen on endothelial cells we need to employ other available models suited for the purpose.
This technique can be used for studying the mechanistic relationships between various cellular and molecular events that regulate BBB hyperpermeability and TJ integrity. Understanding them will provide insight for developing various molecular strategies to attenuate BBB disruption and microvascular permeability.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We acknowledge Scott and White Hospital Research Grants Program for financial support and Texas A&M Health Science Center College of Medicine Integrated Imaging Laboratory for the use of the confocal laser microscope. We acknowledge Mr. Glen Cryer for help with manuscript editing.
References
- Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, et al. Lycium barbarum polysaccharides reduce intestinal ischemia/reperfusion injuries in rats. Chem Biol Interact. 2013;204(3):166–172. doi: 10.1016/j.cbi.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5(1):71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13) 1:S52–S57. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovasc Res. 1998;39:106–120. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovineendothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990;85(4):1090–108. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulueta JJ, Sawhney R, Yu FS, Cote CC, Hassoun PM. Intracellular generation of reactive oxygen species in endothelial cellsexposed to anoxia-reoxygenation. Am J Physiol. 1997;272(5 Pt 1):L897–L902. doi: 10.1152/ajplung.1997.272.5.L897. [DOI] [PubMed] [Google Scholar]
- Zhu H, et al. Baicalin reduces the permeability of the blood-brain barrier during hypoxia in vitro by increasing the expression of tight junction proteins in brain microvascular endothelial cells. J Ethnopharmacol. 2012;141(2):714–720. doi: 10.1016/j.jep.2011.08.063. [DOI] [PubMed] [Google Scholar]
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27(5):1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundimeda U, et al. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cepsilon. J Biol Chem. 2012;287(41):34694–34708. doi: 10.1074/jbc.M112.356899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Alluri H, et al. Reactive Oxygen Species-Caspase-3 Relationship in Mediating Blood-Brain Barrier Endothelial Cell Hyperpermeability Following Oxygen-Glucose Deprivation and Reoxygenation. Microcirculation. 1111;21(2):187–195. doi: 10.1111/micc.12110. [DOI] [PubMed] [Google Scholar]
- Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13(3):237–247. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- Doggett TM, Breslin JW. Study of the actin cytoskeleton in live endothelial cells expressing GFP actin. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]


