Abstract
Background. A/H3N2 variant (H3N2v) influenza may sustain human-to-human transmission, and an available candidate vaccine would be important.
Methods. In this phase I, randomized, observer-blind, dose-ranging study, 627 healthy subjects ≥3 years of age were randomized to receive 2 vaccinations with H3N2c cell-culture-derived vaccine doses containing 3.75 µg, 7.5 µg, or 15 µg hemagglutinin antigen of H3N2v with or without MF59 (registered trademark of Novartis AG) adjuvant (an oil-in-water emulsion). This paper reports Day 43 planned interim data.
Results. Single MF59-adjuvanted H3N2c doses elicited immune responses in almost all subjects regardless of antigen and adjuvant dose; the Center for Biologics Evaluation Research and Review (CBER) licensure criteria were met for all groups. Subjects with prevaccination hemagglutination inhibition titers <10 and children 3–<9 years achieve CBER criteria only after receiving 2 doses of nonadjuvanted H3N2c vaccine. Highest antibody titers were observed in the 7.5 µg + 0.25 mL MF59 groups in all age cohorts. MF59-adjuvanted H3N2c vaccines showed the highest rates of solicited local and systemic events, predominately mild or moderate.
Conclusions. A single dose of H3N2c vaccine may be immunogenic and supports further development of MF59-adjuvanted H3N2c vaccines, especially for pediatric populations.
Clinical Trials Registration. ClinicalTrials.gov identifier NCT01855945 (http://clinicaltrials.gov/ct2/show/NCT01855945).
Keywords: A/H3N2 variant, cell culture–derived, immunogenicity, influenza, safety, vaccine, virus
A/H3N2 variant (H3N2v) swine influenza virus containing the matrix (M) gene from the 2009 A/H1N1 pandemic virus was first identified in pigs in 2010 and subsequently detected in 12 people in the United States in 2011 [1]. The following year, 309 confirmed cases of H3N2v infection were reported across 12 states, resulting in 1 death and 16 hospitalizations [2]. The majority of cases were in children and adolescents; most cases reported agricultural fair attendance and/or contact with swine prior to illness [3]. Consequently, the US Department of Health and Human Services requested a clinical evaluation of candidate H3N2v influenza vaccines as part of their pandemic preparedness program. There were only 19 confirmed cases in 2013, suggesting restricted transmission of H3N2v virus [2, 4]. However sporadic person-to-person transmission demonstrates the epidemic potential of this emerging virus [3]. In addition, animal models have shown that H3N2v viruses have the capacity for efficient replication and transmission in mammals [5].
The aim of this Phase 1 study was to assess the safety and immunogenicity of MF59-adjuvanted and nonadjuvanted cell-culture-derived, inactivated swine origin H3N2v influenza monovalent subunit (H3N2c) vaccines in children, adolescents, adults, and the elderly, and to help identify the optimal antigen and adjuvant dose to be used in further clinical development in case the H3N2v virus threat increases. Here we present results from a planned interim analysis at Day 43; safety and antibody data from a 12-month follow-up will be presented separately.
METHODS
Study Design and Objectives
This phase I multicenter, randomized, observer-blind, dose-ranging study was conducted in 11 centers in the United States. The protocol was approved by appropriate Institutional Review Boards, and the study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Before enrolment, written informed consent was obtained from subjects ≥18 years and from the parent/guardian of younger participants. Subjects from 4 stratified age cohorts (3–<9 years, 9–<18 years, 18–<65 years, and ≥65 years of age) were randomized (1:1:1) to receive 2 vaccinations, 3 weeks apart, of 1 of 3 H3N2c vaccines: 3.75 µg hemagglutinin antigen (HA) of H3N2v with 0.125 mL MF59 (the half-dose group), 7.5 µg HA of H3N2v with 0.25 mL MF59 (the full-dose group), or 15 µg HA of H3N2v without MF59 adjuvant (the nonadjuvanted group). Subjects were randomized using a web-based randomization system. The study was performed in 2 parts: adult/elderly subjects (≥18 years) were vaccinated first, and pediatric subjects (3–<18 years) were vaccinated following a safety review. This allowed vaccination of younger age cohorts only in the absence of any safety concerns in the older cohorts.
Participants
Eligible study participants were healthy males and females aged ≥3 years. The main exclusion criteria were: a history of or ongoing chronic or progressive disease, or any illness that posed additional risk to the subjects; a history of allergy to any vaccine component; confirmed or suspected illness from H3N2v swine flu; a body temperature >38°C or any acute illness within 3 days of study vaccination; receipt of vaccine containing H3N2v swine flu virus; receipt of a seasonal influenza vaccine 2 weeks prior to enrolment or intention to receive seasonal influenza vaccine up to Day 43 of the study; receipt of any other vaccine 2 weeks (for inactivated vaccines) or 4 weeks (for live vaccines) prior to enrolment or intention to receive any vaccine within 4 weeks from the study vaccinations; or an impaired or altered immune system. Female subjects of childbearing age could not be pregnant at the time of enrolment, and had to commit to the use of adequate birth control measures for at least 3 weeks after the second study vaccination.
Vaccines
The cell-culture-derived subunit inactivated monovalent MF59-adjuvanted or nonadjuvanted H3N2c vaccines (Novartis Vaccines, Holly Springs, NC) were based on purified surface antigens (HA) from the H3N2v A/Minnesota/11/2010 influenza strain, based on published methods for pandemic influenza vaccine [6]. Vaccines were supplied for injection in a prefilled syringe containing 0.5 mL fluid, with a half dose ring mark, and stored between 2°C and 8°C. A full dose of MF59 (Novartis Vaccines, Marburg, Germany) contains 9.75 mg squalene, 1.175 mg polysorbate 80, 1.175 mg sorbitan trioleate, 0.66 mg sodium citrate, and 0.04 mg citric acid. The half-dose regimen used the same prefilled syringes and MF59 formulation as the full-dose regimen, but only half the volume was administered. Vaccines were administered as intramuscular injections in an observer-blind fashion by designated unblinded site staff. The investigator and all other study personnel remained blinded to study treatment.
Safety Assessment
After each vaccination, subjects were observed for 30 minutes to monitor for immediate adverse reactions. Solicited local and systemic adverse events (AEs) were recorded by the subject or their parent/guardian on a diary card for 7 days after each vaccination. Solicited local reactions included: injection site induration, erythema, ecchymosis (all ages); injection site tenderness (subjects <6 years only) or injection site pain (subjects ≥6 years only). Solicited systemic reactions in subjects <6 years of age were change in eating habits, sleepiness, irritability, vomiting, diarrhea, and fever (body temperature ≥38.0°C); in subjects ≥6 years of age they were nausea, generalized myalgia, generalized arthralgia, headache, fatigue, loss of appetite, malaise, vomiting, diarrhea, and fever. Reports of unsolicited AEs and serious AEs (SAEs) were collected throughout the study to Day 43. The investigators assessed the AEs for severity and relation to study vaccines. The severity of AEs was categorized as mild (transient with no limitation in normal daily activity), moderate (some limitation in normal daily activity), or severe (unable to perform normal daily activity). Assessments of the causal relationship of AEs to the study vaccines were classified as not related, possibly related, or probably related.
Immunogenicity Assessment
Blood was drawn from each subject on Day 1 right before priming, Day 22 before boosting, and on Day 43. Antibody titers were determined by a hemagglutination inhibition (HI) assay, according to standard methods [7] at the Novartis Vaccines laboratory in Marburg, Germany.
Immunogenicity Objectives
The primary immunogenicity objective was to evaluate HI antibody responses for each H3N2c vaccine group, in terms of the percentage of subjects with HI titer ≥40 on Days 1, 22, and 43; and the percentage of subjects achieving seroconversion on Days 22 and 43. Seroconversion is defined as an HI titer ≥40 for subjects with a baseline HI titer <10, or a minimum 4-fold increase in HI titer for subjects with a baseline HI titer ≥10. The secondary immunogenicity objectives were to evaluate immunogenicity defined by the Center for Biologics Evaluation Research and Review (CBER) and the Committee for Medicinal Products for Human Use (CHMP) criteria [8, 9]. The CBER criteria for seroconversion and HI titers ≥40 were applied for subjects <65 years or ≥65 years, while the CHMP criteria for seroconversion, HI titers ≥40, and geometric mean ratio (GMR) were applied for subjects 18–60 years or ≥61 years. As no specific CHMP criteria are established for the pediatric population, criteria for adult subjects (18–60 years) were applied instead.
Statistical Analyses
A total of 600 subjects were to be enrolled into the 4 age cohorts with about 150 subjects in each, approximately 50 subjects for each age and vaccine group. There were 200 subjects planned in total for each vaccine group. Assuming a 15% dropout rate, 170 eligible subjects were considered sufficient to provide a descriptive summary of the safety and immunogenicity for each vaccine group, based on previous monovalent pandemic vaccines [6, 10]. No formal statistical hypotheses were tested for the immunogenicity and safety data. The percentages of subjects achieving seroconversion and with HI titers ≥40, with their 2-sided 95% Clopper-Pearson confidence intervals (CIs), were summarized for each vaccine group and time point, overall and within each age cohort. Log10-transformed geometric mean titers (GMTs) and GMRs and their 95% CIs were determined using analysis of covariance with factors for vaccine group, age group, and center and a covariate for the effect defined by the log-transformed prevaccination antibody titer. Immunogenicity analyses were performed on the full analysis set (FAS), which included subjects who received at least 1 dose of study vaccination and provided evaluable serum samples at both prevaccination and at least 1 postvaccination time points. The safety set included all enrolled subjects who received a study vaccination and had either postvaccination adverse event or reactogenicity records.
RESULTS
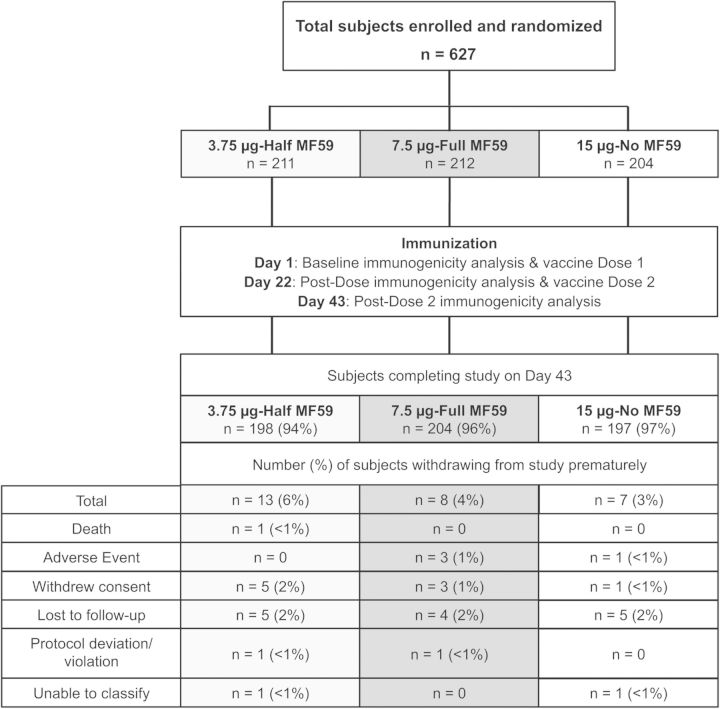
A total of 627 healthy subjects 3 to ≥65 years were enrolled from 20 May 2013 through 12 September 2013. Overall, 624 subjects received at least 1 dose of assigned H3N2c vaccine, and 94% (198 of 211 subjects in half-dose group), 96% (204 of 212 subjects in full-dose group), and 97% (197 of 204 subjects in the nonadjuvanted group) completed the study to Day 43, respectively (Figure 1). There were no imbalances in the demographic and baseline characteristics across the 3 vaccine groups except for the proportion of enrolled females, which was lower in the full-dose group (48% vs 58% in the half-dose group and 55% in the nonadjuvanted group) (Table 1).
Figure 1.
Study design and subject disposition.
Table 1.
Study Population Demographics and Baseline Characteristics
| 3.75 µg-Half MF59 (N = 211) | 7.5 µg-Full MF59 (N = 212) | 15 µg-No MF59 (N = 204) | Total (N = 627) | |
|---|---|---|---|---|
| Age, mean ± SD (years) | 32.6 ± 26.8 | 33.2 ± 27.4 | 33.9 ± 28.5 | 33.2 ± 27.5 |
| Gender, n (%) | ||||
| Female | 122 (58) | 102 (48) | 112 (55) | 336 (54) |
| Male | 89 (42) | 110 (52) | 92 (45) | 291 (46) |
| Weight, mean ± SD (kg) | 58.3 ± 25.5 | 55.7 ± 25.7 | 57.6 ± 27.0 | 57.2 ± 26.0 |
| Height, mean ± SD (cm) | 153.9 ± 22.5 | 152.6 ± 23.6 | 153.2 ± 23.7 | 153.2 ± 23.2 |
| BMI, mean ± SD (kg/m²) | 23.0 ± 5.8 | 22.2 ± 5.8 | 22.8 ± 5.9 | 22.7 ± 5.9 |
| Had previous influenza vaccination, n (%) | 136 (64) | 133 (64) | 120 (59) | 389 (62) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Safety
Overall, solicited AEs were reported at a lower frequency in subjects receiving the nonadjuvanted formulation. Among the MF59-adjuvanted vaccines, the highest local reactogenicity rates were observed in the full-dose group (Table 2 and Supplementary Table 1). After any vaccination, the most frequently reported local reaction in subjects <6 years was tenderness (55%), and among patients ≥6 years it was injection site pain (53%); the most frequently reported systemic reactions were malaise and irritability (21%) among subjects <6 years and fatigue (28%) among patients ≥6 years. In addition, the frequency of subjects reporting solicited AEs was higher following the first than the second vaccination (62% vs 48%) (Table 2).
Table 2.
Overview of Solicited Adverse Events and Other Indicators of Reactogenicity in the Overall Population During a 7-day Period Following the First Vaccination and Second Vaccination
| After First Vaccination |
After Second Vaccination |
|||||||
|---|---|---|---|---|---|---|---|---|
| 3.75 µg-Half MF59 (N = 204) | 7.5 µg-Full MF59 (N = 207) | 15 µg-No MF59 (N = 200) | Total (N = 611) | 3.75 µg-Half MF59 (N = 195) | 7.5 µg-Full MF59 (N = 196) | 15 µg-No MF59 (N = 197) | Total (N = 588) | |
| Any reaction, n (%) | 124 (61) | 153 (74) | 103 (52) | 380 (62) | 95 (49) | 98 (50) | 90 (46) | 283 (48) |
| Local reactions, n (%) | 102 (50) | 129 (62) | 78 (39) | 309 (51) | 79 (41) | 88 (45) | 65 (33) | 232 (39) |
| Systemic reactions, n (%) | 80 (39) | 93 (45) | 65 (33) | 238 (39) | 56 (29) | 56 (29) | 51 (26) | 163 (28) |
| Other reactions, n (%) | 15 (7) | 21 (10) | 7 (4) | 43 (7) | 6 (3) | 8 (4) | 9 (5) | 23 (4) |
Spontaneously reported adverse reactions were reported in a total of 23% of subjects, and were higher among those who received the nonadjuvanted vaccine (28% vs 21% in the MF59-adjuvanted formulations). In 7% of cases, unsolicited AEs were considered to be possibly or probably related to study vaccine, and were reported with a similar frequency in all vaccine groups. SAEs including 1 death were reported in a total of 2 cases (<1%: hepatic enzymes increased and breast cancer), none of them possibly or probably related to vaccinations. Finally, 4 AEs causing premature withdrawal accounted for 1% of cases overall; 3 were reported in the MF59-adjuvanted groups (1%), and 1 (<1%) in the nonadjuvanted group.
Immunogenicity
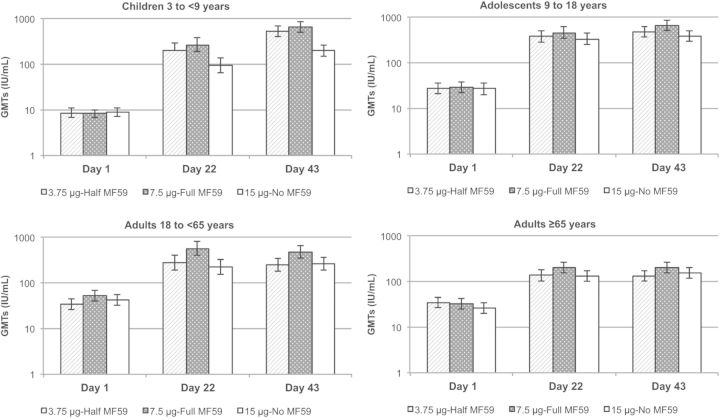
In the overall population aged ≥3 years, the proportions of subjects with baseline (Day 1) HI titers ≥40 against the H3N2v homologous virus strain were 41%, 46% and 44% in the half-dose, full-dose, and nonadjuvanted vaccine groups, respectively (Table 3). Three weeks after the first vaccination (Day 22), almost all subjects achieved HI titers ≥40 regardless of the H3N2c vaccine received (average 95% across groups); 3 weeks after the second vaccine dose (Day 43) there was a slight increase in percentages of subjects with HI titers ≥40 (average 99% across groups). Seroconversion rates followed a similar trend, with percentages ranging from 71% to 89% across H3N2c vaccine groups at Day 22 after the first vaccine dose. There was a slight increase in seroconversion at Day 43 for MF59-adjuvanted vaccines (84% and 93% in the half-dose and full-dose groups, respectively), while the percentage in the nonadjuvanted group increased from 71% to 79%. There were substantial increases in HI GMTs following vaccination that were maximal after the first dose (GMRs: 9.6, 14.0, and 7.8 for the half-dose, full-dose, and nonadjuvanted groups, respectively), with a slight increase after the second vaccination (GMRs: 12.0, 18.0, and 10.0 for the half-dose, full-dose, and nonadjuvanted groups, respectively). At both postvaccination time points, the increase in GMTs was lower for subjects receiving the nonadjuvanted vaccine compared with those receiving MF59-adjuvanted vaccines (Figure 2). All H3N2c vaccines met the CBER or CHMP licensure criteria after either 1 or 2 doses (Table 3).
Table 3.
Percentage of Subjects (95% CI) With HI Titers ≥40 at Day 1, Day 22 and Day 43, Percentage of Subjects Achieving Seroconversion at Day 22 and 43, and GMRs
| 3.75 µg-Half MF59 | 7.5 µg-Full MF59 | 15 µg-No MF59 | |
|---|---|---|---|
| Subjects with HI titers ≥40, % (95% CI) | N = 194–209 | N = 203–211 | N = 199–203 |
| Day 1 | 41 (34–48) | 46 (39–53) | 44 (37–51) |
| Day 22 | 97 (93–99)a,b | 100 (97–100)a,b | 90 (85–94)a,b |
| Day 43 | 98 (96–100)a,b | 100 (98–100)a,b | 97 (94–99)a,b |
| Seroconversion, % (95% CI) | N = 194 | N = 203 | N = 199 |
| Day 22 | 79 (72–84)a,b | 89 (84–93)a,b | 71 (64–77)a,b |
| Day 43 | 84 (78–88)a,b | 93 (88–96)a,b | 79 (73–85)a,b |
| GMRs, (95% CI) | N = 194–200 | N = 203–205 | N = 199–201 |
| Day 22/Day 1 | 9.6 (8.0–12)b | 14 (12–17)b | 7.8 (6.4–9.4)b |
| Day 43/Day 1 | 12 (10–15)b | 18 (15–22)b | 10 (8.3–12)b |
Seroconversion was defined as HI titers ≥40 for subjects with a baseline HI titer <10 or a minimum 4-fold increase in HI titer for subjects with a baseline HI titer ≥10.
Abbreviations: GMR, geometric mean ratio; HI, hemagglutination inhibition.
a Outcomes meet the Center for Biologics Evaluation and Research (CBER) licensure criteria (for both subjects 18 to 65 years and >65 years) [9].
b Outcomes meet the European Committee for Proprietary Medicinal Products (CHMP) licensure criteria (for both subjects 18 to 60 years and >60 years) [8].
Figure 2.
Geometric mean antibody titers (GMTs) measured by hemagglutination inhibition assay against the A/H3N2v homologous strain at baseline and 3 weeks after the first (Day 22) and second (Day 43) vaccine doses. GMTs and their 95% confidence intervals were determined using analysis of covariance with factors for vaccine group, age group, and center and a covariate for the effect defined by the log-transformed prevaccination antibody titer.
We further analyzed the effect of prevaccination HI titers on immune response (Table 4). We observed that subjects with baseline HI titers ≥10, <40, or ≥40 had a strong immune response following 1 and 2 doses regardless of the administered vaccine, and all met the CBER or CHMP licensure criteria. Similarly, the percentage of subjects with prevaccination HI titers <10 who achieved HI titers ≥40 after 1 dose was high among those receiving adjuvanted vaccines (94% [95% CI, 81–99] for the half dose, and 97% [95% CI, 87–100] for the full dose, respectively). However, the proportion in the nonadjuvanted group was 73% (95% CI, 58–85), which does not meet CBER licensure criteria, and a second vaccination was needed.
Table 4.
Percentage of Subjects (95% CI) With HI Titers ≥40 at Day 1, Day 22 and Day 43, Percentage of Subjects Achieving Seroconversion at Day 22 and 43, and GMRs According to Baseline HI Titers
| Baseline HI | 3.75 µg-Half MF59 |
7.5 µg-Full MF59 |
15 µg-No MF59 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1:40 | ≥1:40 | <1:10 | ≥1:10 | <1:40 | ≥1:40 | <1:10 | ≥1:10 | <1:40 | ≥1:40 | <1:10 | ≥1:10 | |
| Percentages (95% CI) of Subjects With HI Titers ≥40 | ||||||||||||
| N = 123 | N = 86 | N = 43 | N = 166 | N = 114 | N = 97 | N = 40 | N = 171 | N = 114 | N = 89 | N = 45 | N = 158 | |
| Day 1 | 0 (0–3) | 100 (96–100) | 0 (0–8) | 52 (44–60) | 0 (0–3) | 100 (96–100) | 0 (0–9) | 57 (49–64) | 0 (0–3) | 100 (96–100) | 0 (0–8) | 56 (48–64) |
| Day 22 | 94 (88–97)a,b | 100 (96–100)a,b | 94 (81–99)a,b | 97 (93–99)a,b | 99 (95–100)a,b | 100 (96–100)a,b | 97 (87–100)a,b | 100 (98–100)a,b | 82 (74–89)a,b | 100 (96–100)a,b | 73 (58–85)b | 95 (90–98)a,b |
| Day 43 | 97 (92–99)a,b | 100 (96–100)a,b | 97 (85–100)a,b | 99 (96–100)a,b | 100 (97–100)a,b | 100 (96–100)a,b | 100 (91–100)a,b | 100 (98–100)a,b | 96 (90–99)a,b | 100 (96–100)a,b | 95 (85–99)a,b | 98 (94–100)a,b |
| Percentages (95% CI) of Subjects with Seroconversion | ||||||||||||
| N = 114 | N = 86 | N = 36 | N = 164 | N = 111 | N = 94 | N = 39 | N = 166 | N = 113 | N = 88 | N = 45 | N = 156 | |
| Day 22 | 89 (81–94)a,b | 65 (54–75)a,b | 94 (81–99)a,b | 75 (68–81)a,b | 96 (91–99)a,b | 81 (71–88)a,b | 97 (87–100)a,b | 87 (81–92)a,b | 78 (69–85)a,b | 63 (52–73)a,b | 73 (58–85)a,b | 71 (63–78)a,b |
| Day 43 | 93 (86–97)a,b | 71 (60–81)a,b | 97 (85–100)a,b | 81 (74–86)a,b | 98 (94–100)a,b | 86 (77–92)a,b | 100 (91–100)a,b | 91 (85–95)a,b | 89 (82–94)a,b | 67 (56–76)a,b | 95 (85–99)a,b | 75 (67–81)a,b |
| Geometric Mean ratios (95% CI) | ||||||||||||
| N = 123 | N = 86 | N = 43 | N = 166 | N = 114 | N = 97 | N = 40 | N = 171 | N = 114 | N = 89 | N = 45 | N = 158 | |
| Day 22/Day 1 | 16 (12–20)b | 5.0 (3.9–6.4)b | 33 (21–52)b | 7.3 (6.1–8.9)b | 25 (20–33)b | 7.1 (5.5–9.0)b | 43 (27–70)b | 11 (9.0–13)b | 11 (8.7–14)b | 4.9 (3.9–6.3)b | 15 (10–24)b | 6.3 (5.2–7.7)b |
| Day 43/Day 1 | 22 (17–29)b | 5.4 (4.3–6.9)b | 68 (46–99)b | 8.6 (7.1–10)b | 37 (29–48)b | 7.9 (6.3–10)b | 103 (70–152)b | 12 (10–14)b | 15 (12–20)b | 5.8 (4.6–7.3)b | 32 (22–45)b | 7.3 (6.1–8.9)b |
Seroconversion was defined as HI titers ≥40 for subjects with a baseline HI titer <10 or a minimum 4-fold increase in HI titer for subjects with a baseline HI titer ≥10.
Abbreviations: GMR, geometric mean ratio; HI, hemagglutination inhibition.
a Outcomes meet the Center for Biologics Evaluation and Research (CBER) licensure criteria (for both subjects 18 to 65 years and >65 years) [9].
b Outcomes meet the European Committee for Proprietary Medicinal Products (CHMP) licensure criteria (for both subjects 18 to 60 years and >60 years) [8].
Analyses of immunogenicity outcomes stratified by age cohorts showed similar patterns to the overall population following vaccination, but with striking differences between age cohorts prevaccination (Table 5; Figure 2). The average percentage of subjects with baseline HI titers ≥40 was much lower among children 3 to <9 years (7%–14% across vaccine groups) than in adolescents 9 to <18 years (44%–59%), adults 18 to <65 years (58%–67%), or the elderly ≥65 years (46%–55%). However, the response to vaccination was much stronger in children than in the other age cohorts: the percentage with HI titers ≥40 was 96%–100% after 1 and 2 doses of MF59-adjuvanted vaccines. Following 1 dose of nonadjuvanted vaccine, the percentage of children with HI titers ≥40 was 78% (95% CI, 63–88), which does not meet CBER licensure criteria, while 2 doses induced HI titers ≥40 at a frequency comparable to MF59-adjuvanted vaccines (96%; 95% CI, 86–99).
Table 5.
Percentage of Subjects (95% CI) With HI Titers ≥40 at Day 1, Day 22 and Day 43, and Percentage of Subjects Achieving Seroconversion at Day 22 and 43, and GMRs According to Age Group (Years)
| 3.75 µg-Half MF59 |
7.5 µg-Full MF59 |
15 µg-No MF59 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 to 9 | 9 to 18 | 18 to <65 | ≥65 | 3 to 9 | 9 to 18 | 18 to <65 | ≥65 | 3 to 9 | 9 to 18 | 18 to <65 | ≥65 | |
| Percentages (95% CI) of Subjects With HI Titers ≥40 | ||||||||||||
| Day 1 | 8 (2–19) | 44 (31–59) | 58 (43–71) | 55 (40–69) | 7 (2–18) | 59 (44–72) | 67 (53–79) | 52 (37–66) | 14 (6–27) | 49 (35–63) | 67 (52–80) | 46 (32–61) |
| Day 22 | 96 (85–99)a,b | 100 (93–100)a,b | 98 (90–100)a,b | 92 (81–98)a,b | 98 (90–100)a,b | 100 (93–100)a,b | 100 (93–100)a,b | 100 (93–100)a,b | 78 (63–88)b | 98 (90–100)a,b | 88 (75–95)a,b | 96 (87–100)a,b |
| Day 43 | 100 (92–100)a,b | 100 (93–100)a,b | 98 (89–100)a,b | 96 (86–100)a,b | 100 (93–100)a,b | 100 (93–100)a,b | 100 (93–100)a,b | 100 (93–100)a,b | 96 (86–99)a,b | 100 (93–100)a,b | 98 (89–100)a,b | 96 (86–100)a,b |
| Percentages (95% CI) of Subjects with Seroconversion | ||||||||||||
| Day 22 | 96 (85–99)a,b | 89 (77–96)a,b | 75 (60–86)a,b | 56 (41–70)a,b | 98 (90–100)a,b | 98 (89–100)a,b | 89 (77–96)a,b | 71 (57–83)a,b | 71 (57–83)a,b | 85 (72–93)a,b | 67 (52–80)a,b | 61 (46–74)a,b |
| Day 43 | 100 (92–100)a,b | 94 (84–99)a,b | 84 (71–93)a,b | 57 (42–71)a,b | 100 (93–100)a,b | 98 (89–100)a,b | 92 (81–98)a,b | 80 (66–90)a,b | 92 (80–98)a,b | 91 (79–97)a,b | 71 (56–83)a,b | 64 (49–77)a,b |
| Geometric Mean ratios (95% CI) | ||||||||||||
| Day 22/Day 1 | 22 (15–32)b | 14 (9.8–19)b | 7.8 (5.6–11)b | 3.8 (2.7–5.2)b | 33 (23–47)b | 15 (10–22)b | 11 (8.2–16)b | 6.6 (4.8–9.3)b | 11 (7.5–16)b | 13 (8.9–18)b | 5.4 (3.8–7.5)b | 5.1 (3.7–7.1)b |
| Day 43/Day 1 | 59 (43–81)b | 18 (13–25)b | 7.3 (5.4–9.7)b | 3.8 (2.8–5.2)b | 82 (62–109)b | 21 (15–30)b | 9.6 (7.2–13)b | 6.6 (4.8–8.9)b | 23 (17–31)b | 14 (10–20)b | 6.3 (4.6–8.4)b | 5.9 (4.4–8.1)b |
Seroconversion was defined as HI titers ≥40 for subjects with a baseline HI titer <10 or a minimum 4-fold increase in HI titer for subjects with a baseline HI titer ≥10.
Abbreviations: GMR, geometric mean ratio; HI, hemagglutination inhibition.
a Outcomes meet the Center for Biologics Evaluation and Research (CBER) licensure criteria (for both subjects 18 to 65 years and >65 years) [9].
b Outcomes meet the European Committee for Proprietary Medicinal Products (CHMP) licensure criteria (for both subjects 18 to 60 years and >60 years) [8].
Immune responses in the adolescent group (9 to <18 years) were the highest of all the age cohorts, with brisk increases in GMTs (Figure 2) and a high rate of HI titers ≥40 and seroconversion after the first dose regardless of the H3N2c vaccine received. These responses did not improve after the second dose (Table 5). In adult and elderly subjects (18 to <65 years and ≥65 years), the immune response was slightly poorer than in children and adolescents, especially in elderly subjects (Figure 2), who showed the lowest seroconversion rates of all age cohorts (Table 5). In both older cohorts, the rates of HI titers ≥40 and seroconversion were comparable between MF59-adjuvanted vaccines and slightly lower for the nonadjuvanted vaccine. There was no benefit of a second dose in older cohorts regardless of the H3N2c vaccine received (Table 5). Except for the single dose of nonadjuvanted vaccine in children, CBER and CHMP licensure criteria were met for all age cohorts in each of the 3 H3N2c vaccine groups after 1 and 2 doses (Table 5).
Subgroups of age cohorts with prevaccination HI titers <40 were calculated to assess whether adjuvanted A/H3N2v vaccines have any benefit for inducing specific immunity in naive populations of different ages: these confirmed that the adjuvanted vaccines achieved CBER criteria after a single dose in all ages, while for the nonadjuvanted vaccine, children 3 to <9 years and adults 18 to <65 years only reached that response criteria after the second dose (Supplementary Table 2). The second dose conferred more benefit to the nonadjuvanted group.
DISCUSSION
This is the only study of adjuvanted H3N2v vaccines in the general US population, and the first data on H3N2v vaccines in pediatric subjects. We evaluated the safety and immunogenicity of 3 MF59-adjuvanted or nonadjuvanted cell-culture-derived H3N2c monovalent subunit vaccines in healthy children, adolescent, adult, and elderly subjects. The percentages of all subjects with HI titers ≥40 ranged between 90% and 100% after a single dose across all 3 H3N2c vaccine groups, which slightly increased to 97%–100% after 2 doses. This pattern was also observed with seroconversion rates, which ranged between 71% and 89% after 1 dose, and increased up to 79%–93% after a second dose across the 3 vaccine groups.
Overall, the immune responses induced by the H3N2c vaccines were similar to those described for licensed pandemic 2009 (A/H1N1) swine influenza vaccines across all age cohorts, including children and the elderly [11–13]. In our study, 1 dose of MF59-adjuvanted H3N2c vaccine (regardless of the HA content) or nonadjuvanted H3N2c vaccine generated similar responses at all ages except in children 3 to <9 years, who needed 2 doses of the latter in order to achieve comparable immunogenicity. Also in parallel with pandemic A/H1N1 vaccines [11–15], MF59-adjuvanted H3N2c vaccines were more immunogenic than nonadjuvanted H3N2c vaccine in all age cohorts. Notably, among MF59-adjuvanted H3N2c vaccines, the full dose induced slightly higher rates of seroconversion and HI titers ≥40 than the low dose. The fact that MF59-adjuvanted H3N2c vaccines are more immunogenic relative to nonadjuvanted H3N2c vaccine is of further clinical and epidemiological interest given MF59 adjuvant enhances functional antibody responses, including a higher virus-neutralizing capacity against pandemic strains [16].
Of note, the overall proportion of subjects who had HI titers ≥40 against H3N2v virus at baseline was between 41%–46%, which is in accordance with rates reported in previous seroepidemiological studies conducted in Norway, the United States, and Canada [17–19]. This phenomenon has been attributed to the presence of serum cross-reactive antibodies to H3N2v induced by prior exposure to related nonvariant human seasonal H3N2 strains circulating from the early 1990s [20, 21]. Moreover, and also in agreement with previous reports [17–19], we found that the seroprevalence of cross-reactive antibodies was age-dependent, with the rates of seropositivity much lower among children 3 to <9 years (7% to 14% across vaccine groups), and a progressive increase in adolescents, adults, and the elderly (44% to 67% across vaccine groups and age cohorts). This could be explained by the lack of exposure to these circulating nonvariant H3N2 viruses of children born after the late 1990s. Coupled with available epidemiologic data, this raises the possibility that baseline immunity may have a protective role against infection with H3N2v virus strain in older age cohorts [22]. However, further research is warranted in order to see whether natural exposure is correlated with actual protection against H3N2v, as has been previously described for 2009 pandemic influenza A H1N1 infection [23, 24]. Moreover, the observed early and robust rise in HI antibodies induced by a single dose of H3N2v vaccines could be due to the recruitment of preexisting cross-reactive memory B cells [25]. Actually, in our study, only subjects who had baseline HI titers <10, and who received nonadjuvanted H3N2c vaccine, needed a second dose to achieve a titer level above 40. This coincides with the observation that subjects 3 to <9 years need a second dose of nonadjuvanted H3N2c vaccine, and suggests that this is a consistent phenomenon in naive subjects.
The safety results of the present study are also in agreement with previous clinical trials, large observational surveillance studies, and meta-analyses conducted with seasonal and pandemic A (H1N1) MF59-adjuvanted vaccines [11–13, 26–28]. As also previously reported elsewhere, the oil-in-water adjuvanted vaccines showed the highest rates of solicited local and systemic adverse events, predominately of mild or moderate severity, but without an apparent increase of unsolicited AEs or SAEs [11, 12].
From a regulatory perspective, a single dose of MF59-adjuvanted H3N2c vaccine (either full or half dose) was sufficient to meet all US (CBER) and European (CHMP) licensure criteria for pandemic influenza vaccines in all age populations. The nonadjuvanted H3N2c was comparable, but required 2 doses to achieve CBER criteria in children <9 years or in subjects with baseline HI titers <10.
This dose-ranging Phase 1 study has limitations that must be acknowledged. First, it was descriptive and not designed to compare safety and immunogenicity of different H3N2c vaccines, and thus enrolled a limited number of subjects in each vaccine and age cohort. Second, the interim data presented prevent an accurate assessment of delayed or infrequent adverse events after immunization, which may be assessed through long-term follow-up.
In summary, this study demonstrated that MF59-adjuvanted H3N2c vaccines with 3.75 and 7.5 µg H3N2v HA content induced effective immune responses meeting CBER criteria with a single dose at all ages. Nonadjuvanted H3N2c vaccine with 15 µg H3N2v HA content generated similar immunogenicity after a single dose in all ages except children, who may require a second dose. Together with an acceptable safety and tolerability profile, these data support further clinical development of MF59-adjuvanted H3N2c vaccines for target pediatric populations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We acknowledge David Hering, David Lee, Bikash Verma, Robin Wallace, Linda Carter, and Jenny Beygo for operational support of the study conduct within Novartis Vaccines. We also acknowledge Dr Monica Gratacos, MD, PhD, and Dr Amanda Prowse, PhD (CHC Europe) and Dr Debaditya Das, PhD (Novartis Vaccines) for providing support in the manuscript preparation, revision, and editing.
Financial support. This work was supported by Novartis Vaccines and Diagnostics, Inc, and was funded in whole or in part with Federal funds from Health and Human Services (HHS) Office of the Assistant Secretary for Preparedness and Response (ASPR), Biomedical Advanced Research and Development Authority (BARDA), under contract no. HHSO100201200014I. Please note that the content of this publication does not necessarily reflect the views or policies of the HHS, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Potential conflict of interest. C. J. and T. P. received financial support from Novartis to conduct the study under standard contracts. M. H., P. J. and N. K. T. are employees or consultants of Novartis Vaccines and Diagnostics, Inc, which manufactures and sells influenza vaccines. M. H. and N. K. T. are Novartis shareholders.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention (CDC). Influenza A (H3N2) variant virus. http://www.cdc.gov/flu/swineflu/h3n2v-cases.htm Accessed March 2014.
- 2.Centers for Disease Control and Prevention (CDC). Case count: detected U.S. human infections with H3N2v by state since August 2011. http://www.cdc.gov/flu/swineflu/h3n2v-case-count.htm Accessed March 2014.
- 3.Jhung MA, Epperson S, Biggerstaff M, et al. Outbreak of variant influenza A(H3N2) virus in the United States. Clin Infect Dis 2013; 57:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray GC, Cao WC. Editorial commentary: variant influenza A(H3N2) virus: looking through a glass, darkly. Clin Infect Dis 2013; 57:1713–4. [DOI] [PubMed] [Google Scholar]
- 5.Pearce MB, Jayaraman A, Pappas C, et al. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc Natl Acad Sci USA 2012; 109:3944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keitel W, Groth N, Lattanzi M, et al. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine 2010; 28:840–8. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect Dis 2004; 4:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). 1997.
- 9.Center for Biologics Evaluation and Research (CBER). Food and Drug Administration (FDA). Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. 2009.
- 10.Clark TW, Pareek M, Hoschler K, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009; 361:2424–35. [DOI] [PubMed] [Google Scholar]
- 11.Manzoli L, Ioannidis JP, Flacco ME, De Vito C, Villari P. Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses. Hum Vaccin Immunother 2012; 8:851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzoli L, De Vito C, Salanti G, D'Addario M, Villari P, Ioannidis JP. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLOS One 2011; 6:e24384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin JK, Khandaker G, Rashid H, Heron L, Ridda I, Booy R. Immunogenicity and safety of pandemic influenza A (H1N1) 2009 vaccine: systematic review and meta-analysis. Influenza Other Respir Viruses 2011; 5:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatz C, von Sonnenburg F, Casula D, Lattanzi M, Leroux-Roels G. A randomized clinical trial to identify the optimal antigen and MF59((R)) adjuvant dose of a monovalent A/H1N1 pandemic influenza vaccine in healthy adult and elderly subjects. Vaccine 2012; 30:3470–7. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Aragon J, Grande Tejada AM, Marquez-Pelaez S, Molina Linde JM, Yang R. [Assessment of the MF59-adjuvanted pandemic influenza A/H1N1 vaccine. Systematic review of literature]. An Pediatr (Barc) 2013; 79:208–17. [DOI] [PubMed] [Google Scholar]
- 16.Khurana S, Verma N, Yewdell JW, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011; 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waalen K, Kilander A, Dudman SG, Ramos-Ocao R, Hungnes O. Age-dependent prevalence of antibodies cross-reactive to the influenza A(H3N2) variant virus in sera collected in Norway in 2011. Euro Surveill 2012; 17:1–5. [PubMed] [Google Scholar]
- 18.Skowronski DM, Janjua NZ, De Serres G, et al. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v). J Infect Dis 2012; 206:1852–61. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Antibodies cross-reactive to influenza A (H3N2) variant virus and impact of 2010–11 seasonal influenza vaccine on cross-reactive antibodies—United States. MMWR Morb Mortal Wkly Rep 2012; 61:237–41. [PubMed] [Google Scholar]
- 20.Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–60. [DOI] [PubMed] [Google Scholar]
- 21.Webby RJ, Swenson SL, Krauss SL, Gerrish PJ, Goyal SM, Webster RG. Evolution of swine H3N2 influenza viruses in the United States. J Virol 2000; 74:8243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambhir M, Swerdlow DL, Finelli L, et al. Multiple contributory factors to the age distribution of disease cases: a modeling study in the context of influenza A(H3N2v). Clin Infect Dis 2013; 57(suppl 1):S23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 2010; 375:1100–8. [DOI] [PubMed] [Google Scholar]
- 24.Hardelid P, Andrews NJ, Hoschler K, et al. Assessment of baseline age-specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol Assess 2010; 14:115–92. [DOI] [PubMed] [Google Scholar]
- 25.Faenzi E, Zedda L, Bardelli M, et al. One dose of an MF59-adjuvanted pandemic A/H1N1 vaccine recruits pre-existing immune memory and induces the rapid rise of neutralizing antibodies. Vaccine 2012; 30:4086–94. [DOI] [PubMed] [Google Scholar]
- 26.Liang XF, Li L, Liu DW, et al. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med 2011; 364:638–47. [DOI] [PubMed] [Google Scholar]
- 27.Vellozzi C, Broder KR, Haber P, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009–January 31, 2010. Vaccine 2010; 28:7248–55. [DOI] [PubMed] [Google Scholar]
- 28.O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines 2007; 6:699–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.