Abstract
Objectives
Enterococcus faecalis (Efc) and Enterococcus faecium (Efm) are frequently resistant to vancomycin and β-lactams (BLs). In vitro data suggest synergy between several BLs and glycopeptides or lipopeptides against resistant pathogens. Our objective was to conduct combination MIC and time–kill experiments to evaluate BL synergy with daptomycin against enterococci.
Methods
Fifteen Efc and 20 Efm strains were evaluated for daptomycin enhancement via combination MICs. Daptomycin MICs were obtained by microdilution in the absence and presence of ceftaroline, ertapenem, cefepime, ceftriaxone, cefotaxime, cefazolin and ampicillin. Two Efc strains (R6981 and R7808) and one isogenic daptomycin-susceptible/daptomycin-non-susceptible Efm pair (8019/5938) were evaluated in time–kill experiments. Daptomycin at 0.5 × MIC was used in combination with BL at biological free concentration. Strain 5938 was evaluated for enhancement of daptomycin binding in fluorescently labelled daptomycin (BoDipy) experiments.
Results
Ceftaroline reduced daptomycin MIC values the most against all strains. In time–kill experiments, ceftaroline, ertapenem, cefepime, ceftriaxone and ampicillin demonstrated synergy with daptomycin against all strains, cefazolin demonstrated none and cefotaxime demonstrated synergy against only R7808. Bacterial reduction at 24 h was greater for daptomycin + ceftaroline, ertapenem, cefepime, ceftriaxone or ampicillin for all strains compared with any single agent or daptomycin + cefazolin or cefotaxime (P < 0.001). In BoDipy daptomycin experiments, ceftaroline enhanced daptomycin binding most compared with all other agents (P < 0.001).
Conclusions
The data support the potential use of daptomycin/BL combination therapy in infections caused by VRE. Combination regimens, other than those involving cefazolin and cefotaxime, provide better kill compared with daptomycin alone. Further clinical research involving daptomycin combinations is warranted.
Keywords: bacteria, combination therapy, infection, in vitro, time–kill
Introduction
Enterococcus faecalis and Enterococcus faecium are together the fourth leading cause of hospital-acquired infection in the USA, accounting for 12% of hospital-acquired infections in recent epidemiological data.1 Enterococcal infections are often due to MDR strains. Recent data demonstrate that 0.4%–5.2% and 70%–92.6% of E. faecalis and E. faecium are resistant to ampicillin, respectively, and vancomycin resistance is present in 1%–12.5% of E. faecalis and 7%–79.7% of E. faecium.2–4 The presence of VRE alone is associated with increased mortality.5 Furthermore, enterococci are often responsible for deep-seated infections such as infective endocarditis, complicating treatment.6 Linezolid, an oxazolidinone antibiotic recommended for vancomycin-resistant E. faecium, is limited by its static activity and potential to cause myelosuppression with long-term use.6,7
Daptomycin is a lipopeptide antibiotic with rapid bactericidal activity against Gram-positive bacteria, and it is frequently employed in the setting of resistant enterococci.8 Daptomycin is FDA approved at doses of 4–6 mg/kg daily, although clinical and in vitro data suggest improved efficacy at higher doses.7,9–11 Daptomycin retains excellent in vitro activity against E. faecalis and E. faecium, with MIC50/90 values of 1/2 and 2/4 mg/L, respectively.12 However, daptomycin non-susceptibility among enterococci, currently defined as MIC >4 mg/L, is a growing concern.13 Although 100% of isolates reported in recent SENTRY data retained daptomycin susceptibility, another recent survey of US hospitals revealed that up to 0.5% of E. faecalis and 4.7% of E. faecium possessed daptomycin MIC values of ≥4 mg/L, placing several isolates on the border of susceptibility and non-susceptibility.14,15 Further data suggest that even among enterococcal strains with MIC values between 2 and 4 mg/L, mutations may be present that confer non-susceptibility to daptomycin and therefore may render an important therapeutic option unusable.16 Therefore, there is importance in finding novel strategies to prevent daptomycin non-susceptibility.
Several in vitro studies have demonstrated synergistic activity against enterococci with the combination of daptomycin and other antibiotics, specifically β-lactams. Combinations of daptomycin with ampicillin, ceftriaxone and ceftaroline specifically have demonstrated bactericidal activity, and ceftaroline has demonstrated the ability to restore daptomycin susceptibility to daptomycin-non-susceptible strains.17 Mechanistically, it appears that both lowering of cell surface charge and increased daptomycin binding enhance daptomycin's antimicrobial activity.17–19 Case reports have also demonstrated the clinical efficacy of daptomycin in combination with ampicillin against endocarditis caused by both E. faecalis and E. faecium, and the combination of daptomycin and ceftaroline has proved effective against E. faecalis.19–21
Owing to the data describing the synergistic effects of a number of β-lactam agents and daptomycin, there is the potential that synergy among daptomycin and β-lactams is a class effect. Therefore, the objective of this study was to evaluate the effects of a variety of β-lactams on daptomycin activity through combination broth microdilution, fluorescent daptomycin binding studies and time–kill assays.
Materials and methods
Bacterial strains
Fifteen vancomycin-resistant E. faecalis and 20 vancomycin-resistant E. faecium strains were chosen for combination broth microdilution MIC testing. Two clinical strains of E. faecalis and one clinical, isogenic strain pair of E. faecium were selected for this study. Both clinical strains of E. faecalis (R6981 and R7808) were chosen from our library at the Anti-Infective Research Laboratory. The isogenic E. faecium strain pair featured one daptomycin-susceptible strain (8019) and one daptomycin-non-susceptible strain (5938) and has been previously described.22 The E. faecalis strains were chosen due to their elevated resistance profiles to all β-lactams tested, and the E. faecium strains were chosen due to our knowledge of their genetics.
Antimicrobials
Daptomycin was purchased commercially from Cubist Pharmaceuticals (Lexington, MA, USA). Cefazolin, cefotaxime, ceftriaxone, cefepime, ampicillin and ertapenem were purchased commercially from Sigma Chemical Co. (St Louis, MO, USA). Ceftaroline analytical powder was obtained from Forest Laboratories, Inc. (San Francisco, CA, USA).
Susceptibility testing
MIC values of studied antimicrobials were determined in duplicate by broth microdilution at ∼106 cfu/mL according to CLSI guidelines.13 Owing to the elevated MIC values of β-lactams for these organisms, combination MIC values for daptomycin were determined by supplementing broth with concentrations of β-lactam antimicrobials at their respective biological free peaks, as it would be impossible to attain 0.5× the MIC value in the clinical setting. All samples were evaluated after incubation at 35°C for 24 h. Daptomycin MIC fold reduction from baseline was calculated as the standard broth microdilution daptomycin MIC divided by the daptomycin MIC in the presence of the specified antibiotic.
Time–kill experiments
Time–kill experiments were performed in Mueller–Hinton broth (MHB; Difco, Detroit, MI, USA) supplemented with 50 mg/L calcium (MHB50) and 12.5 mg/L magnesium as growth medium. Each well received an initial bacterial inoculum of ∼106 cfu/mL. Experiments were performed in duplicate for all antibiotic regimens. Daptomycin was tested at 0.5 × MIC for each organism. Ceftaroline, cefazolin, cefotaxime, ceftriaxone, cefepime, ampicillin and ertapenem were tested at biological free peak concentrations of 17.04, 37, 68, 25.7, 134.5, 64 and 15.5 mg/L, respectively. All agents were tested alone and in combination with daptomycin against each strain. Aliquots of 0.1 mL were obtained from each well at 0, 4, 8 and 24 h, serially diluted to the appropriate concentrations, and plated using automatic spiral plating (WASP, DW Scientific, West Yorkshire, UK) for best enumeration of cfu/mL and avoidance of antibiotic carryover. After 24 h of growth on brain heart infusion agar (BHIA; Difco), bacterial colonies were counted using a laser colony counter (ProtoCOL, Synoptics Limited, Frederick, MD, USA). Time–kill curves were generated by plotting mean colony counts (log10 cfu/mL) versus time to compare 24 h killing effects of single agents and combination antimicrobial exposure. Synergy was defined as a ≥100-fold increase in bacterial killing compared with the most active constituent. Bactericidal activity was defined as a ≥3 log10 cfu/mL reduction from baseline.
Binding of fluorescent daptomycin
E. faecium strain 5938 was chosen for assessment of binding of fluorescent daptomycin. This strain was chosen for its elevated resistance to daptomycin. Bacteria were grown to an OD600 of 0.6, grown for an additional 1 h without β-lactam treatment or in the presence of 5 mg/L ceftaroline, 20 mg/L ceftriaxone or 10 mg/L imipenem, and then incubated with 8 mg/L daptomycin–BoDipy (boron-dipyrromethene) for 20 min, washed three times in medium to remove unincorporated label, stained with 1 mg/L DAPI and placed on a 1% agarose pad for imaging in an Applied Precision deconvolution fluorescence microscope as described previously.23 For quantification of daptomycin–BoDipy fluorescence, images from each sample were collected using identical camera exposures. The average fluorescence intensity of individual pixels for the background was also measured and subtracted from the cells to generate an accurate measurement of daptomycin–BoDipy binding.
Statistical analysis
Changes in cfu/mL at 24 h were compared by one-way analysis of variance for time–kill assays. A P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS Statistical Software (Release 21, SPSS, Inc., Chicago, IL, USA).
Results
Susceptibility testing
Daptomycin MIC values for the 15 E. faecalis and 20 E. faecium strains ranged from 2 to 128 mg/L. All isolates were resistant to vancomycin and ampicillin. Daptomycin MIC values were reduced in the presence of ceftaroline, ampicillin, ertapenem, cefotaxime, ceftriaxone, cefepime and cefazolin in both species. Against E. faecium, ceftaroline demonstrated the greatest reduction in daptomycin MIC value compared with other antimicrobials (average reduction 8.4 ± 8.3-fold, median reduction 6-fold, range 4- to 32-fold). In descending order, ampicillin, ertapenem, ceftriaxone, cefepime, cefazolin and cefotaxime provided daptomycin MIC reduction as well (Table 1). Against E. faecalis, ceftaroline again demonstrated the greatest daptomycin MIC fold reduction (average 19.1 ± 17.6, median 8, range 2–64). Ceftaroline was followed by cefepime, ceftriaxone, ampicillin, ertapenem, cefazolin and cefotaxime (Table 1). Daptomycin MIC reductions for the strains selected for time–kill studies, R6981, R7808, 8019 and 5938, are illustrated graphically in Figure 1.
Table 1.
Daptomycin combination MIC reductions against 15 E. faecalis and 20 E. faecium strains
| Daptomycin MIC (fold reduction from baseline) |
||||
|---|---|---|---|---|
| mean | SD | median | range | |
| E. faecalis | ||||
| DAP + CPT | 19.07 | 17.58 | 8 | 2–64 |
| DAP + FEP | 12.00 | 18.98 | 2 | 1–64 |
| DAP + AMP | 5.00 | 2.38 | 4 | 4–32 |
| DAP + ERT | 4.27 | 3.37 | 4 | 2–16 |
| DAP + CRO | 7.73 | 15.76 | 4 | 1–64 |
| DAP + CTX | 1.80 | 1.01 | 2 | 1–4 |
| DAP + CFZ | 3.33 | 0.98 | 4 | 2–4 |
| E. faecium | ||||
| DAP + CPT | 8.40 | 8.30 | 6 | 4–32 |
| DAP + FEP | 3.20 | 2.04 | 3 | 1–8 |
| DAP + AMP | 6.00 | 2.31 | 6 | 4–8 |
| DAP + ERT | 4.00 | 2.25 | 4 | 2–8 |
| DAP + CRO | 3.30 | 2.18 | 2 | 2–8 |
| DAP + CTX | 2.70 | 2.45 | 2 | 1–8 |
| DAP + CFZ | 2.80 | 2.40 | 2 | 1–8 |
CPT, ceftaroline; FEP, cefepime; AMP, ampicillin; ERT, ertapenem; CRO, ceftriaxone; CTX, cefotaxime; CFZ, cefazolin.
Figure 1.
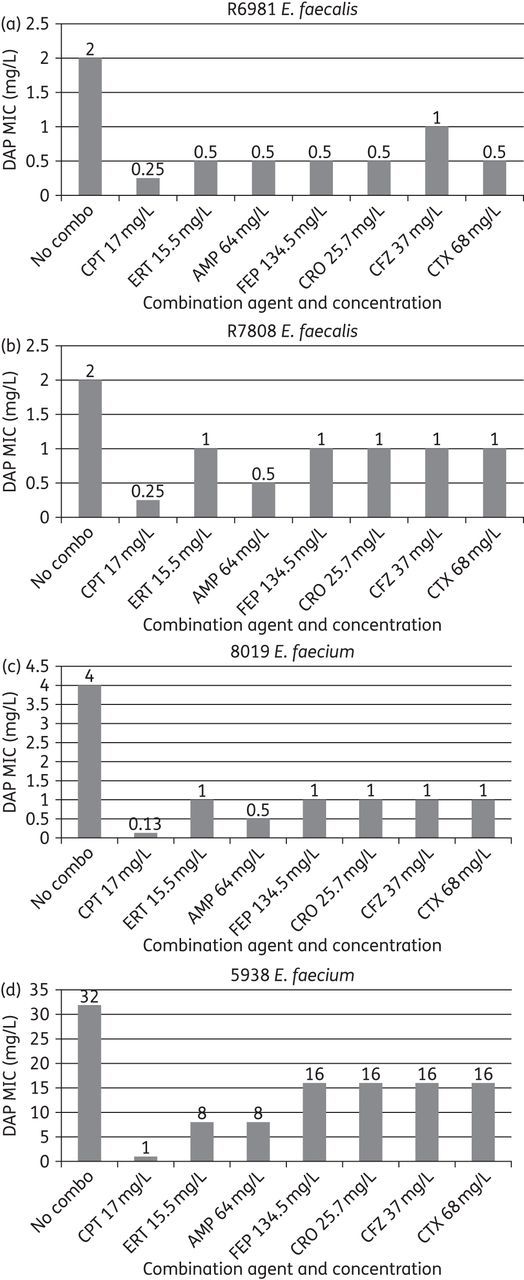
Daptomycin (DAP) MIC values in the presence of several β-lactam agents against strains (a) R6981, (b) R7808, (c) 8019 and (d) 5938. CPT, ceftaroline; ERT, ertapenem; AMP, ampicillin; FEP, cefepime; CRO, ceftriaxone; CFZ, cefazolin; CTX, cefotaxime; No combo, DAP MIC values in MHB50 without the presence of a β-lactam.
Time–kill studies
Against strains R6981, 8019 and 5938, synergy with daptomycin was demonstrated for ceftaroline, ampicillin, ertapenem, ceftriaxone and cefepime, while cefazolin and cefotaxime demonstrated no synergy (Figure 2a, c and d). Against strain R7808, all tested antimicrobials except cefazolin demonstrated synergy with daptomycin (Figure 2b). Antimicrobial activity was similar among all successful synergistic combinations against all strains with the exception of the combination of daptomycin and ertapenem against strain 5938. The combination of daptomycin and ertapenem provided statistically superior killing compared with the other combinations (P < 0.05). Bactericidal activity was not achieved against any Enterococcus isolates with any of the combinations.
Figure 2.
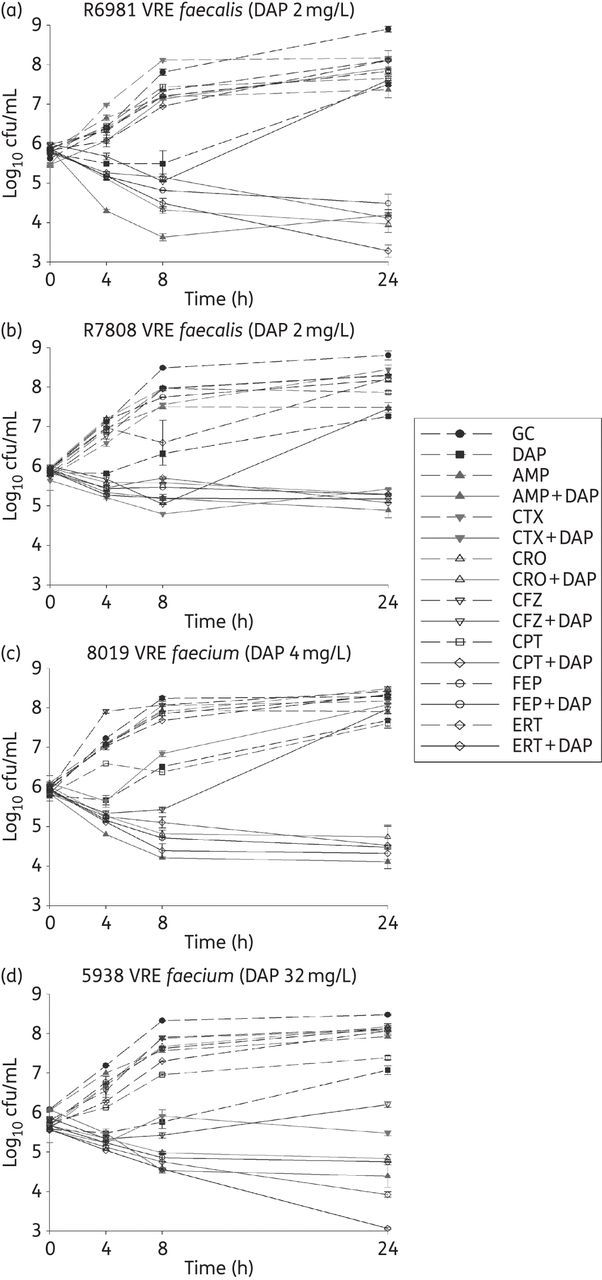
Twenty-four hour time–kill curves against strains (a) R6981, (b) R7808, (c) 8019 and (d) 5938. Broken lines, single agents; continuous lines, combination regimens. DAP, daptomycin; AMP, ampicillin; CTX, cefotaxime; CRO, ceftriaxone; CFZ, cefazolin; CPT, ceftaroline; FEP, cefepime; ERT, ertapenem; GC, drug-free growth control.
Binding of fluorescent daptomycin
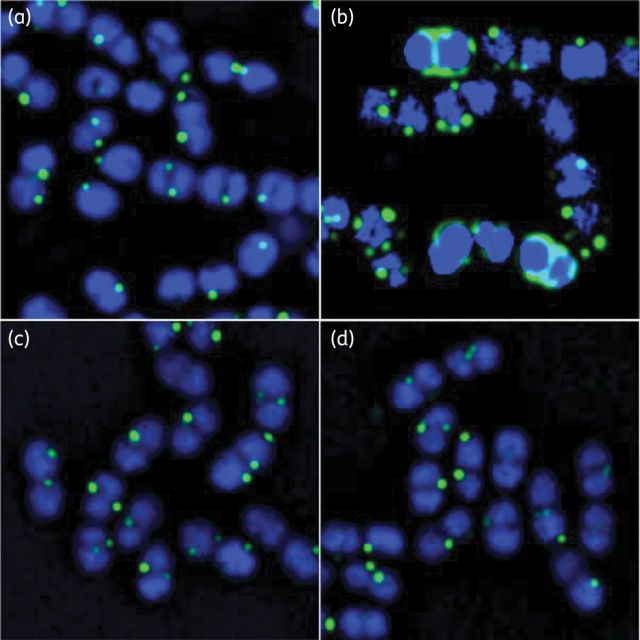
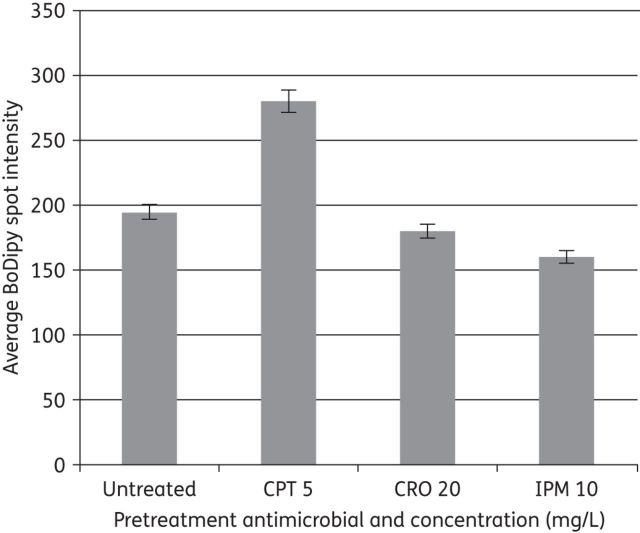
After pretreatment with subinhibitory concentrations of β-lactams ceftaroline, imipenem and ceftriaxone, or no β-lactam exposure, images of E. faecium 5938 were taken, and fluorescent daptomycin was visualized in green (light shading in print; Figure 3). Quantification of daptomycin binding revealed significantly more binding in the presence of ceftaroline compared with any other antimicrobials or no pretreatment (P < 0.001; Figure 4). Imipenem and ceftriaxone pretreatment produced similar fluorescent daptomycin binding compared with no pretreatment.
Figure 3.
Binding of fluorescent daptomycin (green or light shading) at 8 mg/L to E. faecium 5938 in cells (blue or dark shading). (a) Not pretreated with antimicrobial, (b) pretreated with 5 mg/L ceftaroline for 20 min, (c) pretreated with 10 mg/L imipenem for 20 min and (d) pretreated with 20 mg/L ceftriaxone for 20 min. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 4.
Average intensity of fluorescently labelled daptomycin against E. faecium 5938 after pretreatment with several β-lactam antimicrobials. CPT, ceftaroline; CRO, ceftriaxone; IPM, imipenem. Error bars indicate standard deviation.
Discussion
To the best of our knowledge, our study is the largest comparison of multiple β-lactam agents with regard to their synergistic effects on daptomycin efficacy in VRE. Here we have demonstrated that several β-lactams, including ceftaroline, ertapenem, ampicillin, ceftriaxone and cefepime, both lower the daptomycin MIC values and provide synergistic activity in time–kill assays against E. faecalis and E. faecium strains. Interestingly, the effect does not seem to be present within the entire class, as cefotaxime and cefazolin demonstrated little to no ability to enhance daptomycin activity in time–kill assays. The inactivity of cefotaxime and cefazolin may be due to the PBP profiles of these agents.
β-Lactam resistance among enterococci is often mediated through mutations that result in altered PBP profiles. In particular, resistant enterococci frequently possess an abundance of PBP5, a PBP with low binding affinity for β-lactams that allows survival in the presence of β-lactam therapy.24 A recent study has demonstrated the significantly enhanced binding affinity of ceftaroline to PBP5 compared with several other cephalosporin agents, perhaps helping to explain its ability to provide synergistic activity with daptomycin against enterococci.25 The same study demonstrated the binding affinity of ceftaroline to enterococcal PBPs 1–4, perhaps suggesting that saturation of several PBPs is important for antimicrobial activity. Previous findings suggest that saturation of PBPs 1–5 with a combination β-lactam regimen increases activity against E. faecalis, and recent clinical data describing the effective combination of ampicillin and ceftriaxone further establish this possibility.26–28 The results of our study suggest that saturation of PBPs provided by ceftaroline, and to a lesser extent ertapenem, ampicillin, ceftriaxone and cefepime, may play an important role in synergistic activity with daptomycin. It is plausible that cefotaxime and cefazolin lack the ability to provide adequate PBP binding to either a broad spectrum of PBPs or PBP5 to enhance daptomycin's efficacy and provide synergistic activity.
Another possible facet of synergistic activity may be the mutations specific to E. faecalis and E. faecium that confer non-susceptibility to daptomycin. Ampicillin has been demonstrated to restore daptomycin activity against E. faecium with mutations within LiaFSR, a system involved in regulation of the cell stress response.29 These mutations are frequently found in E. faecium possessing MIC values of 2–4 mg/L.16 It is possible that several of these isolates may possess these mutations, and further genetic workup may demonstrate this. If this is indeed the case, it appears that several β-lactam agents may restore daptomycin's activity when this mutation is present. Further study regarding LiaFSR mutations, along with mutations present within the cardiolipin synthase (cls) gene, which confers changes in the membrane orientation of cardiolipins in the E. faecalis cell membrane, and yyCFGHIJ, a regulator of cell wall homeostasis, is warranted to determine whether specific mutations are amenable to β-lactam synergy.29,30
Daptomycin non-susceptibility among E. faecalis has been previously demonstrated to be mediated in part by sequestration away from the cellular divisome, the primary site of bactericidal activity.30 Similarly, E. faecium that are daptomycin non-susceptible demonstrate a lack of daptomycin binding.17,19 We have demonstrated that in the presence of a subinhibitory concentration of ceftaroline, daptomycin binding is increased against even a daptomycin-non-susceptible strain (5938). Ceftaroline was the only β-lactam among those tested to have this effect against this strain, further supporting its synergistic effect and possibly demonstrating the importance of PBP5 binding.
The successful augmentation of daptomycin by other β-lactams in combination broth microdilution MIC testing is notable. Ampicillin is frequently employed in combination therapy for enterococcal infections, and ceftaroline has been demonstrated to provide synergistic activity with daptomycin in clinical cases.6,20 Ertapenem is a broad-spectrum antibiotic, potentially limiting its targeted use.31 However, ertapenem may be advantageous in the setting of acute, polymicrobial infections that do not harbour Pseudomonas aeruginosa or Acinetobacter baumannii, and its once daily dosing regimen allows simpler outpatient therapy compared with other β-lactams in the setting of prolonged antibiotic courses. Our data suggest that ertapenem may receive consideration for combination enterococcal therapy in these settings.
There are some limitations to the current study, as the data presented here are from short-duration experiments and demonstrate only in vitro efficacy. In addition, the limited number of strains investigated in this study warrants further study to confirm the reproducibility of these results in other enterococcal strains.
Conclusions
With the increasing presence of vancomycin- and ampicillin-resistant enterococci, novel therapeutic approaches are necessary. When deep-seated infections require prolonged, intensive therapy, the currently available options are limited by bacteriostatic activity or adverse effects. Daptomycin is a viable option, but the emergence of daptomycin non-susceptibility is a concern. The results of our study demonstrate the ability of several β-lactams to provide synergistic activity with daptomycin. Given the recent data suggesting the emergence of daptomycin non-susceptibility among enterococci when daptomycin is given alone, our study provides promising evidence for the early use of high-dose daptomycin in combination with a β-lactam against deep-seated VRE infections.
Funding
This study was supported by internal funding. Ceftaroline analytical powder was provided by Forest Laboratories.
Transparency declarations
The authors have not received any financial reimbursement from any pharmaceutical companies for any of the work presented in this article. The authors do not own any stocks or shares relevant to this study, nor has any funding agency been involved in the study design. No professional medical writers have been involved in this manuscript preparation, and no reimbursement has been received for preparation of this article.
G. S. has received grant support from, consulted for or provided lectures for Cubist, Astellas, Pfizer and Ortho-McNeil Pharmaceuticals. M. J. R. has received grant support from R21 AI109266-01 from the NIH. He has also received grant support from, consulted for or provided lectures for Astellas, Cubist, Forest, Pfizer and Novartis. J. R. S., K. E. B., A. R. and M. A.: none to declare.
Acknowledgements
We thank Forest Laboratories for the use of ceftaroline powder.
References
- 1.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34: 1–14. [DOI] [PubMed] [Google Scholar]
- 2.Rathnayake IU, Hargreaves M, Huygens F. Antibiotic resistance and virulence traits in clinical and environmental Enterococcus faecalis and Enterococcus faecium isolates. Syst Appl Microbiol 2012; 35: 326–33. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa K, Marchaim D, Vidaillac C, et al. Growing prevalence of vancomycin-resistant Enterococcus faecalis in the region with the highest prevalence of vancomycin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2011; 32: 922–4. [DOI] [PubMed] [Google Scholar]
- 4.Billington EO, Phang SH, Gregson DB, et al. Incidence, risk factors, and outcomes of Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis 2014; 26: 76–82. [DOI] [PubMed] [Google Scholar]
- 5.Carmeli Y, Eliopoulos G, Mozaffari E, et al. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med 2002; 162: 2223–8. [DOI] [PubMed] [Google Scholar]
- 6.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111: e394–434. [DOI] [PubMed] [Google Scholar]
- 7.Casapao AM, Kullar R, Davis SL, et al. Multicenter study of high-dose daptomycin for treatment of enterococcal infections. Antimicrob Agents Chemother 2013; 57: 4190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev 2013; 26: 759–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akins RL, Rybak MJ. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 2001; 45: 454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall AD, Steed ME, Arias CA, et al. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother 2012; 56: 3174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kullar R, Casapao AM, Davis SL, et al. A multicentre evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J Antimicrob Chemother 2013; 68: 2921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sader HS, Farrell DJ, Flamm RK, et al. Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005–2012). Int J Antimicrob Agents 2014; 43: 465–9. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-second Informational Supplement M100-S22. CLSI; Wayne, PA, USA, 2012. [Google Scholar]
- 14.Edelsberg J, Weycker D, Barron R, et al. Prevalence of antibiotic resistance in US hospitals. Diagn Microbiol Infect Dis 2014; 78: 255–62. [DOI] [PubMed] [Google Scholar]
- 15.Jones RN, Sader HS, Flamm RK. Update of dalbavancin spectrum and potency in the USA: report from the SENTRY Antimicrobial Surveillance Program (2011). Diagn Microbiol Infect Dis 2013; 75: 304–7. [DOI] [PubMed] [Google Scholar]
- 16.Munita JM, Panesso D, Diaz L, et al. Correlation between mutations in liaFSR of Enterococcus faecium and MIC of daptomycin: revisiting daptomycin breakpoints. Antimicrob Agents Chemother 2012; 56: 4354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoulas G, Rose W, Nonejuie P, et al. Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2014; 58: 1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall Snyder A, Werth BJ, Barber KE, et al. Evaluation of the novel combination of daptomycin plus ceftriaxone against vancomycin-resistant enterococci in an in vitro pharmacokinetic/pharmacodynamic simulated endocardial vegetation model. Antimicrob Agents Chemother 2014; 69: 2148–54. [DOI] [PubMed] [Google Scholar]
- 19.Sakoulas G, Bayer AS, Pogliano J, et al. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2012; 56: 838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakoulas G, Nonejuie P, Nizet V, et al. Treatment of high-level gentamicin-resistant Enterococcus faecalis endocarditis with daptomycin plus ceftaroline. Antimicrob Agents Chemother 2013; 57: 4042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra-Hoffman M, Iznaola O, Goodwin M, et al. Combination therapy with ampicillin and daptomycin for treatment of Enterococcus faecalis endocarditis. Antimicrob Agents Chemother 2012; 56: 6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphries RM, Kelesidis T, Tewhey R, et al. Genotypic and phenotypic evaluation of the evolution of high-level daptomycin nonsusceptibility in vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2012; 56: 6051–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogliano J, Osborne N, Sharp MD, et al. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol 1999; 31: 1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice LB, Carias LL, Hutton-Thomas R, et al. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother 2001; 45: 1480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry X, Verlaine O, Amoroso A, et al. Activity of ceftaroline against Enterococcus faecium PBP5. Antimicrob Agents Chemother 2013; 57: 6358–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mainardi JL, Gutmann L, Acar JF, et al. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother 1995; 39: 1984–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Hidalgo N, Almirante B, Gavalda J, et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin Infect Dis 2013; 56: 1261–8. [DOI] [PubMed] [Google Scholar]
- 28.Liao CH, Huang YT, Tsai HY, et al. In vitro synergy of ampicillin with gentamicin, ceftriaxone and ciprofloxacin against Enterococcus faecalis. Int J Antimicrob Agents 2014; 44: 85–6. [DOI] [PubMed] [Google Scholar]
- 29.Diaz L, Tran TT, Munita JM, et al. Whole genome analyses of Enterococcus faecium with diverse daptomycin minimal inhibitory concentrations. Antimicrob Agents Chemother 2014; 58: 4527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran TT, Panesso D, Mishra NN, et al. Daptomycin-resistant Enterococcus faecalis diverts the antibiotic molecule from the division septum and remodels cell membrane phospholipids. mBio 2013; 4: e00281–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odenholt I. Ertapenem: a new carbapenem. Expert Opin Investig Drugs 2001; 10: 1157–66. [DOI] [PubMed] [Google Scholar]