Abstract
Human infection with typhoidal Salmonella serovars causes a febrile systemic disease, termed enteric fever. Here we establish that in response to a temperature equivalent to fever (39°C–42°C) Salmonella enterica serovars Typhi, Paratyphi A, and Sendai significantly attenuate their motility, epithelial cell invasion, and uptake by macrophages. Under these feverlike conditions, the residual epithelial cell invasion of S. Paratyphi A occurs in a type III secretion system (T3SS) 1–independent manner and results in restrained disruption of epithelium integrity. The impaired motility and invasion are associated with down-regulation of T3SS-1 genes and class II and III (but not I) of the flagella-chemotaxis regulon. In contrast, we demonstrate up-regulation of particular Salmonella pathogenicity island 2 genes (especially spiC) and increased intraepithelial growth in a T3SS-2–dependent manner. These results indicate that elevated physiological temperature is a novel cue controlling virulence phenotypes in typhoidal serovars, which is likely to play a role in the distinct clinical manifestations elicited by typhoidal and nontyphoidal salmonellae.
Keywords: enteric fever, pyrexia, paratyphoid, Salmonella, pathogenicity, motility, flagella, invasion
The species Salmonella enterica is one of the most prevalent human and animal pathogens, consisting of >2500 serovars. In immunocompetent humans, nontyphoidal Salmonella serovars, such as Typhimurium and Enteritidis, normally cause a localized self-limiting inflammation of the terminal ileum and colon, known as gastroenteritis. In contrast, host-adapted serovars typically cause a systemic disease in one or a limited number of host species [1]. S. Typhi, S. Paratyphi A and B, and S. Sendai are human-adapted serovars that cause enteric fever (also known as typhoid or paratyphoid fever). This is an invasive life-threatening systemic disease with a global annual estimate of >25 million cases, resulting in >200 000 deaths [2].
Active invasion into nonphagocytic cells is one of the hallmarks of S. enterica and is pivotal for its pathogenicity. One of the main mechanisms used by Salmonella to enter host cell is the “trigger” mechanism, which induces intense cytoskeletal rearrangements known as membrane ruffles [3]. This process is mediated by a type III secretion system (T3SS), encoded on Salmonella pathogenicity island (SPI) 1, which injects a wide array of translocated effectors directly into the host cell cytoplasm, enabling Salmonella engulfment and transport through the intestinal barrier. After passing the intestinal mucosa, typhoidal Salmonella gain access to underlying lymphoid tissues and multiply intracellularly within mononuclear phagocytes. Systemic infection with bacteremia and fever develop 7–21 days after infection, accompanied by intensive bacterial spreading from the intestine to the mesenteric lymph nodes, liver, spleen, bone marrow, and gallbladder epithelium [4]. Secondary infection of typhoidal organisms to the small bowel can occur via secretion in the bile through the enterohepatic cycle [5].
Here, we demonstrate for the first time that at temperatures that occur during fever (39°C–42°C), motility, epithelial cell invasion, and uptake by macrophages are markedly attenuated in the human-adapted typhoidal serovars Paratyphi A, Typhi, and Sendai. These thermoregulated phenotypes involve down-regulation of the T3SS-1 genes and class II and III (but not class I) of the flagella-chemotaxis regulon. On the other hand we found induction of specific SPI-2 genes (eg, spiC) and increased intraepithelial replication. These results suggest that typhoidal Salmonella have evolved to use pyrexia as an environmental cue to control key virulence programs, required during different stages of the infection.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
Bacterial strains used in this study are listed in Supplementary Table 1. Bacterial cultures were routinely maintained in Lennox Luria-Bertani (LB) broth or in LB broth supplemented with 0.17 mol/L sodium chloride.
Cloning and Mutant Construction
All primers used to construct deletion mutants and gene cloning are listed in Supplementary Table 2. In-frame deletions of invA, invG, hilA, and ssaR in S. Paratyphi A and the invG and hilA mutants in S. Typhimurium were constructed by the λ Red–mediated recombination system [6]. A C-terminal 2-hemagglutinin (2HA) tagged version of SipB, SopB, SptP, PrgJ, and FliC from S. Typhimurium SL1344 or S. Paratyphi A 45157 were constructed within pWSK29 or pACYC184.
Tissue Cultures, Invasion, and Replication Assays
All cell lines were purchased from the American Type Culture Collection. Epithelial cells (Caco-2) and macrophages (THP-1) were seeded at 5 × 104 and 2.5 × 105 cells/mL, respectively, in a 24-well tissue culture dish 18–24 hours before bacterial infection. Cell infection experiments were carried out using the gentamicin protection assay, as described elsewhere [7] and more details are provided in the Supplementary Data. Host cells were infected with stationary-phase Salmonella cultures grown under microaerophilic conditions in LB broth supplemented with 0.17 mol/L sodium chloride. Under these conditions S. Paratyphi A invasion was maximal in comparison with other examined infection conditions described elsewhere [8] (data not shown).
Transepithelial Electric Resistance Assay
A transepithelial electric resistance (TEER) assay was used to monitor the integrity of the epithelial monolayer using the EVOM Epithelial Voltohmmeter (World Precision Instruments). Caco-2 cells were seeded (2.5 × 105 cells/cm2) and grown in Transwell filter inserts (pore size, 0.4-µm). For more details see the Supplementary Data.
Western Blotting
Bacterial pellets were resuspended in 1 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Boiled samples were separated on 12% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories). Blots were probed with anti-2HA antibody (Abcam ab18181) or anti-DnaK antibody (Abcam ab69617). Goat anti-mouse antibody conjugated to horseradish peroxidase (Abcam ab6721) was used as a secondary antibody, followed by detection with enhanced chemiluminescence reagents (Amersham Pharmacia).
Quantitative Reverse-Transcription Polymerase Chain Reaction
After RNA was extracted from Salmonella cultures using the Qiagen RNAprotect Bacteria Reagent and the RNeasy Mini Kit, 200 ng of DNase I-treated RNA was subjected to a first-strand complementary DNA (cDNA) synthesis, using the iScript cDNA synthesis kit (Bio-Rad Laboratories). Real-time polymerase chain reactions (RT-PCRs) were performed on an Applied Biosystems 7500 Fast Real-Time PCR System. Each reaction was carried out in a total volume of 20 µL containing 10 µL of FastStart Universal SYBR Green Master (ROX) mix (Roche Applied Science), 2 µL of cDNA, and 2 gene-specific primers (Supplementary Table 2) at a final concentration of 0.3 µmol/L each. Relative quantification of transcripts was evaluated using the comparative cycle threshold (Ct) method. The housekeeping gene, rpoD was used as the endogenous normalization control.
Motility Assay
After 10 µL of overnight Salmonella cultures grown in LB broth at 37°C were placed onto 0.3% agar LB plates, the plates were incubated for 5–15 hours at 37°C, 39°C, or 42°C without being inverted.
RESULTS
Impairment of Host Cell Entry by S. Paratyphi A at Fever Temperature
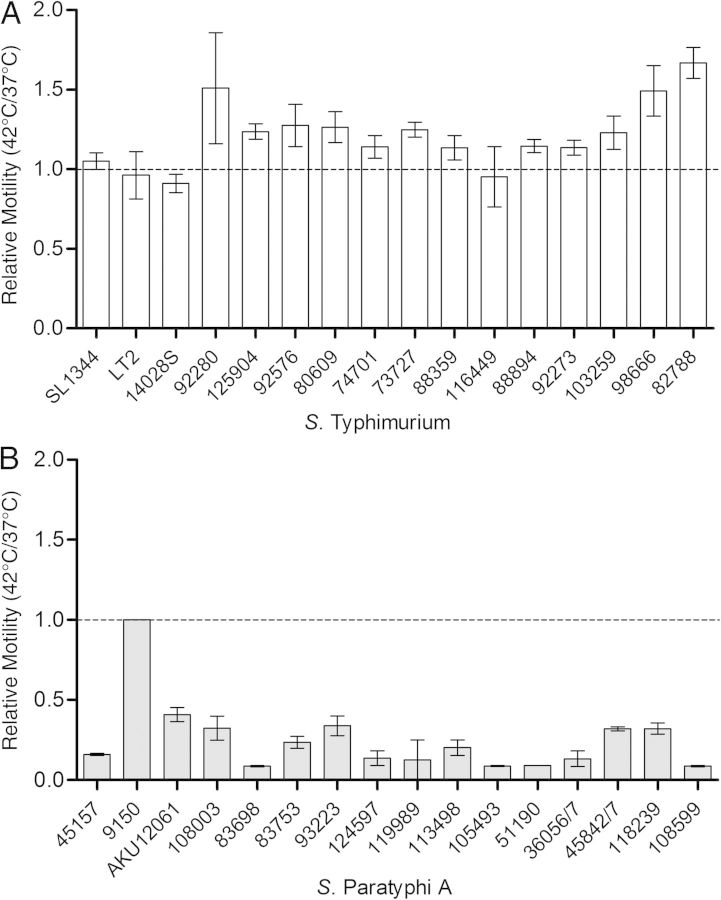
One of the main clinical symptoms characterizing human infection with typhoidal serovars is the manifestation of a sustained fever [4]. Because various bacterial virulence functions have been shown to be temperature regulated [9], we were interested in understanding how elevated physiological temperature affects the virulence of S. Paratyphi A. To address this, we studied the invasion of 16 S. Paratyphi A and 16 S. Typhimurium reference and clinical strains (Supplementary Table 1) into human colonic epithelial cells after bacterial growth at 37°C and 42°C. Although considerable intraserovar variation was observed, a clear and significant reduction in the invasion of all 16 S. Paratyphi A strains was demonstrated after growth at 42°C, presenting, on average, only 18% (median, 13%) of their invasion capability after growth at 37°C (Figure 1A). In comparison, 14 of 16 S. Typhimurium (88%) strains showed either similar or even increased invasion at 42°C relative to 37°C (Figure 1B), while presenting an average invasion of 116% at the elevated temperature. Equivalent results were also obtained when the strains were grown at 39°C (Supplementary Figure 1A).
Figure 1.
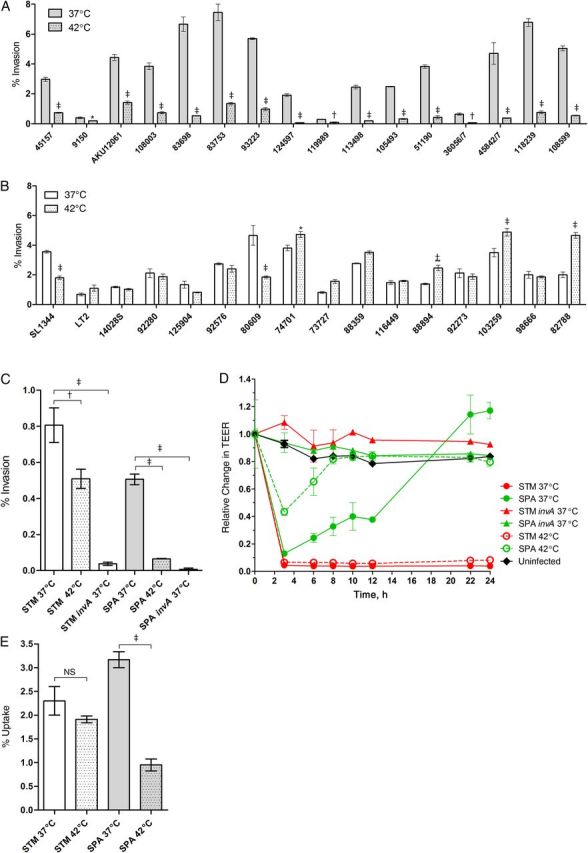
Salmonella Paratyphi A invasion is significantly compromised at fever temperature. A, B, Sixteen S. Paratyphi A (A) and 16 Salmonella Typhimurium strains (B) were grown in Lennox Luria-Bertani (LB) broth supplemented with 0.17 mol/L sodium chloride to the stationary phase under microaerophilic conditions at 37°C (open bars) or 42°C (dotted bars) and used to infect Caco-2 cells at a multiplicity of infection (MOI) of 20:1. Invasion, as assessed by the gentamicin protection assay, is shown as the percentage of intracellular bacteria (colony-forming units [CFUs]) recovered at 2 hours after infection compared with the total number of CFUs used to infect the cells. Values represent the mean, and error bars represent the standard error of the mean (SEM) for ≥3 independent infections. The unpaired, 2-tailed Student t test was used to determine statistical differences. *P < .05; †P < .001; ‡P < .0001. C, S. Typhimurium (STM) strain SL1344, S. Paratyphi A (SPA) strain 45157 and their invA derivatives were grown at 37°C (open bars) or 42°C (dotted bars) and used to infect polarized Caco-2 cells using the gentamicin protection assay. Invasion is shown as the percentage of intracellular bacteria recovered at 2 hours after infection from the total number of the bacteria used to infect the cells. Values represent the mean and SEM for 3 independent infections in 1 of 2 representative experiments. One-way analysis of variance with the Dunnett multiple-comparison test was used to determine differences between data sets. D, Integrity of the epithelial monolayer was determined using polarized Caco-2 cells infected at an MOI of 10:1 at 0, 3, 6, 8, 10, 12, 22, and 24 hours after infection and is shown as the change in transepithelial electric resistance (TEER) from the time of infection. Data represent the mean and SEM for 3 independent infections in 1 of 2 representative experiments. E, Salmonella cultures were grown in LB broth supplemented with 0.17 mol/L sodium chloride to stationary phase under microaerophilic conditions at 37°C or 42°C and used to infect human-derived monocytes THP-1 cells at an MOI of 2:1. Uptake is shown as the percentage of intracellular bacteria recovered at 2 hours after infection from the total number of the bacteria used to infect the cells. Values represent the mean and SEM for 3 independent infections in 1 of 2 representative experiments. The unpaired, 2-tailed Student t test was used to determine statistical differences; Abbreviation: NS, not significant.
To further investigate differences in the response of these serovars to elevated temperature, we chose to focus on the sequenced strains S. Typhimurium SL1344 (PRJNA86645) and S. Paratyphi A 45157 (PRJNA168852) as representative virulent strains [7, 10] that showed similar levels of invasion into host cell at 37°C. We next examined whether impaired S. Paratyphi A invasion at an elevated temperature also occurs in polarized (differentiated) epithelial monolayers of Caco-2 cells. As a control, we included the S. Typhimurium and S. Paratyphi A invA mutants, deficient in invasion of cultured epithelial cells [11]. Similar to the results obtained with nonpolarized cells, S. Paratyphi A invasion into an epithelial monolayer was significantly reduced by about 8-fold at 42°C, whereas S. Typhimurium showed only a moderate decrease in the invasion at 42°C (Figure 1C). In agreement with these results, we also showed that whereas invasion of S. Typhimurium that were grown at 37°C or 42°C induced similar and prominent reduction in the TEER of the monolayers, S. Paratyphi A grown at 42°C caused a lower reduction in TEER that was recovered much faster than S. Paratyphi A grown at 37°C (Figure 1D).
Next we asked how feverlike temperature affects bacterial invasion to THP-1 human macrophages. Similar to the results obtained with epithelial cells, we found that bacterial growth at 42°C reduces the invasion of S. Paratyphi A, but not that of S. Typhimurium, by >3-fold (Figure 1E). Because a similar growth was found for both strains in liquid LB medium at 42°C (data not shown), we concluded that inducing S. Paratyphi A at 39°C–42°C significantly impairs its ability to enter host cells in vitro.
Role of the T3SS-1 in the Impaired Invasion of S. Paratyphi A at Fever Temperature
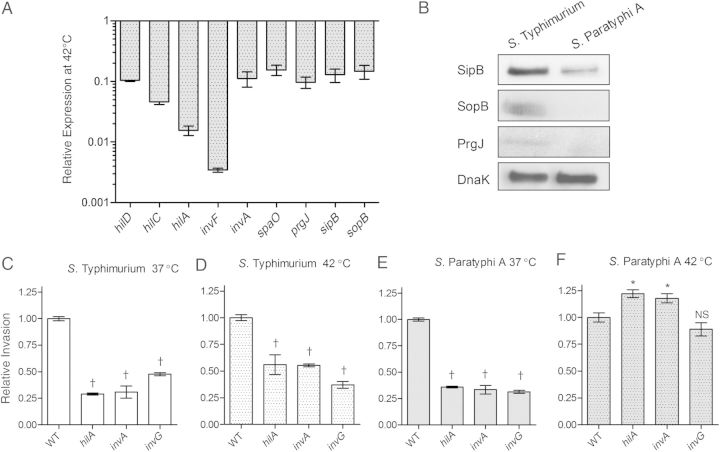
The T3SS encoded within SPI-1 is used by Salmonella to translocate effector proteins required for bacterial engulfment and transport through the intestinal barrier [12]. Considering the differences between the invasion of S. Typhimurium and Paratyphi A at 42°C shown above, we sought to study their SPI-1 gene expression under these conditions. Quantitative reverse transcription PCR (qRT-PCR) was used to determine the relative expression of hilD, hilC, hilA, and invF (SPI-1 regulators), invA, spaO, and prgJ (T3SS-1 structural genes) and sipB and sopB (SPI-1 effectors) in S. Paratyphi A and S. Typhimurium at 42°C. This analysis showed 7–300-fold lower levels of SPI-1 transcripts at 42°C in S. Paratyphi A relative to S. Typhimurium (Figure 2A). Immunostaining by Western blot against SopB, SipB, and PrgJ tagged with a hemagglutinin epitope confirmed the qRT-PCR results and demonstrated lower levels of these T3SS-1 proteins in S. Paratyphi A than in S. Typhimurium at 42°C (Figure 2B).
Figure 2.
The impaired invasion of Salmonella Paratyphi A at fever temperature occurs in a type III secretion system 1–independent manner. A, The ratio between the expression of the Salmonella pathogenicity island (SPI) 1 genes hilD, hilC, hilA, invF, invA, spaO, prgJ, sipB and sopB in S. Paratyphi A and Salmonella Typhimurium at 42°C was determined by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Total RNA was harvested from S. Paratyphi A 45157 and S. Typhimurium SL1344 cultures grown in Lennox Luria-Bertani broth to the late logarithmic phase and gene expression was normalized using the housekeeping gene rpoD. Values represent the mean and standard error of the mean (SEM) for 3 independent qRT-PCR analyses based on 3 independent RNA extractions. B, Western blot analysis of bacterial cell lysate from S. Typhimurium and S. Paratyphi A expressing SipB-2HA, SopB-2HA, and PrgJ-2HA. Protein fractions were probed using anti–hemagglutinin antibody and anti-DnaK as a control. C, D, S. Typhimurium SL1344 and its hilA, invA, and invG null mutant strains were grown at 37°C (C) or 42°C (D) and used to infect Caco-2 cells (multiplicity of infection, 20:1), using the gentamicin protection assay. *P < .05; †P < .001. E, F, S. Paratyphi A 45157 and its hilA, invA, and invG null mutant strains were grown at 37°C (E) or 42°C (F). The invasion of SPI-1 mutants in panels C–F is shown relative to the wild-type (WT) parental strain. Values represent the mean and SEM for ≥3 independent infections in 1 of 3 representative experiments. Analysis of variance with Dunnett multiple-comparison test was used to determine differences between data sets; Abbreviation: NS, not significant.
To further characterize the impaired invasion of S. Paratyphi A at 42°C, we studied the contribution of the T3SS-1 to host cell entry. Wild-type (WT), hilA, invA, and invG null mutants of S. Typhimurium and S. Paratyphi A were compared for their invasion ability at 37°C and 42°C. S. Typhimurium strains lacking these SPI-1 genes, demonstrated a 2–3-fold reduction in their invasion into Caco-2 cells both at 37°C (Figure 2C) and 42°C (Figure 2D). Nevertheless, while S. Paratyphi A invasion was reduced in the absence of HilA, InvA or InvG by 3-fold after growing at 37°C (Figure 2E), no further reduction in the invasion relative to the wild-type was observed at 42°C (Figure 2F). To ensure that this phenotype is not isolate specific, an invA mutant was also constructed in an additional S. Paratyphi A strain (isolate 118 239), and the same impaired yet InvA-independent invasion was found at 42°C, but not at 37°C (data not shown). These results are consistent with the reduced expression of SPI-1 genes in S. Paratyphi A compared with S. Typhimurium at 42°C and together suggest that the T3SS-1 does not play a role in the residual invasion of S. Paratyphi A into Caco-2 cells at elevated temperatures, in contrast to S. Typhimurium, which uses a T3SS-1–dependent invasion both at the permissive and restricted temperatures.
Significant Impairment of S. Paratyphi A but Not S. Typhimurium Motility at Fever Temperature
Another phenotype central to Salmonella pathogenicity is motility, which has been shown to play roles in multiple virulence-associated processes, including host cell adherence, biofilm formation, protein secretion, and invasion [13]. Therefore, we next studied the motility phenotype of 16 S. Typhimurium and 16 S. Paratyphi A strains. The motility of all S. Typhimurium strains on soft (0.3%) agar plates was similar (or even slightly higher for 13 of 16 strains) at 42°C than at 37°C (Figure 3A). The mean motility ratio between 42°C and 37°C for all 16 S. Typhimurium strains was 1.21 (median, 1.18). Nevertheless, in sharp contrast (P < .0001), the motility of the S. Paratyphi A strains (with the exception of strain 9150, which was barely motile in both conditions) was significantly (2–12-fold) decreased at 42°C compared with their motility at 37°C, with a mean motility ratio of only 0.25 (median, 0.18) for all S. Paratyphi A strains (Figure 3B). Similar results were also obtained when we repeated this analysis at 39°C (Supplementary Figure 1B), and hence we concluded that S. Paratyphi A, but not S. Typhimurium motility is significantly inhibited under feverlike conditions.
Figure 3.
Salmonella Paratyphi A motility is significantly impaired at fever temperature. A, B, Ten microliters of overnight cultures from 16 Salmonella Typhimurium (A) and 16 S. Paratyphi A (B) strains grown at 37°C were inoculated onto a soft (0.3%) agar plates and incubated at 37°C or 42°C for 5.5 hours. Bars represent mean and standard error of the mean for the ratio between their motility (swim halo radius) at 42°C and 37°C in 2 independent experiments. The S. Paratyphi A 9150 strain was nonmotile (1 mm of swimming during 5.5 hours) at both temperatures.
Down-regulation of the Flagella-Chemotaxis Regulon in S. Paratyphi A at Fever Temperatures
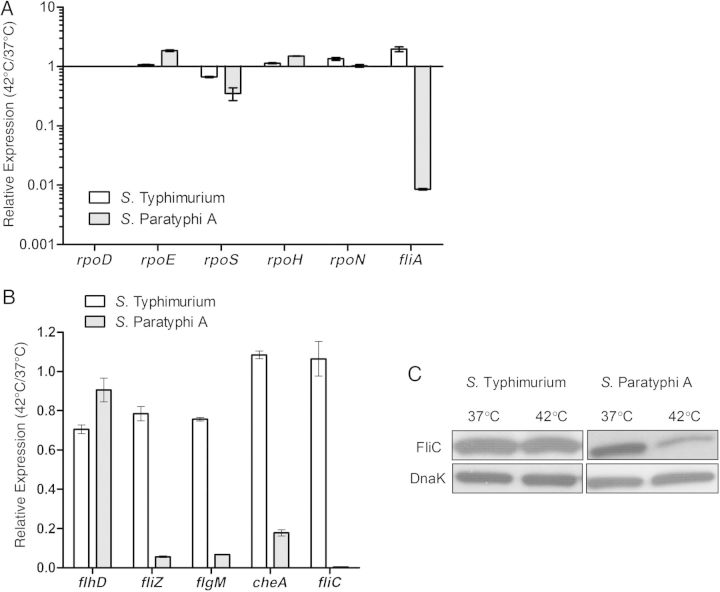
To gain further insights into a possible regulatory mechanism underlying this phenotype, we determined the expression levels of the 6 Salmonella sigma factors, including σD/70 (encoded by rpoD), σE/24 (rpoE), σH/32 (rpoH), σS/38 (rpoS), σN/54 (rpoN), and σ28 (fliA) at 37°C and 42°C in both serovars. The relative expression of the 5 sigma factor genes rpoD, rpoE, rpoH, rpoS, and rpoN at 42°C relative to their expression at 37°C was similar in S. Typhimurium and S. Paratyphi A, but the expression of fliA was found to be about 100-fold lower in S. Paratyphi A than in S. Typhimurium at 42°C (Figure 4A).
Figure 4.
The flagella-chemotaxis regulon in Salmonella Paratyphi A is down-regulated at fever temperature. A, Total RNA was harvested from Salmonella Typhimurium SL1344 and S. Paratyphi A 45157 cultures grown at 37°C and 42°C to the late logarithmic phase and was subjected to quantitative reverse-transcription polymerase chain reaction (qRT-PCR). The fold change in the abundance of the transcripts (normalized to rpoD) in S. Typhimurium (open bars) or S. Paratyphi A (gray bars) grown at 42°C relative to their expression at 37°C is shown. Values represent mean and standard error of the mean (SEM) for 3 independent qRT-PCR experiments. B, Abundance of flhD, fliZ, flgM, cheA, and fliC transcript in S. Typhimurium SL1344 (open bars) or in S. Paratyphi A 45 157 (gray bars), grown at 42°C relative to 37°C. The qRT-PCR results show the mean and the SEM for 3 independent experiments. C, Western blot analysis of bacterial cell lysate from S. Typhimurium and S. Paratyphi A expressing FliC-2HA grown at 37°C or 42°C. Protein fractions were probed using anti-hemagglutinin antibody and anti-DnaK as a control.
In Escherichia coli and Salmonella, the flagellar, motility, and chemotaxis genes constitute a hierarchical regulon of >60 genes, organized into 3 classes that are temporally expressed in a cascade manner [14]. At the top of the hierarchy is a class I master regulator encoded by the single operon flhDC, necessary for the activation of fliA and other class II genes. FliA, in turn, is required for the expression of the third class genes. To broaden the transcription analysis, we further studied the expression of class I (flhD), class II (fliZ and flgM), and class III (cheA and fliC) genes in both serovars at 42°C versus 37°C. Although the expression of these genes did not change (cheA and fliC) or only marginally decreased (flhD, fliZ, and flgM) in S. Typhimurium at 42°C, in S. Paratyphi A class II and III but not class I genes were sharply reduced (by 6–250-fold) when grown at 42°C (Figure 4B). A reduced expression of FliC at 42°C in S. Paratyphi A, but not in S. Typhimurium, was also confirmed on the protein level using Western blot analysis against FliC tagged with a 2HA epitope (Figure 4C).
We concluded from these experiments that specifically class II and III genes of the flagella-chemotaxis regulon in S. Paratyphi A, but not in S. Typhimurium, are markedly down-regulated at fever temperature. These results are highly consistent with the impaired motility of S. Paratyphi A, suggesting that the lower motility at fever temperature is mediated by the repression of classes II and III of the flagella-chemotaxis regulon.
Impaired Motility and Invasion at Fever Temperature in Typhoidal Serovars
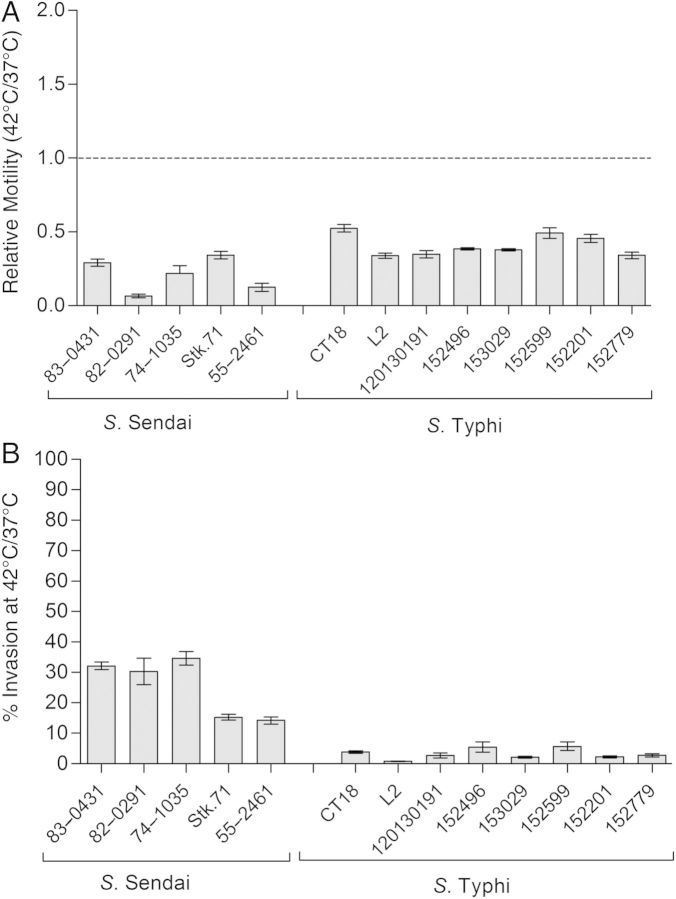
To investigate whether the impaired motility and invasion at 42°C is characteristic of other typhoidal serovars, we studied these phenotypes in 8 S. Typhi and 5 S. Sendai strains. As in S. Paratyphi A, a clear decrease of 5- and 2.5-fold, on average, was measured in the motility of S. Sendai and S. Typhi, respectively, at 42°C relative to 37°C (Figure 5A). Also, the average invasion of S. Sendai and S. Typhi to Caco-2 cells was prominently reduced by 5- and 40-fold, respectively, at the elevated temperature relative to their invasion at the permissive temperature (Figure 5B). We concluded from these experiments that the impaired motility and compromised invasion observed at fever temperature are common phenotypes to all 3 human-adapted typhoidal serovars: Typhi, Paratyphi A ,and Sendai.
Figure 5.
Impaired motility and invasion at fever temperature are common to typhoidal serovars. A, Ten microliters of overnight cultures grown at 37°C were inoculated onto a soft (0.3%) agar plate and incubated at 37°C or 42°C for 12 hours (Salmonella Sendai) or 15 hours (Salmonella Typhi). Bars represent the mean ratio of Salmonella motility at 42°C and 37°C in 3 experiments, and error bars the standard error of the mean (SEM). B, S. Typhi and S. Sendai strains were grown in Lennox Luria-Bertani broth supplemented with 0.17 mol/L sodium chloride to the stationary phase under microaerophilic conditions at 37°C or 42°C and used to infect Caco-2 cells at a multiplicity of infection of 20:1. Invasion at 42°C relative to the invasion at 37°C is shown (as %). Values represent the mean and SEM for ≥3 independent infections.
Contribution of fever temperature to intraepithelial replication of S. Paratyphi A
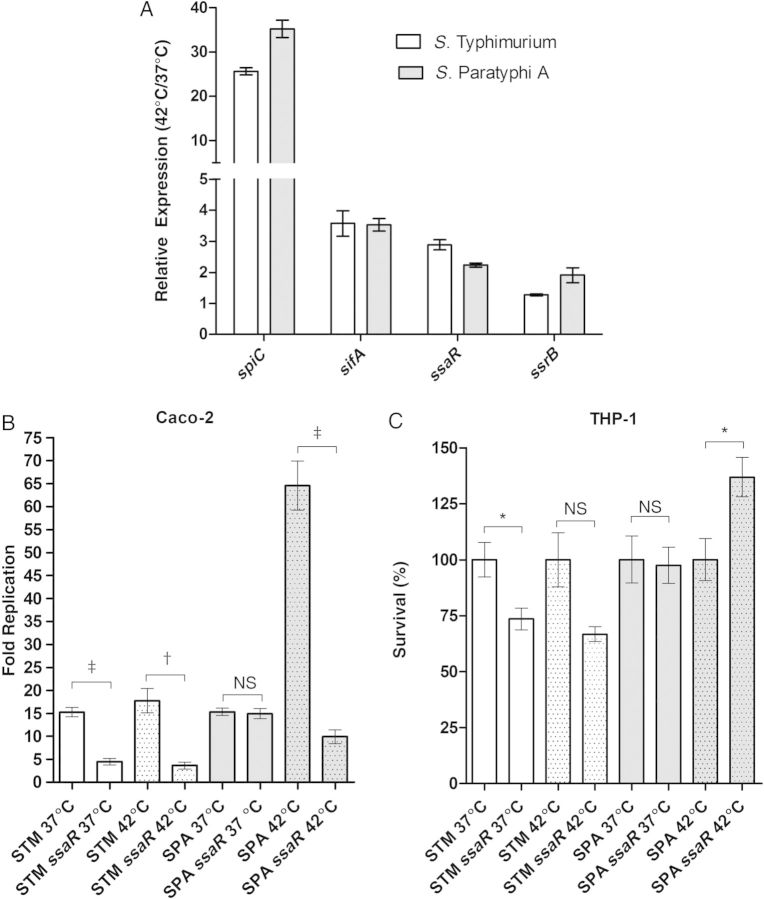
The reduced expression of SPI-1 and motility genes at an elevated temperature prompted us to investigate the effect of such a cue on SPI-2 expression. Thus, we analyzed the expression level of 2 SPI-2 effectors (spiC and sifA) and T3SS-2 structural (ssaR) and SPI-2 master regulator (ssrB) genes in S. Typhimurium and S. Paratyphi A grown to the stationary phase at 42°C, compared with 37°C. Interestingly, in contrast to the SPI-1 and motility regulons, we found a moderate (ssrB, ssaR and sifA) to high (spiC) up-regulation in the expression of these SPI-2 genes at the elevated temperature, in both S. Typhimurium and S. Paratyphi A (Figure 6A). We concluded from this analysis that at least some SPI-2 genes expression is induced at a feverlike temperature.
Figure 6.
Intraepithelial growth of Salmonella Paratyphi A increases at fever temperature. A, Total RNA was harvested from Salmonella Typhimurium SL1344 (open bars) and S. Paratyphi A 45157 (gray bars) cultures grown to stationary phase under microaerophilic conditions at 37°C or 42°C and was subjected to quantitative reverse-transcription polymerase chain reaction. Expression (normalized to rpoD) is shown as the abundance of transcripts in cultures grown at 42°C relative to their expression at 37°C. Values represent mean and standard error of the mean (SEM) for 3 independent experiments. B, S. Typhimurium (STM; open bars), and S. Paratyphi A (SPA; gray bars) were grown in Lennox Luria-Bertani broth supplemented with 0.17 mol/L sodium chloride to stationary phase under microaerophilic conditions at 37°C or 42°C and used to infect Caco-2 cells at a multiplicity of infection (MOI) of 20:1. C, Salmonella cultures grown as in (B) at 37°C or 42°C were used to infect human derived monocytes THP-1 cells at an MOI 2:1. A null ssaR (type three secretion system 2 structural gene) strain was included for both serovars. Intracellular replication in B and C is shown as the ratio of recovered colony-forming units at 24 hours to those at 2 hours after infection. Values represent mean and SEM for ≥3 independent infections in 1 of 2 representative experiments. The unpaired, 2-tailed Student t test was used to determine statistical differences. *P < .05; †P < .001. Abbreviation: NS, not significant.
To further establish the importance of the elevated temperature on SPI-2 expression, we compared the ability of Salmonella to replicate within epithelial cells when bacteria were grown at 37°C or 42°C before Caco-2 cell infection. Interestingly, while S. Typhimurium replication was similar under both conditions (approximately 15-fold increase in the intracellular colony-forming unit count within 24 hours), when S. Paratyphi A was primed at 42°C its intracellular replication was >4-fold higher than for cells infected with cultures grown at 37°C, and it displayed about a 65-fold growth increase (Figure 6B). Furthermore, the contribution of the T3SS-2 to S. Paratyphi A replication was much more pronounced at 42°C (P = .001) than at 37°C, indicating that elevated temperature supports S. Paratyphi A intracellular replication in the human epithelial cell line. Interestingly, an increased growth of S. Paratyphi A after induction at 42°C was found to be specific to epithelial cells and was not observed in the human monocyte–like cell line THP-1 (Figure 6C), suggesting a cell type–specific phenotype.
DISCUSSION
Human infection with different S. enterica serovars may result in diverse clinical manifestations. Whereas infection with the majority of the subspecies-1 serovars induces a self-limiting gastroenteritis, the human-restricted serovars, Typhi, Paratyphi A, and Sendai, elicit a systemic enteric fever, completely different from the clinical presentation of gastroenteritis [15]. Although this dichotomy in disease manifestations is well recognized, the mechanisms underlying these different diseases in humans are far from being understood. One of the accepted notions in the field suggests that the polysaccharide capsular antigen Vi in S. Typhi enables this pathogen to resist phagocytosis and complement killing [16] and to evade detection by pattern recognition receptors, in a way that limits neutrophil influx and small bowel inflammation [17, 18]. Nevertheless, because the Vi capsule is largely restricted to serovar Typhi and is absent from serovars Paratyphi A and Sendai, it cannot explain the extremely similar clinical manifestation caused by other typhoidal serovars. Furthermore, the fact that Vi-negative strains of S. Typhi are still able to cause a typhoid illness in human volunteers [19], or even typhoid outbreaks [20] suggests that additional mechanisms are involved.
Human infection with typhoidal Salmonella that lead to enteric fever is almost always characterized by a high and sustained fever, during the acute stage of the infection [4]. Here, we established that at elevated physiological temperature of 39°C–42°C, both epithelial cell invasion and motility are fundamentally repressed in the 3 typhoidal serovars Paratyphi A, Typhi, and Sendai. On the other hand, under these conditions S. Paratyphi A presents an improved intracellular growth in a T3SS-2–dependent manner, within epithelial cells but not in macrophages. On the molecular level, we showed that these phenotypes are associated with down-regulation of flagellar and T3SS-1 gene expression and up-regulation of specific SPI-2 genes, including spiC.
Taken together, our results indicate that physiological elevated temperature is used by typhoidal serovars as a regulatory cue controlling key virulence phenotypes. We hypothesize that this cue may signal the pathogen to switch from the initial invasive stage of the infection (SPI-1 and motility dependent) to the later persistent phase. It is possible that reducing invasion, uptake by phagocytic cells and motility at a later stage of the infection facilitates the persistence of the pathogen in niches such as the gallbladder epithelium [21, 22] and lessens repetitive cycles of reseeding from the gut back to systemic sites. An additional possible benefit from such a thermoregulated response might be limiting the number of organisms crossing epithelial tissues to the lymphatic system to control bacterial load in the blood, avoid sepsis, and, consequently, prevent host death. Indeed, typhoidal serovars are rarely associated with septic shock, in contrast to many other gram-negative pathogens [23].
An increasing body of data suggests that lower flagellin expression in Salmonella contributes to the ability of the pathogen to disseminate and induce a systemic disease. The nonflagellate S. enterica serovars Gallinarum and Pullorum cause a systemic disease in poultry, termed fowl typhoid [24]. Similarly, S. Typhimurium definitive phage type 2, associated with systemic disease in pigeons, has been also shown to repress flagella expression at the avian host temperature [25], and overexpression of fliC or flhDC attenuate S. Typhimurium virulence in vivo [26]. The S. Typhi SPI-7–encoded regulator TviA has been also shown to repress genes involved in flagella-mediated motility and epithelial invasion and was speculated to contribute to the ability of S. Typhi to cause systemic disease [27]. Here we show that fever temperature has a similar effect on the expression of the flagella resulting in reduced motility of 3 typhoidal serovars, including those that do not harbor the tviA locus (ie, Paratyphi A and Sendai).
To summarize, we have shown that at fever temperature, S. Paratyphi A manifests compromised host cell entry (Figure 1 and Supplementary Figure 1) and significantly impaired motility (Figure 3 and Supplementary Figure 1) that are associated with lower levels of SPI-1 genes (Figure 2) and down-regulation of the flagellar regulon (Figure 4). On the other hand, under these feverlike conditions, S. Paratyphi A up-regulates specific SPI-2 genes expression and enhances its intraepithelial growth (Figure 6). These results suggest that clinical fever (pyrexia) is used by typhoidal serovars as an environmental regulatory cue controlling key virulence-associated phenotypes. Because these phenotypes were also found in S. Typhi and S. Sendai (Figure 5) but were not prominent in serovar Typhimurium (Figures 1B and 3A), it is likely that the different response to fever contributes to the distinct mechanisms required by typhoidal and nontyphoidal salmonellae.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful for the staff of the Ministry of Health Central Laboratories and specifically to Dr Israel Nissan, Dr Lea Valinsky, and Dr Vered Agmon for sharing clinical isolates. We also thank prof Ilan Rosenshine for critical reading of the manuscript.
Financial support. This work was supported by the German-Israeli Foundation for Scientific Research and Development (grant 1096-39.11/2010 to O. G.-M.), the Israel Science Foundation (grant 999/14 to O. G.-M.), the National Institutes of Health (grants HHSN272200900040C, AI039557 AI052237, AI073971, AI075093, AI077645 AI083646, USDA 2009-03579, and 2011-67017-30127 to M. M.), the Binational Agricultural Research and Development Fund (M. M.), and the Center for Produce Safety (M. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Uzzau S, Brown DJ, Wallis T, et al. Host adapted serotypes of Salmonella enterica. Epidemiol Infect 2000; 125:229–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ 2004; 82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Velge P, Wiedemann A, Rosselin M, et al. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. MicrobiologyOpen 2012; 1:243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med 2002; 347:1770–82. [DOI] [PubMed] [Google Scholar]
- 5.Gordon MA. Salmonella infections in immunocompromised adults. J Infect 2008; 56:413–22. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 2000; 97:6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gal-Mor O, Suez J, Elhadad D, et al. Molecular and cellular characterization of a Salmonella enterica serovar Paratyphi a outbreak strain and the human immune response to infection. Clin Vaccine Immunol 2012; 19:146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartera C, Metcalf ES. Osmolarity and growth phase overlap in regulation of Salmonella typhi adherence to and invasion of human intestinal cells. Infect Immun 1993; 61:3084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurme R, Rhen M. Temperature sensing in bacterial gene regulation—what it all boils down to. Mol Microbiol 1998; 30:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 1981; 291:238–9. [DOI] [PubMed] [Google Scholar]
- 11.Galan JE, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol 1992; 174:4338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou D, Galan J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect 2001; 3:1293–8. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JK, Smith TG, Hoover TR. Sense and sensibility: flagellum-mediated gene regulation. Trends Microbiol 2010; 18:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terashima H, Kojima S, Homma M. Flagellar motility in bacteria structure and function of flagellar motor. Int Rev Cell Mol Biol 2008; 270:39–85. [DOI] [PubMed] [Google Scholar]
- 15.Sabbagh SC, Forest CG, Lepage C, Leclerc JM, Daigle F. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett 2010; 305:1–13. [DOI] [PubMed] [Google Scholar]
- 16.Robbins JD, Robbins JB. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis 1984; 150:436–49. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci U S A 2004; 101:17492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RP, Raffatellu M, Chessa D, Winter SE, Tukel C, Baumler AJ. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol 2008; 10:876–90. [DOI] [PubMed] [Google Scholar]
- 19.Hone DM, Attridge SR, Forrest B, et al. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun 1988; 56:1326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arya SC. Field effectiveness of Vi polysaccharide typhoid vaccine in the People's Republic of China. J Infect Dis 2002; 185:846. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Escobedo G, Gunn JS. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun 2013; 81:2920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menendez A, Arena ET, Guttman JA, et al. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J Infect Dis 2009; 200:1703–13. [DOI] [PubMed] [Google Scholar]
- 23.Tsolis RM, Young GM, Solnick JV, Baumler AJ. From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol 2008; 6:883–92. [DOI] [PubMed] [Google Scholar]
- 24.Kwon HJ, Park KY, Yoo HS, Park JY, Park YH, Kim SJ. Differentiation of Salmonella enterica serotype gallinarum biotype pullorum from biotype gallinarum by analysis of phase 1 flagellin C gene (fliC). J Microbiol Methods 2000; 40:33–8. [DOI] [PubMed] [Google Scholar]
- 25.Kingsley RA, Kay S, Connor T, et al. Genome and transcriptome adaptation accompanying emergence of the definitive type 2 host-restricted Salmonella enterica serovar Typhimurium pathovar. mBio 2013; 4:e00565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Thornburg T, Suo Z, et al. Flagella overexpression attenuates Salmonella pathogenesis. PloS One 2012; 7:e46828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wangdi T, Winter SE, Baumler AJ. Typhoid fever: “you can't hit what you can't see.” Gut Microbes 2012; 3:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.