Abstract
Pulmonary arterial hypertension (PAH) is a disease affecting distal pulmonary arteries (PA). These arteries are deformed, leading to right ventricular failure. Current treatments are limited. Physiologically, pulsatile blood flow is detrimental to the vasculature. In response to sustained pulsatile stress, vessels release nitric oxide (NO) to induce vasodilation for self-protection. Based on this observation, this study developed a protocol to assess whether an artificial pulmonary pulsatile blood flow could induce an NO-dependent decrease in pulmonary artery pressure. One group of piglets was exposed to chronic hypoxia for 3 weeks and compared to a control group of piglets. Once a week, the piglets underwent echocardiography to assess PAH severity. At the end of hypoxia exposure, the piglets were subjected to a pulsatile protocol using a pulsatile catheter. After being anesthetized and prepared for surgery, the jugular vein of the piglet was isolated and the catheter was introduced through the right atrium, the right ventricle and the pulmonary artery, under radioscopic control. Pulmonary artery pressure (PAP) was measured before (T0), immediately after (T1) and 30 min after (T2) the pulsatile protocol. It was demonstrated that this pulsatile protocol is a safe and efficient method of inducing a significant reduction in mean PAP via an NO-dependent mechanism. These data open up new avenues for the clinical management of PAH.
Keywords: Medicine, Issue 99, Piglets, pulmonary arterial hypertension, right heart catheterization, pulmonary artery pressure, vascular pulsatility, vasodilation, nitric oxide
Introduction
Pulmonary arterial hypertension is a life-threatening disease affecting the pulmonary vasculature. There is agreement in the field that an imbalance between an increase in vasoconstrictors (endothelin, serotonin) and a decrease in vasodilators (NO, prostacyclin) contributes to the development of PAH. Over time, this pro-constrictive phenotype evolves into a complex pro-proliferative and anti-apoptotic phenotype, contributing to the development of vascular lesions 1.
Prolonged exposure to vasoconstrictors leads to a significant and sustained increase in [Ca2+]i in pulmonary artery smooth muscle cells, allowing the activation of several calcium-regulated transcription factors, such as NFAT 2-4, promoting PASMC proliferation and resistance to an apoptosis phenotype 5. This phenotype leads to pulmonary vascular lesions, contributing to an increase in both PA pressure and pulmonary resistance, which ultimately leads to fatal right heart failure 6.
Currently, there is no treatment available that reverses PAH although there are several that improve patients’ quality of life 7. Among these treatments, the effectiveness of inhaled NO treatment has been demonstrated but because of its short half-life it is difficult to use in clinical practice. For this reason, more stable and durable treatments have been preferred, such as prostacyclin analogs, or endothelin receptor blockers 7. To develop better treatments, it is essential to improve and extend knowledge of the pathophysiology of PAH.
Pulsatility is a well-known stimulus activating shear stress-induced vasodilation, protecting the non-elastic distal artery from high-pressure flow injuries 8,9. In a model of PAH secondary to aortopulmonary surgical shunting, Nour et al. demonstrated intrapulmonary shear stress-mediated endothelial function enhancement 10. Several studies have demonstrated that NO, prostacyclin and ET-1 expression are closely regulated by changes in pulsatile flow. Indeed, a moderate increase in pulsatile flow increases eNOS activity and prostacyclin levels, both of which are reduced in PAH. Pulsatile flow modulation is probably implicated in the etiology of PAH and artificially increasing it is an attractive and novel way of increasing NO and prostacyclin production within the pulmonary circulation.
The present study aims to assess the effects of a 10 min pulsatile flow using a newly developed pulsatile catheter on hemodynamic measurements in a pulmonary hypertension (PH) model in piglets in whom hypoxia has been induced. It has been hypothesized that increasing pulmonary artery pulsatility induces vasorelaxation of the pulmonary arteries, thereby decreasing pulmonary artery pressure.
Right heart catheterization (RHC) is a critical clinical intervention for the diagnosis and follow-up of PAH patients. Indeed, it is the most reliable way of diagnosing PAH and allows physicians to assess vascular reactivity 11,12 as well as disease progression. In fact every PAH patient undergoes RHC several times. The present study in large animals aims to demonstrate the efficacy and safety of pulsatile catheters in assessing and treating PAH during a regular RHC procedure. Because pulsatile catheters are already available and RHC is routinely performed in PAH patients, this study provides all the information required to be able to conduct clinical trials rapidly.
Protocol
NOTE: This study was authorized by Ethics committee number CEEA34.PB.103.12.
1. Use of Piglets as an Animal Model
Perform the in two groups (n = 6 in each group), matched in terms of sex, age (15 ± 3 months) and weight (30 ± 10 kg) (control group and chronic hypoxia (CH) group). House the CH group for 3 weeks in a hypobaric chamber (0.4 atm), and house the control group in regular normobaric (1 atm) conditions.
Use a hypobaric chamber consisting of a Plexiglas box with a 2 square meter footprint and a height of 1.6 meters, as shown in Figure 1.
Continuously monitor and maintain a temperature of 18 °C and a pressure of 0.4 atm. Ensure adequate ventilation by a vacuum pump, enabling an air renewal rate of 8 m3 per hr.
Place two animals in the chamber of the appropriate litter. Every 48 hr, return the pressure in the box to normobaric conditions for half an hour to clean the box, in the presence of the two animals, with the box kept closedto prevent them escaping. This method of inducing pulmonary hypertension has been widely validated 13.
- Anesthetizing of the animals
- Anesthetize the piglets with an initial intravenous injection of sodium thiopental (10 mg/kg) and maintain anesthesia by continuous inhalation of isoflurane (1.5 to 3.5%). Apply two drops of Carbopol gel into the piglets’ eyes to prevent corneal dryness.
- Place the animals in the left lateral decubitus position with their forelegs tied in a flexed position to expose the chest. Clean the skin with soap and water and then shave using an electric shaver to remove any hair that could impede penetration of the ultrasound through the chest.
Echocardiogram
Monitor the development of PAH longitudinally and non-invasively by echocardiography. Perform an echo every week using a 3 MHz transducer. Record an electrocardiogram by placing 3 electrodes on the right and left paws and on the right side of the chest.
Record two-dimensional and M-mode imaging data in three different incidences (longitudinal, minor axis and apical) using a-Doppler probe. Hold the probe in the right hand and place between the fourth and fifth left intercostal spaces. Move the probe slowly up and down, rotating it right and left until a good image resolution is obtained for the different cardiac structures (i.e. the septum). The acoustic window differs slightly from one animal to another depending on the position of the heart in the chest.
Record the ECG at the same time for each of these incidences and for at least 10 cardiac cycles to enable off-line analysis. End diastole is defined as the point in the cardiac cycle coinciding with the onset of the Q wave on the ECG. End systole coincides with the onset of the T wave.
Measure mitral and tricuspid valve blood flow by apical view Doppler ultrasound. Place the-Doppler probe at the tip of the manubrium sterni; the sampling zone was located just above the valve to record the blood flow crossing the valve.
Record the blood velocity during the cardiac cycle by Doppler flowmetry to obtain the velocity-time integral of the blood flow through the mitral and tricuspid valves and the aortic and pulmonary valves. Use the video graphics array of the echograph to store images at a frame rate of 25 per sec in order to get fixed images or sequence images provided by the echograph.
Measure the dimensions and surface area of the heart cavities using measuring instruments and the contours of the surface areas proposed by the software integrated into the echograph in accordance with international recommendations 14.
Measure the free wall thickness of the right ventricle during diastole and systole by TM (time-motion) recording in minor axis incidence 14.
Measure the pulmonary artery root diameter at the tip of the pulmonary valves on the minor axis view 14.
Measure the septum and the posterior wall of the left ventricle during diastole in TM motion on the longitudinal view at the beginning of the Q waves on the ECG and in systole at the tip of the T wave on the ECG 14.
Measure the left ventricular end-diastolic diameters (LVEDD) and left ventricular end-systolic diameters (LVESD). Calculate fractional shortening (FS) using the formula FS (%) = (LVEDD-LVESD)/LVEDD. Measure the diameter of the aorta and the surface area of the right and left atrium.
From the velocity-time integral of the mitral and tricuspid blood flows, measure the following: maximum amplitude of the E and A wave, deceleration half-time of the E wave, flow duration, velocity-time index 14.
From Doppler measurements of the pulmonary artery, measure the following: maximum velocity, ascension half-time, flow duration and its integral 14.
Assess the left ventricular volume using the Simpson 15 method.
Store the data for each piglet in a database for subsequent statistical analysis on the whole group.
2. Right Heart Catheterization
- Animal preparation
- Before pulsatile catheter placement, pig is placed in a hypobaric chamber during 3 weeks in order to induce pulmonary hypertension.
- Fast the animals for 24 hr before surgery (24 hr for solid food, 8 to 12 hr for water).
- 24 hr prior to RHC, have a well-trained vet perform a pre-anesthesia clinical exam to assess mucous membrane color, capillary refill time, global lung and heart functions using a stethoscope and body temperature using a rectal thermometer.
- Administer injections of Midazolam (intramuscular, 0.5 mg/kg) and morphine hydrochloride (intramuscular, 0.1 mg/kg) 15 to 30 min before the induction of anesthesia. Repeat injection of morphine hydrochloride (0.05 to 0.5 mg/kg, intramuscular) during induction every 4 to 6 hr.
- Administer sodium thiopental (10 mg/kg, intravenous) via an initial bolus injection of 5 mg/kg then by partial injection until it was effective. Follow this by bag and mask ventilation until endotracheal intubation.
- Piglet intubation
- Lubricate the tube with pramocaine gel. Insert a metal stylus into the tube to stiffen it and facilitate the intubation process.
- Touch the eyelid to ensure deep anesthesia. Perform intubation by direct visualization of the larynx, using a laryngoscope to raise the tongue and avoid injury to the vocal cord. Inflate the tube balloon to prevent regurgitation-related issues.
- Control the respiratory rate at 10-12 breaths per minute; current volume of 7-10 ml/kg, insufflation pressure of 25 to 30 cm H2O and an inspiratory phase of 2 sec with a positive end-expiratory pressure of 5 cm H2O.
- Anesthetize the animal with isoflurane in 100% oxygen (induction 3-5% with oxygen flow of 2 to 3 L/min, maintenance of 1.5-2.5% oxygen flow at 1 L/min). Apply carbopol gel to the cornea as in step 1.5.1.
- Insert a heparinized catheter into the caudal auricular artery (5 ml of 0.9% saline spiked with 5,000 IU/ml heparin) via subcutaneous infusion with green cannula fixed in place with a suture stitch.
- Infuse lactated Ringer’s solution (10-20 ml/kg/hr).
- Place the animal on a slightly tilted examination table. Keep the head inclined slightly downwards to promote salivary flow.
- Monitoring
- Every 5 min, check and record the following values on the individual anesthesia case report: mucous membrane color and capillary refill time, mandibular muscle tone and eyeball position, miosis/mydriasis, palpebral reflex.
- Continuously monitor the heart and respiratory rate, pulse oximetry, body temperature and electrocardiograms. Insert an arterial introducer into the femoral artery. Insert a large-caliber 10 cm long catheter, into the femoral artery and monitor the systemic blood pressure.
- Setting up of the pulsatile catheter NOTE: This medical device consists of two catheters placed side-by-side and welded together. The distal part of the first one is connected to a standard balloon with a diameter of 20 mm and a maximum volume capacity of 5 ml. The second catheter enables insertion of a wire to facilitate positioning in the pulmonary artery. The device is 750 mm long with an internal gauge of 0.035 and an external diameter of 12 Fr.
- For the experiments, perform pulsation with a small Harvard 683 animal ventilator, which applies an active vacuum to the balloon during deflation and positive pressure during inflation, with a volume of 2.5 ml for each pulse. Use helium as the propellant gas for the balloon pump in order to prevent gas embolism.
- Record the surface electrocardiographic tracings continuously to document any heart rhythm disturbances during the protocol. In the left femoral artery, connect a sensor device to a 20 gauge catheter, connected to a hemodynamic monitoring system.
- Right heart catheterization
- Wash and shave the neck of the animal. Clean the skin with a cutaneous antiseptic solution (Betadine scrub) using a gauze compress. To demarcate the surgical site, place sterile drapes around the right jugular between the right shoulder and the manubrium sterni.
- Make a 4 cm longitudinal incision with sterile scissors half-way between the right shoulder and the manubrium sterni.
- Carefully remove the skin and muscle layers with forceps. Then gently remove the connective tissue surrounding the vein over a length of approximately 5 cm. Clip the distal side to prevent bleeding. Place a binder wire around the proximal side to be able to control the vein opening after hemi-section.
- Using a specific small very sharp chisel, cut the vein in half transversely. Ensure that the edges of the incision are neat. Using thin foam, raise one edge of the incision and gently push the catheter into the proximal side of the vein. Control bleeding with binder wire.
- Introduce the catheter into the jugular vein and successively push through the superior vena cava, right atrium, right ventricle and, finally, the pulmonary artery.
- Record the pressures over 10 stable cardiac cycles, on each cardiac cavity and the pulmonary artery (T0). Measure cardiac blood flow three times at 1 min intervals.
- Position the balloon catheter in the pulmonary artery under radioscopic control. Inflate and deflate the balloon (pulsation) with 1 cm3 of helium. Continue pulsation for 10 min. Record pressures over 10 stable cardiac cycles, in each cardiac cavity and the pulmonary artery and measure the cardiac blood flow after 10 min (T1).
- Measure the cardiac blood flow again 30 min after the pulsatility protocol (T2).
3. NO Measurement
Connect a Douglas bag to the exhaled respiratory gas outlet pipe until it is completely filled. Place it over the in-pipe of a NO breath analyzer.
Slowly and constantly force the exhaled air into the analyzer by compressing the elastic Douglas bag. Measure the bag outflow by the flowmeter of the analyzer to maintain a constant flow.
4. Histology Measurements
Under anesthesia, inject 30 ml of Dolethal (injectable solution for euthanasia) into the pulsatile catheter placed in the heart.
Immediately after euthanasia, open the chest by sawing the manubrium longitudinally, slightly move the heart and hold the right lung. Then using dissecting scissors, cut two 2 or 3 cm3 samples from the middle lobe of the lung.
Snap freeze one sample in liquid nitrogen and stored at -80 °C. Fix the second sample in 3.7% paraformaldehyde for 24 h and then embed in paraffin for subsequent histological analysis.
Perform histology measurements as previously described 16. Measure the PA wall thickness as follows: 2 measurements/artery in 10 arteries/piglet and in 6 piglets/group.
- Perform statistical analysis. Values were expressed as fold change ± SEM.
- To compare two means, use an unpaired Student’s t test. To compare more than two means, use a one-way ANOVA followed by a Dunn’s test. A p <0.05 was considered statistically significant (*).
Representative Results
Increasing Pulmonary Artery Pulsatile Flow Improves Induced Chronic Hypoxic Pulmonary Hypertension in Piglets
Prior to exposing animals to an increase in pulsatile flow, ultrasound was used non-invasively to check that the piglets had developed pulmonary hypertension. As shown in Figure 2, three weeks of chronic hypoxia induced the development of pulmonary hypertension in piglets, characterized by a significant reduction in pulmonary artery acceleration time (Doppler) and an increase in RV hypertrophy (M-mode). During RHC, invasive measurements of both right ventricular systolic pressure (RVSP) and mean PA pressure confirmed the presence of pulmonary hypertension in chronic hypoxic piglets vs. control piglets (values T0) (Figure 3). Diastolic pressure in the right ventricle was of -3 ± 1 mmHg in the control group and -2 ± 1 in the PAH group and the atrial pressure was -4 ± 2 mmHg in each group. The systemic pressure was 106 ± 13 vs 95 ± 18 mmHg in the control group vs the PAH group. Pulmonary hypertension was also confirmed by quantification of vascular remodeling on histological slices. To assess the probable therapeutic effect of pulsatile flow on pulmonary hypertension, a pulsatile catheter was used to generate an artificial pulsatile pulmonary flow for 10 min. As shown in Figure 3 an increase in the pulsatile flow for 10 min induced a significant reduction in both RVSP and mean PA pressure (T1 values) compared to baseline (T0 values). Since the cardiac output was not changed, pulsatility reduced the vascular pulmonary artery resistance by 26 ± 3% in the control group and by 41 ± 4% in the PAH group. To ensure that the decrease in PA pressure was sustained over time, animals in which the right catheter did not generate further pulsatile flow stayed in place for a further 30 min. As shown (T2 values), both RVSP and mean PA pressure continued to decrease compared to baseline (T0 values). It should be noted that systemic vascular parameters were not affected by the generation of pulsatile flow in the pulmonary artery and that neither systemic pressure nor cardiac output changed significantly.
Exhaled NO was measured for 6 chronically hypoxic piglets (T0) and 40 min after the generation of the artificial pulsatile flow (T2). This preliminary result, which needs to be confirmed - demonstrated a significant (p <0.001) increase in exhaled NO from 2 ± 1 ppm to 22 ± 8 ppm.
Finally, the pulsatile flow showed no significant effects on circulating ET-1 and 5-HT levels, suggesting that the decrease in PA pressure was mainly due to an increase in NO generation.
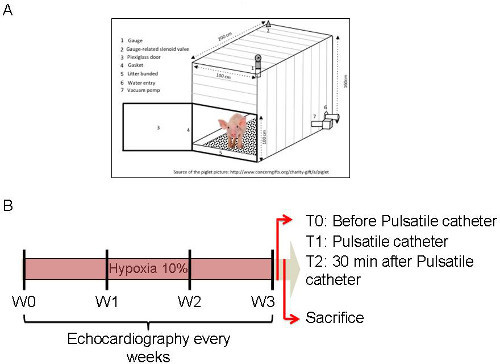 Figure 1: Diagram of the Protocol. (A) Diagram of the hypoxic box with the equipment required. (B) Timeline of the experiment. Echocardiographs were performed every week for 3 weeks before, during and after hypoxia. Please click here to view a larger version of this figure.
Figure 1: Diagram of the Protocol. (A) Diagram of the hypoxic box with the equipment required. (B) Timeline of the experiment. Echocardiographs were performed every week for 3 weeks before, during and after hypoxia. Please click here to view a larger version of this figure.
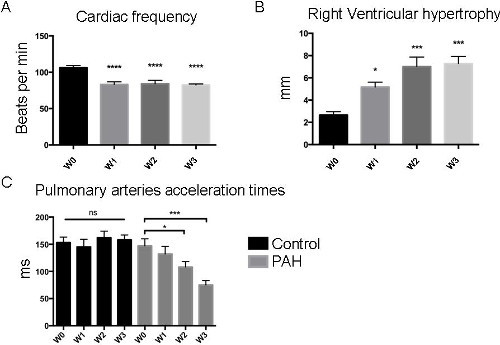 Figure 2: Echocardiography Results. Typical analysis measured non-invasively. (A) Over the 3 weeks (W1, W2, W3) of chronic hypoxia exposure (0.4 atmospheres), modifications in heart rate were observed as right ventricular hypertrophy progressively increased (B). In addition, pulmonary artery acceleration time (PAAT) measurements (C) demonstrated that PAAT decreased significantly in PAH piglets as PAH progressed, whereas no modifications occurred in the control group. An ANOVA statistical analysis adapted to the small number of animals concerned was performed (* p <0.05; ** p <0.005; *** p <0.001). Please click here to view a larger version of this figure.
Figure 2: Echocardiography Results. Typical analysis measured non-invasively. (A) Over the 3 weeks (W1, W2, W3) of chronic hypoxia exposure (0.4 atmospheres), modifications in heart rate were observed as right ventricular hypertrophy progressively increased (B). In addition, pulmonary artery acceleration time (PAAT) measurements (C) demonstrated that PAAT decreased significantly in PAH piglets as PAH progressed, whereas no modifications occurred in the control group. An ANOVA statistical analysis adapted to the small number of animals concerned was performed (* p <0.05; ** p <0.005; *** p <0.001). Please click here to view a larger version of this figure.
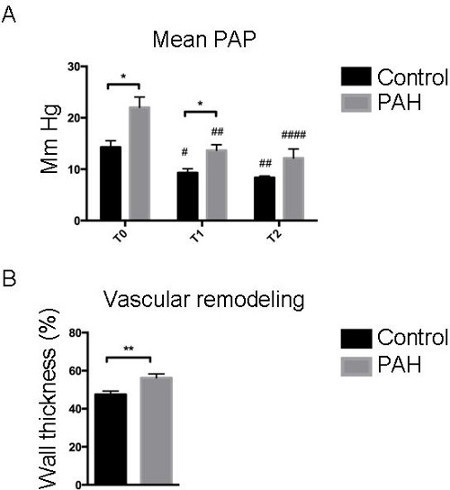 Figure 3: Pulmonary Pressure was Reversed Following Pulsatile Catheter Exposure. (A) Piglets were exposed to pulsatile catheterization for 10 min. Mean pulmonary artery pressure (PAPcm H2O) was evaluated before (T0), immediately after (T1), and 30 min after the pulsation (T2). Each time, the pressure was measured over 10 stable cardiac cycles in order to obtain a representative value for the results. (B) Vascular remodeling was quantified and was increased in PAH piglets compared to control piglets. An ANOVA statistical analysis adapted to the small number of animals concerned was performed (* p <0.05; ** p <0.005; *** p <0.001).
Figure 3: Pulmonary Pressure was Reversed Following Pulsatile Catheter Exposure. (A) Piglets were exposed to pulsatile catheterization for 10 min. Mean pulmonary artery pressure (PAPcm H2O) was evaluated before (T0), immediately after (T1), and 30 min after the pulsation (T2). Each time, the pressure was measured over 10 stable cardiac cycles in order to obtain a representative value for the results. (B) Vascular remodeling was quantified and was increased in PAH piglets compared to control piglets. An ANOVA statistical analysis adapted to the small number of animals concerned was performed (* p <0.05; ** p <0.005; *** p <0.001).
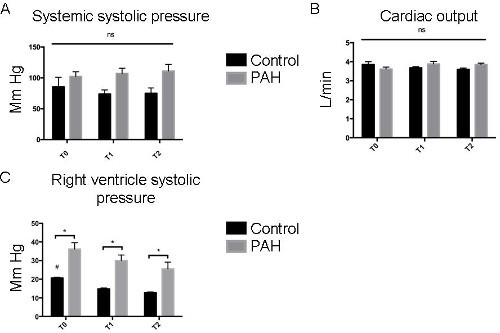 Figure 4: Pulsatile Catheters did not Modify Hemodynamic Parameters. The systemic systolic pressure (A) and the cardiac output (B) were evaluated in PAH and control piglets before, during and after the pulsatile protocol. No significant difference was observed between the groups and the different times. (C) Systolic pulmonary pressures were measured (mmHg) by right catheterization at T0, T1 and T2. An ANOVA statistical analysis adapted to the small number of animals concerned was performed (* p <0.05; ** p <0.005; *** p <0.001). Please click here to view a larger version of this figure.
Figure 4: Pulsatile Catheters did not Modify Hemodynamic Parameters. The systemic systolic pressure (A) and the cardiac output (B) were evaluated in PAH and control piglets before, during and after the pulsatile protocol. No significant difference was observed between the groups and the different times. (C) Systolic pulmonary pressures were measured (mmHg) by right catheterization at T0, T1 and T2. An ANOVA statistical analysis adapted to the small number of animals concerned was performed (* p <0.05; ** p <0.005; *** p <0.001). Please click here to view a larger version of this figure.
Discussion
For the first time, it has been shown that changes in pulmonary pulsatile flow are causally related to the development of PAH secondary to chronic hypoxic exposure. This translational approach provides evidence that inducing an artificial increase in pulmonary pulsatile flow using a specifically designed catheter improves pulmonary hypertension, probably by increasing NO generation.
These findings are not only original, they are also of great therapeutic interest, demonstrating that endogenous NO production can be stimulated mechanically and safely within the pulmonary circulation without affecting systemic functions. The study design complies with the latest preclinical research recommendations in pulmonary hypertension, which have recently recommended tests on large animals before new methods are adopted in clinics. The translational potential of these findings is very high.
However the pathophysiological mechanism of PAH is not unique. Classification of PAH differentiates between five different groups. Group three is representative of medial hypertrophy secondary to chronic hypoxia. Although it can be included in this group, the model does not perfectly represent human PAH. Human PAH secondary to chronic hypoxia is mainly linked to chronic bronchitis in which lesions of the bronchi and pulmonary parenchyma induce peripheral pulmonary-to-systemic shunts. These shunts induce hypoxemia via a mechanism different to that used in the model. Here, the model is closer to life at high altitude than lung disease. However the cardiac hemodynamics of the model are close to those in humans: same heart rate, same cardiac output and same pulmonary pressure.
A previous study by Nour et al. demonstrated coronary 17 and pulmonary circulation 10 vasodilation secondary to pulsation. Their model of PAH was an aortopulmonary shunt. Aortopulmonary shunts increase blood flow in the pulmonary artery. It is our view that placing a balloon in the pulmonary artery creates an obstacle to right ventricular ejection that could interfere with the decrease in pulmonary pressure observed in their experiment. It is for that reason that a model of PAH was chosen that does not affect cardiac output. Instead the cardiac blood flow remains constant throughout theexperiment.
Moreover, their experimental conditions are quite different since the chest of the animal is opened surgically during application of pulsatility, which modifies normal ventilation and pulmonary circulation. It is therefore difficult to compare the two experiments.
The mechanism via which pulsatility decreases PAH has not been demonstrated. The study conducted by Nour showed an increase in e-NOS, while we demonstrated an increase in exhaled NO. These two conclusions are strongly suggestive of a role played by NO in the PAH reduction mechanism. However other mediators may be involved 18. It would be useful to perform further studies to examine the biomarkers involved in pulsation-induced vasodilation.
NO delivery has been reported as being beneficial to PAH patients 19-21. However, NO has a very short half-life and its use to treat patients therefore involves a complicated procedure. In addition, the release of NO secondary to pulsatile flow needs to be more clearly demonstrated. The increase in eNOS and the systemic vasodilation observed in the study conducted by Nour but not in the study suggest that it is necessary to conduct further studies examining NO release induced by pulsatility and its role in vasodilation. This is another reason why NO therapy is not yet used in clinical practice.
Right heart catheterization is a standard procedure used to assess the severity of the condition and to monitor patient treatment 16,18. It would be entirely feasible to use pulsatile catheters instead of regular catheters. This would not require any additional procedures and could be used to assess the severity of PAH as well as to treat patients and improve their condition. Another advantage of this technique is that it has few, if any, toxic effects. Since these devices induce NO generation via a physiological process, any detrimental interaction with other therapies is unlikely. Consequently, pulsatile catheters could be rapidly and safely used in patients requiring ongoing treatment.
Although novel, the findings of this study confirm previous observations made in left heart failure patients who developed secondary pulmonary hypertension. Indeed, patients with pulmonary hypertension associated with chronic congestive heart failure have a significant risk of morbidity and death following heart transplants. Torre-Amione et al. 22 observed that patients who received a pulsatile left ventricular assist device had a significant decrease in pulmonary pressure, enabling them to qualify for heart transplants. Although not explored in the study, NO release could probably explain the improvement in pulmonary pressure, as in this study. This study not only confirms previous findings but suggests that the use of pulsatile catheters should also be explored in left heart failure patients who develop secondary pulmonary hypertension of high clinical significance.
Despite being very promising, this study has several limitations. Firstly, the efficacy of this device has only been tested with one model of pulmonary hypertension. Currently there are very few large animal pulmonary hypertension models 23. It is essential to develop new models. In the light of the findings by Torre-Amione et al. 22 concerning secondary pulmonary hypertension, a pulmonary hypertension model associated with left heart failure would be of great therapeutic interest. The effect of pulsatile catheters on NO release was estimated by measuring exhaled NO and not directly via the NO circulating in the PA. Ozkan et al. 24 have shown that exhaled NO is a good indicator of the amount of NO within the PA circulation. Indeed in their study, they demonstrated that exhaled NO decreased in PAH patients vs. controls and that stimulating NO generation in the PA using epoprostenol was reflected in the exhaled NO. This means that exhaled NO measurement is a valid method of estimating NO levels circulating in the PA. This study also measured PA pressure over 30 min, but the long-term effects (weeks, months and years) have not been studied. Sharing this technique will allow other teams to test different protocols (i.e. combinations with other treatments), analyze the benefits and perhaps discover adverse effects. Above all, it is hoped that this study may lead to rapid and safe use of the device in human PAH patients.
Disclosures
The authors would like to thank the INRA institute, which housed the piglets, and Cardio Innovating systems for the funding.
Acknowledgments
The authors have nothing to disclose.
References
- Malenfant S, et al. Signal transduction in the development of pulmonary arterial hypertension. Pulm Circ. 2013;3(2):278–293. doi: 10.4103/2045-8932.114752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin R, et al. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123(11):1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courboulin A, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208(3):535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007;104(27):11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche J, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129(7):786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- Humbert M, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2014;43(12 Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Archer SL, Michelakis ED. An evidence-based approach to the management of pulmonary arterial hypertension. Curr Opin Cardiol. 2006;21(4):385–392. doi: 10.1097/01.hco.0000231410.07426.9b. [DOI] [PubMed] [Google Scholar]
- Li M, Scott DE, Shandas R, Stenmark KR, Tan W. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Eng. 2009;37(6):1082–1092. doi: 10.1007/s10439-009-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Stenmark KR, Shandas R, Tan W. Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors. J Vasc Res. 2009;46(6):561–571. doi: 10.1159/000226224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour S, et al. Intrapulmonary shear stress enhancement: a new therapeutic approach in pulmonary arterial hypertension. Pediatr Cardiol. 2012;33(8):1332–1342. doi: 10.1007/s00246-012-0322-8. [DOI] [PubMed] [Google Scholar]
- Barst RJ, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Galie N, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25(24):2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Naeije R, Dewachter L. Animal models of pulmonary arterial hypertension. Rev Mal Respir. 2007;24(4 pt 1):481–496. doi: 10.1016/s0761-8425(07)91571-5. [DOI] [PubMed] [Google Scholar]
- Via G, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27(7):e681–e683. doi: 10.1016/j.echo.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Folland ED, et al. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60(4):760–766. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- Meloche J, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc. 2013;2(1):e005157. doi: 10.1161/JAHA.112.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour S, et al. Intrapulmonary shear stress enhancement: a new therapeutic approach in acute myocardial ischemia. Int J Cardiol. 2013;168:4199–4208. doi: 10.1016/j.ijcard.2013.07.107. [DOI] [PubMed] [Google Scholar]
- Barrier M, et al. Today's and tomorrow's imaging and circulating biomarkers for pulmonary arterial hypertension. Cell Mol Life Sci. 2012;69(17):2805–2831. doi: 10.1007/s00018-012-0950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budev MM, Arroliga AC, Jennings CA. Diagnosis and evaluation of pulmonary hypertension. Cleve Clin J Med. 2003;70(1):S9–S17. doi: 10.3949/ccjm.70.suppl_1.s9. [DOI] [PubMed] [Google Scholar]
- Barst RJ, Channick R, Ivy D, Goldstein B. Clinical perspectives with long-term pulsed inhaled nitric oxide for the treatment of pulmonary arterial hypertension. Pulm Circ. 2012;2(2):139–147. doi: 10.4103/2045-8932.97589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991;338(8776):1173–1174. doi: 10.1016/0140-6736(91)92033-x. [DOI] [PubMed] [Google Scholar]
- Zapol WM, Rimar S, Gillis N, Marletta M, Bosken CH. Nitric oxide and the lung. Am J Respir Crit Care Med. 1994;149(5):1375–1380. doi: 10.1164/ajrccm.149.5.8173780. [DOI] [PubMed] [Google Scholar]
- Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G, et al. Reversal of secondary pulmonary hypertension by axial and pulsatile mechanical circulatory support. J Heart Lung Transplant. 2010;29(2):195–200. doi: 10.1016/j.healun.2009.05.030. [DOI] [PubMed] [Google Scholar]


