Abstract
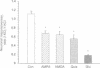
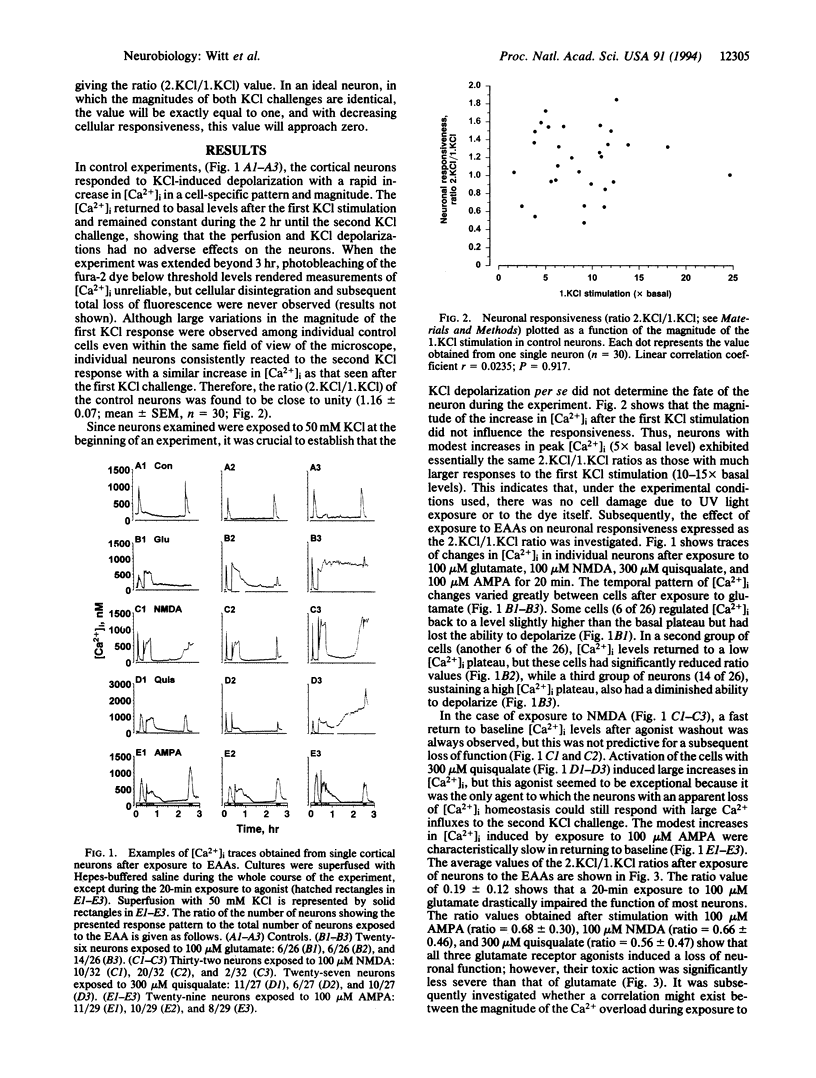
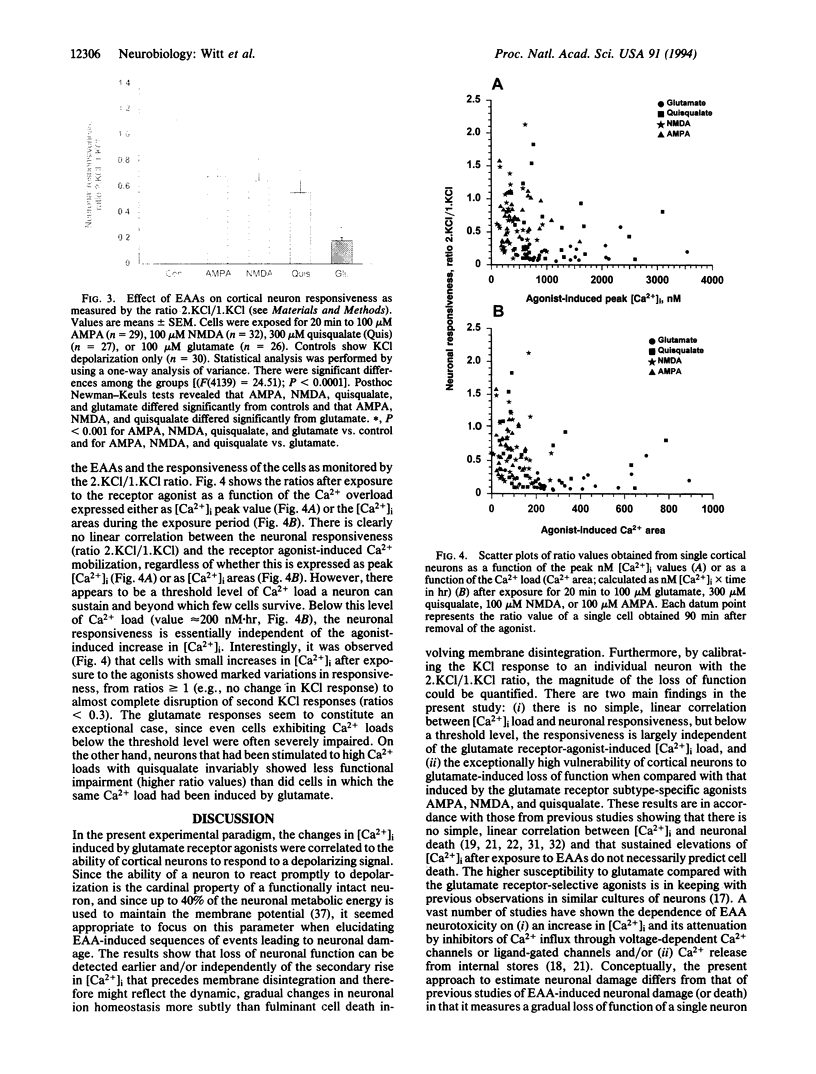
Primary cultures of cerebral cortical neurons and single-cell imaging of intracellular free Ca2+ concentration ([Ca2+]i) with the ratiometric dye fura-2 were used to assess excitatory amino acid (EAA)-induced neurotoxicity; the loss of neuronal function as defined by the ability of the cells to respond to K(+)-induced depolarization by a transient increase in Ca2+ influx was measured. The responsiveness of individual neurons was measured quantitatively as the [Ca2+]i values of the second KCl (2.KCl) stimulation divided by those of the first KCl (1.KCl) stimulation, giving the value of the ratio (2.KCl/1.KCl). Exposure to EAAs led to an increase in [Ca2+]i, but no simple correlation between the increase in [Ca2+]i and neuronal responsiveness could be demonstrated. Rather, below a threshold level of [Ca2+]i (ca. 1 microM), the neuronal responsiveness was largely independent of the glutamate receptor-agonist-induced increase in [Ca2+]i. However, when [Ca2+]i increased above this threshold level, the neurons almost invariably lost the ability to respond to a K(+)-induced depolarization, particularly after exposure to glutamate. Therefore, the cortical neurons were found to be exceptionally vulnerable to the glutamate-induced loss of function when compared with the effect induced by the glutamate receptor subtype-specific agonists, N-methyl-D-aspartate, quisqualate, and 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl) propionate. The findings suggest that the loss of neuronal membrane polarization precedes plasma membrane disruption and is a sensitive marker of EAA-induced neurodegeneration observed at the single-cell level.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Excitotoxic cell death. J Neurobiol. 1992 Nov;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985 Aug 5;58(3):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky J. M., Rothman S. M. Intracellular calcium concentrations during "chemical hypoxia" and excitotoxic neuronal injury. J Neurosci. 1991 Aug;11(8):2545–2551. doi: 10.1523/JNEUROSCI.11-08-02545.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimerl S., Schramm M. Potentiation of 45Ca uptake and acute toxicity mediated by the N-methyl-D-aspartate receptor: the effect of metal binding agents and transition metal ions. J Neurochem. 1993 Aug;61(2):518–525. doi: 10.1111/j.1471-4159.1993.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Forsby A., Witt R., Walum E. Sesquiterpenoid unsaturated dialdehydes increase the concentration of intracellular free Ca2+ in human neuroblastoma SH-SY5Y cells. Nat Toxins. 1994;2(2):89–95. doi: 10.1002/nt.2620020207. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Frandsen A., Drejer J., Schousboe A. Direct evidence that excitotoxicity in cultured neurons is mediated via N-methyl-D-aspartate (NMDA) as well as non-NMDA receptors. J Neurochem. 1989 Jul;53(1):297–299. doi: 10.1111/j.1471-4159.1989.tb07327.x. [DOI] [PubMed] [Google Scholar]
- Frandsen A., Schousboe A. Dantrolene prevents glutamate cytotoxicity and Ca2+ release from intracellular stores in cultured cerebral cortical neurons. J Neurochem. 1991 Mar;56(3):1075–1078. doi: 10.1111/j.1471-4159.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Frandsen A., Schousboe A. Development of excitatory amino acid induced cytotoxicity in cultured neurons. Int J Dev Neurosci. 1990;8(2):209–216. doi: 10.1016/0736-5748(90)90013-r. [DOI] [PubMed] [Google Scholar]
- Frandsen A., Schousboe A. Excitatory amino acid-mediated cytotoxicity and calcium homeostasis in cultured neurons. J Neurochem. 1993 Apr;60(4):1202–1211. doi: 10.1111/j.1471-4159.1993.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Frandsen A., Schousboe A. Mobilization of dantrolene-sensitive intracellular calcium pools is involved in the cytotoxicity induced by quisqualate and N-methyl-D-aspartate but not by 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionate and kainate in cultured cerebral cortical neurons. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2590–2594. doi: 10.1073/pnas.89.7.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum S. R., Scholz W. K., Miller R. J. Acute- and long-term glutamate-mediated regulation of [Ca++]i in rat hippocampal pyramidal neurons in vitro. J Pharmacol Exp Ther. 1990 Jun;253(3):1293–1302. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Lees G. J. Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res Brain Res Rev. 1991 Sep-Dec;16(3):283–300. doi: 10.1016/0165-0173(91)90011-v. [DOI] [PubMed] [Google Scholar]
- Lei S. Z., Zhang D., Abele A. E., Lipton S. A. Blockade of NMDA receptor-mediated mobilization of intracellular Ca2+ prevents neurotoxicity. Brain Res. 1992 Dec 11;598(1-2):196–202. doi: 10.1016/0006-8993(92)90183-a. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Nicoll R. A. The impact of postsynaptic calcium on synaptic transmission--its role in long-term potentiation. Trends Neurosci. 1989 Nov;12(11):444–450. doi: 10.1016/0166-2236(89)90094-5. [DOI] [PubMed] [Google Scholar]
- Manev H., Favaron M., Guidotti A., Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989 Jul;36(1):106–112. [PubMed] [Google Scholar]
- Mattson M. P. Excitatory amino acids, growth factors, and calcium: a teeter-totter model for neural plasticity and degeneration. Adv Exp Med Biol. 1990;268:211–220. doi: 10.1007/978-1-4684-5769-8_24. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Guthrie P. B., Hayes B. C., Kater S. B. Roles for mitotic history in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci. 1989 Apr;9(4):1223–1232. doi: 10.1523/JNEUROSCI.09-04-01223.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels R. L., Rothman S. M. Glutamate neurotoxicity in vitro: antagonist pharmacology and intracellular calcium concentrations. J Neurosci. 1990 Jan;10(1):283–292. doi: 10.1523/JNEUROSCI.10-01-00283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani D., Guidolin D., Facci L., Pozzan T., Buso M., Leon A., Skaper S. D. Excitatory amino acid-induced alterations of cytoplasmic free Ca2+ in individual cerebellar granule neurons: role in neurotoxicity. J Neurosci Res. 1991 Mar;28(3):434–441. doi: 10.1002/jnr.490280317. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. A role for the mitochondrion in the protection of cells against calcium overload? Prog Brain Res. 1985;63:97–106. doi: 10.1016/S0079-6123(08)61978-0. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. The role of Ca2+ in cell killing. Chem Res Toxicol. 1990 Nov-Dec;3(6):484–494. doi: 10.1021/tx00018a001. [DOI] [PubMed] [Google Scholar]
- Ogura A., Miyamoto M., Kudo Y. Neuronal death in vitro: parallelism between survivability of hippocampal neurones and sustained elevation of cytosolic Ca2+ after exposure to glutamate receptor agonist. Exp Brain Res. 1988;73(3):447–458. doi: 10.1007/BF00406601. [DOI] [PubMed] [Google Scholar]
- Olney J. W. Excitotoxic amino acids and neuropsychiatric disorders. Annu Rev Pharmacol Toxicol. 1990;30:47–71. doi: 10.1146/annurev.pa.30.040190.000403. [DOI] [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Randall R. D., Thayer S. A. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992 May;12(5):1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S. M. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985 Jun;5(6):1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1(2):155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- Siman R., Noszek J. C., Kegerise C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J Neurosci. 1989 May;9(5):1579–1590. doi: 10.1523/JNEUROSCI.09-05-01579.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C., Frandsen A., Suzdak P. D., Andersen C. F., Schousboe A. Effects of t-ACPD on neural survival and second messengers in cultured cerebral cortical neurones. Neuroreport. 1993 Sep 10;4(11):1255–1258. doi: 10.1097/00001756-199309000-00011. [DOI] [PubMed] [Google Scholar]
- Tymianski M., Charlton M. P., Carlen P. L., Tator C. H. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993 May;13(5):2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]