Baker-Nigh et al. reveal accumulation of amyloid-β in basal forebrain cholinergic neurons throughout life, and the formation of large intraneuronal oligomers specifically in aged and Alzheimer brains. Accumulation and age-related aggregation of amyloid-β may contribute to the selective vulnerability of cholinergic neurons to degeneration in Alzheimer’s disease.
Keywords: Alzheimer pathology, amyloid-β, amyloid oligomer, basal forebrain cholinergic neurons, intracellular
Baker-Nigh et al. reveal accumulation of amyloid-β in basal forebrain cholinergic neurons throughout life, and the formation of large intraneuronal oligomers specifically in aged and Alzheimer brains. Accumulation and age-related aggregation of amyloid-β may contribute to the selective vulnerability of cholinergic neurons to degeneration in Alzheimer’s disease.
Abstract
The mechanisms that contribute to selective vulnerability of the magnocellular basal forebrain cholinergic neurons in neurodegenerative diseases, such as Alzheimer’s disease, are not fully understood. Because age is the primary risk factor for Alzheimer’s disease, mechanisms of interest must include age-related alterations in protein expression, cell type-specific markers and pathology. The present study explored the extent and characteristics of intraneuronal amyloid-β accumulation, particularly of the fibrillogenic 42-amino acid isoform, within basal forebrain cholinergic neurons in normal young, normal aged and Alzheimer’s disease brains as a potential contributor to the selective vulnerability of these neurons using immunohistochemistry and western blot analysis. Amyloid-β1–42 immunoreactivity was observed in the entire cholinergic neuronal population regardless of age or Alzheimer’s disease diagnosis. The magnitude of this accumulation as revealed by optical density measures was significantly greater than that in cortical pyramidal neurons, and magnocellular neurons in the globus pallidus did not demonstrate a similar extent of amyloid immunoreactivity. Immunoblot analysis with a panel of amyloid-β antibodies confirmed accumulation of high concentration of amyloid-β in basal forebrain early in adult life. There was no age- or Alzheimer-related alteration in total amyloid-β content within this region. In contrast, an increase in the large molecular weight soluble oligomer species was observed with a highly oligomer-specific antibody in aged and Alzheimer brains when compared with the young. Similarly, intermediate molecular weight oligomeric species displayed an increase in aged and Alzheimer brains when compared with the young using two amyloid-β42 antibodies. Compared to cortical homogenates, small molecular weight oligomeric species were lower and intermediate species were enriched in basal forebrain in ageing and Alzheimer’s disease. Regional and age-related differences in accumulation were not the result of alterations in expression of the amyloid precursor protein, as confirmed by both immunostaining and western blot. Our results demonstrate that intraneuronal amyloid-β accumulation is a relatively selective trait of basal forebrain cholinergic neurons early in adult life, and increases in the prevalence of intermediate and large oligomeric assembly states are associated with both ageing and Alzheimer’s disease. Selective intraneuronal amyloid-β accumulation in adult life and oligomerization during the ageing process are potential contributors to the degeneration of basal forebrain cholinergic neurons in Alzheimer’s disease.
Introduction
Neurodegenerative disorders are characterized by cell loss in pathologically vulnerable neuronal populations. The basal forebrain cholinergic neurons (BFCNs) are selectively vulnerable to pathology, such as axonal abnormalities, phosphorylated tau accumulation and neurofibrillary tangle formation, early in the course of ageing and in Alzheimer’s disease (Geula et al., 2008). By end-stage disease up to 95% of BFCNs degenerate (Geula and Mesulam, 1999; Mesulam et al., 2004). Significantly, the BFCNs are vulnerable to degeneration in a number of other neurodegenerative conditions, particularly those affecting the elderly, such as Parkinson’s disease and Lewy body dementia (McKinney, 2005). Because age is the primary risk factor in these disorders, we have hypothesized that age-related changes in human BFCNs are temporally related, and thus are likely to contribute to the vulnerability of these neurons.
The acute causes of cell death in neurodegenerative diseases are not fully understood; however, alterations in intracellular calcium (Ca2+) homeostasis have been implicated in neuronal dysfunction, excitotoxic cascades, and production of toxic proteins (Harkany et al., 2000; Green et al., 2007). We have described a decrease in expression of the Ca2+-binding protein calbindin-D28K in most BFCNs, correlated with advancing age (Geula et al., 2003). Calbindin is involved in rapid buffering of excess intraneuronal Ca2+, therefore calbindin loss is likely to reduce the total Ca2+-buffering capacity of affected BFCNs. We have demonstrated that the subpopulation of BFCNs that lose calbindin are susceptible to increased phosphorylation of tau, its accumulation in pre-tangles and neurofibrillary tangles, and degeneration (Riascos et al., 2011).
In addition to neurofibrillary tangles, a major hallmark of Alzheimer pathology is the plaque, composed of aggregated amyloid-β peptide. Compact plaques are composed of amyloid-β in a highly aggregated fibrillar state and exert local toxic effects in human and non-human primates (Shah et al., 2010). Mice overexpressing mutated human amyloid precursor protein (APP) from which amyloid-β is derived, exhibit synaptic deficits even in the absence of marked plaque formation, implicating soluble amyloid-β oligomers in Alzheimer pathogenesis (Mucke et al., 2000; Tomiyama et al., 2010). Amyloid-β is generated in neurons throughout the brain (Masters et al., 1985; Goedert, 1987), and can also be endocytosed (Knauer et al., 1992). Gouras et al. (2000) observed region-specific accumulation of amyloid-β in pyramidal neurons of the hippocampus, layer II of entorhinal cortex, and, notably, in BFCNs. Although staining intensity and prevalence were not quantified, the authors noted that intraneuronal amyloid-β42 appeared to increase with age, and decreased with severity of dementia and plaque deposition. In the hippocampus of humans and APP transgenic mice, this accumulation was associated with aberrant synaptic structure (Takahashi et al., 2002).
Amyloidogenic cleavage of human APP results primarily in the production of two amyloid-β isoforms, amyloid-β40 and amyloid-β42, with the latter markedly more prone to accumulation and aggregation. Non-human primates share a homologous and nearly (99%) identical APP sequence with humans and express both isoforms of amyloid-β, as well as plaque formation in the process of ageing, although in contrast to humans, amyloid-β40 is the predominant isoform in other primate species (Selkoe et al., 1987; Podlisny et al., 1991; Gearing et al., 1996; Geula et al., 2002). We have established that BFCNs in the lower non-human primate, the common marmoset, display selective age-related calbindin loss similar to that observed in humans (Wu et al., 2003). Our preliminary immunohistochemical observations revealed that BFCNs in the rhesus monkey also exhibit intracellular amyloid-β accumulation, particularly of the amyloid-β40 isoform, and that this accumulation increases with age (unpublished observations). Because this age-related accumulation occurs in the selectively vulnerable BFCNs, it may serve as a model for examination of the role of intracellular amyloid in neuronal vulnerability. Furthermore, several in vitro and in vivo lines of evidence suggest a synergistic relationship between Ca2+ dysregulation and amyloid-β. Oligomers, in particular, modulate ion channel activity/Ca2+ permeable receptors resulting in excess intracellular Ca2+, which in turn seems to further drive amyloid-β production (De Felice et al., 2007; Green et al., 2007; Green and LaFerla, 2008; Renner et al., 2010). The toxicity of amyloid-β is also mediated, at least in part, by increased intracellular calcium, and is prevented by expression of calbindin (Mattson et al., 1991; Guo et al., 1998; Mattson, 2007).
The goal of the present experiments was to investigate whether in conjunction with loss of calbindin, and similar to accumulation of amyloid-β in the non-human primate, the human BFCNs display accumulation of amyloid-β in the course of ageing, and in Alzheimer’s disease. We demonstrate a selective accumulation of amyloid-β42, but not amyloid-β40, in human BFCNs. This accumulation begins early, evident even in the youngest brain examined (age 20), and is consistently observed during ageing, including cognitively unimpaired and Alzheimer brains. Further, although quantitation of staining intensity did not demonstrate an age-related accumulation, western blot analysis of basal forebrain homogenates demonstrated an increase in the size of amyloid-β42 oligomers in ageing that may play a role in the selective vulnerability of BFCNs.
Toxicity of amyloid-β is well established. Exposure to monomeric, oligomeric, and fibrillar forms of amyloid-β causes acute damage to neurons (Emre et al., 1992; Geula et al. 1998; Chromy et al., 2003). Intracellular amyloid-β leads to degenerative changes in neurons and synapses (Takahashi et al., 2002; Tomiyama et al., 2010), and BFCNs in particular are lost in the triple-transgenic mouse model of Alzheimer’s disease, which exhibit substantial intraneuronal amyloid-β accumulation (Oddo et al., 2003; Perez et al., 2011). Thus, intraneuronal accumulation of amyloid-β is likely to contribute to the selective vulnerability of BFCNs in Alzheimer’s disease.
Material and methods
Cases and tissue
Brains from 13 cognitively normal young individuals (20–66 years, seven males, six females), 14 normal, non-demented old individuals (70–99 years, five males, nine females) without any signs of neurological or psychiatric disorders, 21 individuals with Alzheimer dementia (60–95 years, 14 males, seven females), and two clinically identified individuals with superior memory performance (‘SuperAged’, age 90 and 95, both female) were obtained at autopsy and used in these experiments. SuperAged individuals performed on memory tests at a level at least average for 50-65 year-olds and at least average for age on tests of other cognitive domains (Rogalski et al., 2013). Results from SuperAged cases are included as cognitively unimpaired aged controls for statistical analysis. Clinical diagnoses were according to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD; Morris et al., 1989) and McKhann et al. (2011). Pathological diagnoses were rendered according to the CERAD, National Institution on Aging (NIA)-Reagan and National Institution on Ageing-Alzheimer’s Association (NIA-AA) criteria (Mirra et al., 1991; Newell, et al., 1999; Hyman, et al., 2012). No statistically significant difference was present in age between the Alzheimer’s disease and aged control groups [t(35) = 2.028, P > 0.05]. Post-mortem intervals across subjects ranged from 4 to 48 h, with an average duration of 15 h and median of 13 h and there was no significant difference of post-mortem interval between groups [F(2,46) = 0.3443, P < 0.0]. Case details are presented in Supplementary Table 1.
Brains were obtained from Northwestern University Alzheimer’s Disease Centre Brain Bank and from pathologists at institutions across the United States. Blocks of one hemisphere (primarily left) of each brain containing the basal forebrain, superior temporal cortex and insular cortex, all contained within the same blocks, were fixed in 4% paraformaldehyde for 30–36 h at 4°C and taken through sucrose gradients (10–40%) for cryoprotection. Serial 40-µm thick sections were obtained from each block using a freezing microtome and stored in 0.1 M phosphate buffer containing 0.02% sodium azide until use.
Immunohistochemical analysis
Series of sections from each brain were processed immunohistochemically using the avidin-biotin peroxidase (ABC) method employing the Vectastain® Elite ABC kit (Vector Labs) as previously described (Geula et al., 1998). Antibodies used were two polyclonal antibodies against amyloid-β (1282, from Dennis Selkoe, Harvard Medical School; and B7, from Bruce Yanker, Harvard Medical School), the monoclonal anti-amyloid-β 1-16 (6E10), polyclonal anti-amyloid-β42 and anti-amyloid-β40 antibodies (Biosource/Invitrogen), polyclonal anti-amyloid-β42 antibody (Calbiochem), anti-APP monoclonal antibody 22C11, and non-specific mouse and rabbit IgG as controls, processed using the same technique. Choline acetyltransferase (CHAT antibody) a marker of cholinergic phenotype, was used for anatomical localization of BFCNs in adjacent sections. Antigen retrieval techniques, including application of heat, were applied as needed based on antibody performance. Nissl staining (1% Cresyl violet acetate) was performed on 1 in 24–54 series of sections for neuroanatomical comparison with immunostaining. See Supplementary Table 2 for a summary of antibodies used.
Measurement of immunoreactivity / optical density
Amyloid-β-stained BFCNs were identified visually by an experienced observer based on location, morphology, and size. Initial semi-quantitative visual rating was performed to assess staining intensity. A quantitative analysis of staining in individual BFCNs by isoform-specific antibodies was performed using optical density measurement with the ImageJ software (NIH). A quantitative comparison of number of BFCNs immunoreactive for amyloid-β42 (Invitrogen) and CHAT was performed in matched sections (one to four sections assessed per case). Counts were averaged per case and amyloid-β42-positive BFCN counts expressed as a percentage of BFCNs displaying CHAT immunoreactivity. Optical density of cortical pyramidal neurons in superior temporal cortex and insular cortex was assessed in the same sections as a control for the specificity of amyloid-β accumulation in BFCNs, and IgG staining in BFCN and cortex from adjacent sections was qualitatively evaluated as a control for non-specific antibody binding. A comparison was made of APP staining in cortex and BFCNs to establish whether differences in amyloid-β staining may be attributable to differences in APP content.
The frequency of positively-stained neurons in an adjacent magnocellular population, the large neurons of the globus pallidus, was estimated by comparing counts of amyloid-β42-positive cells with counts of large globus pallidus neurons in matched Nissl-stained sections. Counts of five randomly selected regions (0.4 mm2 counting frame) in internal globus pallidus and five regions in external globus pallidus were pooled per area (external and internal globus pallidus) and then by case. With one exception, counts were made in three sections per case (two sections in one case with Alzheimer’s disease). Additionally, a qualitative comparison of staining intensity was made between amyloid-β42 staining in BFCNs (both for Invitrogen and Calbiochem antibodies) and magnocellular globus pallidus neurons using a scale of 1–5 where 1 indicates no staining and 5 the highest intensity. For each case, two to three slides were assessed and qualitative intensity value averaged across slides per cell type. Intraneuronal amyloid-β immunoreactivity in magnocellular globus pallidus neurons was assessed in the same sections containing the BFCNs.
Western and dot immunoblot analysis
Amyloid-β oligomer size and relative levels were measured using western blot of basal forebrain and inferior parietal cortical homogenates in a subgroup of cases. The basal forebrain region containing the nucleus basalis of Meynert cholinergic cell group 4 (nbM-Ch4) component of BFCN was dissected from fresh tissue blocks based on anatomical landmarks and the clearly identifiable grey matter appearance of the compact sector of nbM-Ch4. Dissected tissue samples were homogenized in buffer by 20 passes with a handheld homogenizer and diluted in lysis buffer and sample buffer from whole tissue homogenate to a working protein concentration as established by BCA assay. After heating for 15 min at 95°C, samples were stored at −80°C until used. Tissue homogenates from young, aged, and Alzheimer cases were electrophoresed in parallel on 10% SDS-PAGE gels with 5% stacking gel. After transfer, polyvinylidine fluoride (PVDF) membranes were incubated overnight or longer in primary antibodies including anti-amyloid-β42 (Calbiochem and Invitrogen), the oligomer-specific antibody NU-2 (Lambert et al., 2007), the monoclonal anti-amyloid-β 1-16 (6E10), and the anti-APP monoclonal antibody 22C11. Dot blot analysis using the monoclonal amyloid-β antibody-2 (MOAB-2), which recognizes amyloid-β at residues 1–4 in a pan-specific manner while exhibiting no cross-reactivity to APP (Youmans et al., 2012), was performed with the same prepared samples, which were spotted directly to nitrocellulose membrane, allowed to dry, blocked for 1 h in 5% bovine serum albumin (BSA), and incubated overnight in primary antibody plus 0.1% BSA. Following washes, membranes were incubated in secondary antibody for 4 h and developed. Blots were stripped and re-probed for GAPDH (Cell Signaling Technology) as a loading control. Bands/dots were quantified using ImageJ software (NIH) and protein levels were expressed as a percentage of loading control.
Statistical analysis
Optical density measures of 50–430 BFCNs, 25–125 pyramidal neurons of the superior temporal cortex, and 75–170 pyramidal neurons of the insular cortex were pooled by case for antibodies of interest, assessed from three representative imaging fields per section and per region from two to four sections in a 1 in 24–54 series. Optical density data were then averaged per case and subjected to tests of normality. Where data exhibited normal distributions, one-way ANOVA and the Student-Newman-Keuls post hoc test were applied (InStat GraphPad software, v. 3.0). Where normality was violated, the non-parametric Kruskal-Wallis with Dunn’s post hoc test was used (InStat). For analysis of amyloid-β42 staining between brain regions and groups, two-way mixed factorial ANOVA was used (SPSS, IBM) with brain region as the repeated measure. Correlation of age and optical density in normal subjects was assessed using Pearson’s moment correlation (InStat) employing optical density of amyloid-β42 immunoreactivity. The optical density measures of dot blot results and bands of interest from western blots were analysed in a similar manner, using one-way ANOVA or Student’s t-test (InStat).
Results
Qualitative and quantitative immunohistochemistry of amyloid-β in basal forebrain
All amyloid-β antibodies used resulted in immunoreactivity in plaques, whereas control antibodies did not. Amyloid-β immunoreactive plaques were sparsely distributed among the BFCNs in ageing and Alzheimer’s disease cases. Even in Alzheimer’s disease brains, the observed plaques in basal forebrain regions occupied by the BFCNs were of the diffuse variety and very rarely were compact or dense-cored plaques encountered (Fig. 1). Positive intracellular staining was observed in BFCNs in all groups of brains using polyclonal antibodies 1282 (not shown) and B7 (Fig. 1A–C). Similar immunoreactivity was observed between groups, with old cases presenting marginally higher intracellular staining compared to young and Alzheimer cases based on semi-quantitative visual rating of staining intensity. The monoclonal antibody, 6E10, against amino acids 1–16 of the amyloid-β peptide, resulted in similar staining to polyclonal antibodies 1282 and B7 (Fig. 1D–F), and quantitation of optical density of staining revealed no difference between groups (not shown). With 6E10 some positive intracellular staining was also observed in cortical pyramidal neurons. Optical density of staining using polyclonal antibodies specific for amyloid-β40 and amyloid-β42 isoforms was also assessed. No BFCN immunopositivity was observed with the amyloid-β40 antibody, even at high antibody concentrations (1:300–1:100; not shown), however, BFCN immunostaining was clearly apparent with anti-amyloid-β42 from two different sources (Invitrogen and Calbiochem). All cases examined demonstrated intraneuronal amyloid-β42 in BFCNs regardless of age and cognitive status. Quantitation of optical density of amyloid-β42 staining in BFCNs revealed no difference between young, old, and Alzheimer’s disease cases (P > 0.05).
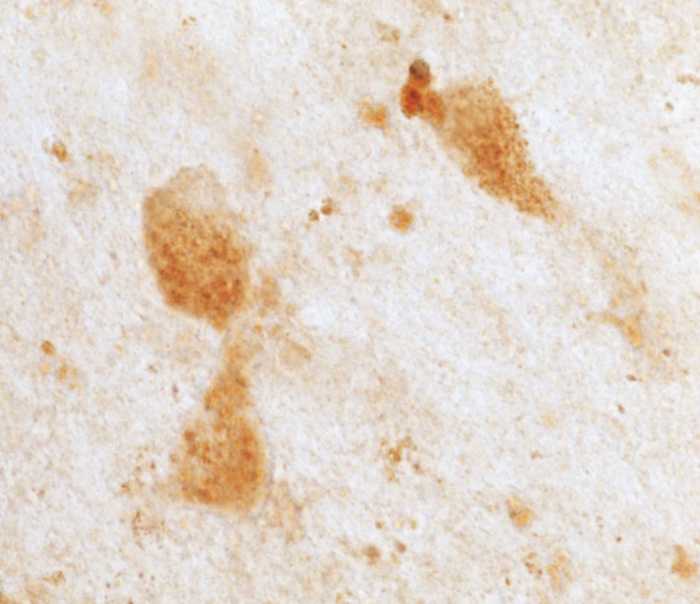
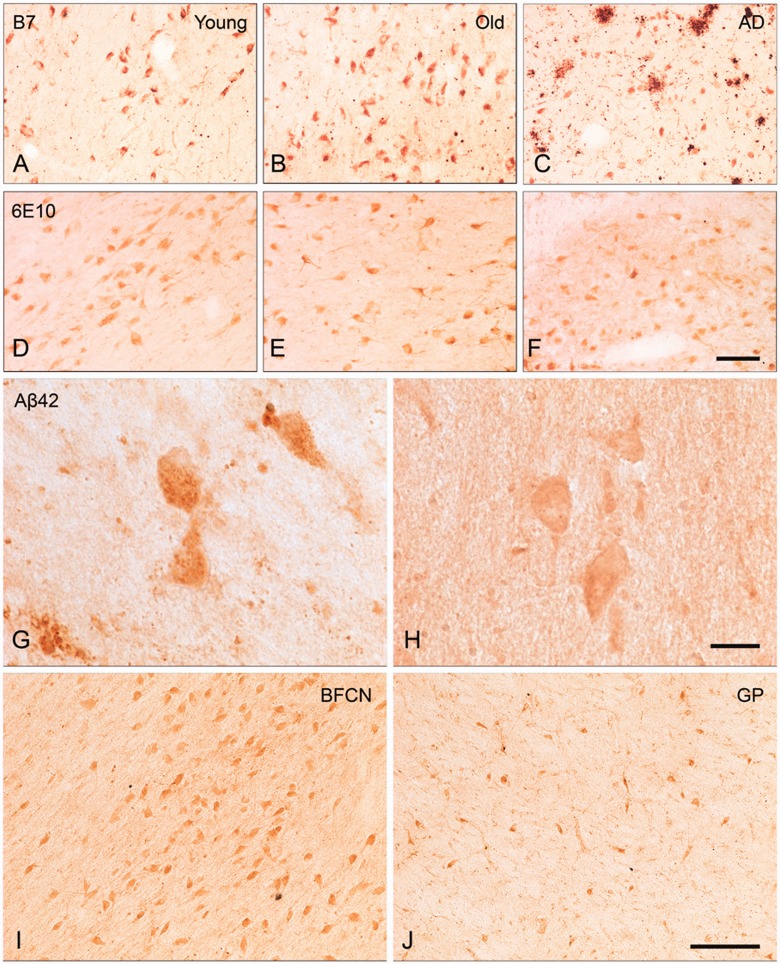
Figure 1.
BFCN immunostaining with B7 and 6E10 antibodies, and amyloid-β42 staining in BFCNs compared with large neurons of globus pallidus. BFCN staining with B7 antibody was present in young (A), old (B), and Alzheimer’s disease (C) cases. No difference was observed between groups by semi-quantitative rating of staining. BFCN staining with 6E10 antibody was observed in young (D), old (E), and Alzheimer’s disease (F) cases. Quantitative analysis of optical density of staining revealed no significant differences in 6E10 immunoreactivity within the BFCNs of the three groups of subjects. Notably, while the region proximal to BFCNs in some Alzheimer’s disease brains contained a few plaques (C), other cases were completely plaque-free (F). BFCN amyloid-β42 staining with the Invitrogen antibody is presented at high magnification, demonstrating granular staining in an Alzheimer's disease case (G) and smooth staining in an old case (H). Calbiochem amyloid-β42 staining was similar between BFCNs and magnocellular globus pallidus (GP) neurons (not shown), whereas with Invitrogen amyloid-β42 staining was occasionally stronger in BFCN (I) than globus pallidus (J), as demonstrated in an old case. Scale bars: A–F and I–J = 100 µm; G and H = 25 µm.
Qualitatively, all amyloid-β antibodies used often demonstrated intracellular staining that was granular or punctate in appearance (Fig. 1G and H). This attribute is likely due to the vesicular packaging of intracellular amyloid-β (Knauer et al., 1992). Punctate amyloid-β immunoreactivity was most apparent when staining intensity was high; neurons with low intensity of immunoreactivity displayed more diffuse cytoplasmic staining. Antibody-dependent differences were also apparent; 6E10 staining seemed more diffuse than B7 (potentially due to cross reactivity of 6E10 with APP). Intracellular staining with amyloid-β42-specific antibodies resulted in the greatest variation in staining intensity. Additionally, stronger cytoplasmic immunoreactivity with a clearly discriminable unstained nucleus was apparent in many BFCNs across all amyloid-β antibodies.
To assess the prevalence of intracellular amyloid-β42, anatomically-paired whole-section counts of amyloid-β42-positive (Invitrogen) and CHAT-positive BFCNs were compared. Results, averaged by group, are presented in Table 1; the entire population of CHAT-positive BFCNs was immunopositive for intracellular amyloid-β42 (98–100%) regardless of group.
Table 1.
Cell counts of ChAT- and amyloid-β42-positive BFCNs
| Group | n | Average amyloid-β42 count | Average ChAT count | %Amyloid-β42/ ChAT | Standard Error |
|---|---|---|---|---|---|
| Young | 2 | 444.4 | 438.7 | >100% | 5.03 |
| Old | 4 | 776.9 | 794.1 | 97.8% | 2.09 |
| Alzheimer’s | 3 | 892.3 | 792.0 | >100% | 1.65 |
Populations of amyloid-β42-positive BFCNs were equivalent to ChAT-stained neurons by cell count. One to four sections were analysed per subject, with total counts averaged per section by subject and then by group. ChAT = choline acetyltransferase.
Specificity of amyloid-β accumulation in basal forebrain cholinergic neurons
Control sections processed with mouse and rabbit IgG exhibited only non-specific staining in BFCNs and neurons in the insular cortex. Both exhibited chromogenicity equivalent to background, indicating specificity of the panel of antibodies used in these experiments.
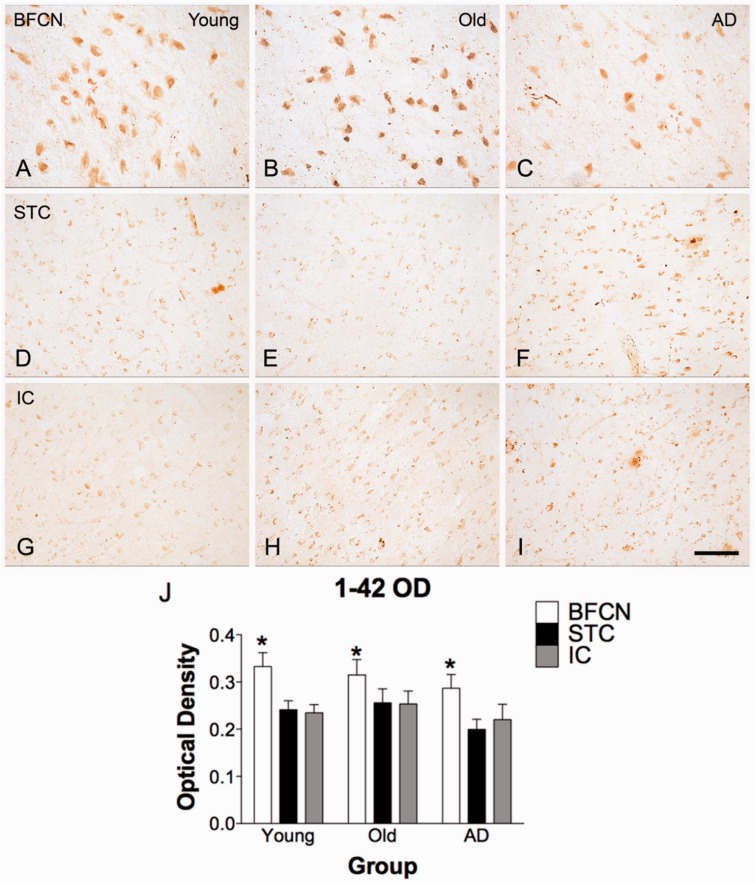
BFCN amyloid-β42 staining was considerably stronger than that observed in cortical pyramidal neurons in the superior temporal and insular cortices (Fig. 2). The intensity of immunoreactivity in all three regions was assessed by quantification of optical density, confirming the qualitative observation that intracellular amyloid-β42 accumulation is more prominent in BFCN when compared with cortical neurons in the superior temporal or insular cortices. The optical density of immunoreactivity was significantly higher in BFCNs than superior temporal cortex (P < 0.05), and insular cortex (P < 0.05), but no differences were detected between superior temporal and insular cortices (P > 0.05). There was no between-group difference for optical density of amyloid-β42 staining in BFCNs (Fig. 2J), and no correlation between optical density of BFCN staining and age in the normal cases (young and aged, P > 0.05). Positive correlations were observed in optical density measures between regions. Immunoreactivity in superior temporal and insular cortices were highly correlated (r = 0.887, P < 1 × 10−8), whereas correlations between BFCNs and either cortical region were weaker (basal forebrain versus superior temporal cortex, r = 0.582, P < 0.005; basal forebrain versus insular cortex, r = 0.640, P < 0.001).
Figure 2.
Amyloid-β42 immunostaining in BFCN and cortical neurons. Immunoreactivity for amyloid-β42 is selective to the BFCNs. Superior temporal cortex (STC; D–F) and insular cortex (IC; G-I) from the same case and the same section as BFCNs images (A–C) exhibit minimal neuronal staining with Invitrogen amyloid-β42. (J) Quantitative determination of immunoreactivity for amyloid-β42. In a two-way mixed factorial ANOVA (SPSS), the main effect of region (BFCNs, superior temporal cortex, insular cortex) was significant; F(2,42) = 21.143, P < 0.001, with optical density measures in BFCNs significantly higher than in either cortical region (BFCNs versus insular cortex, P < 0.05, BFCNs versus superior temporal cortex, P < 0.05). Optical density of staining in cortical regions superior temporal cortex and insular cortex did not differ significantly from each other (P > 0.05). For BFCNs and superior temporal cortex, n = 7 young, 10 old, and 8 Alzheimer’s disease cases; for superior temporal cortex, n = 7 young, 10 old, and 7 Alzheimer’s disease cases. Scale bar = 100 µm.
Although no statistically significant differences in amyloid-β42 optical density were present between groups, several case-specific characteristics were noted. The highest optical density recorded occurred in an aged case (Subject O5, Supplementary Table 1). Notably, the lowest optical density at any age was a SuperAged case (Subject S1), and the other available SuperAged case (Subject S2) was also among the lower values recorded (fourth lowest among 10 old and SuperAged cases). Two Alzheimer’s disease cases (Subjects AD16 and AD20) of eight available exhibited staining that was lower than normal, non-SuperAged cases at any age (data not shown). A similar pattern of optical density measures by case was observed in both cortical regions.
To further assess the specificity of amyloid-β accumulation in BFCNs, another magnocellular forebrain neuronal type was examined to determine whether neuronal size is associated with accumulation. A subpopulation of large neurons located in the globus pallidus is anatomically proximal to basal forebrain but resistant to degeneration in Alzheimer’s disease. The soma of large cells in globus pallidus were approximately one-half to two-thirds the diameter of BFCNs. Other globus pallidus neurons are one-tenth the size of BFCNs and were excluded from analysis. A quantitative comparison between amyloid-β42-positive and anatomically identified Nissl-stained cells was made to estimate the percentage of magnocellular globus pallidus neurons that exhibit amyloid-β42 staining with each amyloid-β42-specific antibody. BFCNs invading the globus pallidus at its borders, usually a distinct grouping with similar ‘streaming’ orientation, were excluded from globus pallidus neuron assessment. Nissl-stained cell counts were slightly higher in external globus pallidus (average 7.7 per counting area) than in internal globus pallidus (average 7.1 per counting area), so counts were averaged by region before averaging by case to estimate cell density. A lower proportion of magnocellular globus pallidus cells per region were immunoreactive with each amyloid-β42 antibody compared to BFCNs (52–76% globus pallidus, Table 2, versus nearly 100% BFCNs, Table 1).
Table 2.
Quantitative comparison of globus pallidus amyloid-β42-positive cell density
| Nissl Stain |
Invitrogen amyloid-β42 |
Calbiochem amyloid-β42 |
||||||
|---|---|---|---|---|---|---|---|---|
| Group | n | GP | n | GP | %Nissl | n | GP | %Nissl |
| Young | 6 | 6.44 | 6 | 4.56 | 70.8% | 3 | 3.81 | 59.2% |
| Old | 5 | 8.01 | 9 | 5.24 | 65.5% | 3 | 4.17 | 52.0% |
| Alzheimer’s | 5 | 7.46 | 8 | 5.64 | 75.5% | 6 | 5.18 | 69.4% |
A subpopulation of magnocellular globus pallidus neurons are amyloid-β42 positive by cell density comparison (average number of positively stained cells per counting region) with Nissl staining. GP = globus pallidus.
Qualitative comparisons were also made between intensity of amyloid-β42-specific antibody staining in magnocellular globus pallidus neurons and BFCNs. Results for both amyloid-β42 antibodies assessed indicated that cellular staining intensity was nearly equivalent between BFCNs and globus pallidus neurons. However, with the Invitrogen antibody, BFCN staining was rated as slightly darker in normal cases but not Alzheimer cases (Fig. 1I and J).
Amyloid precursor protein in basal forebrain and cortex
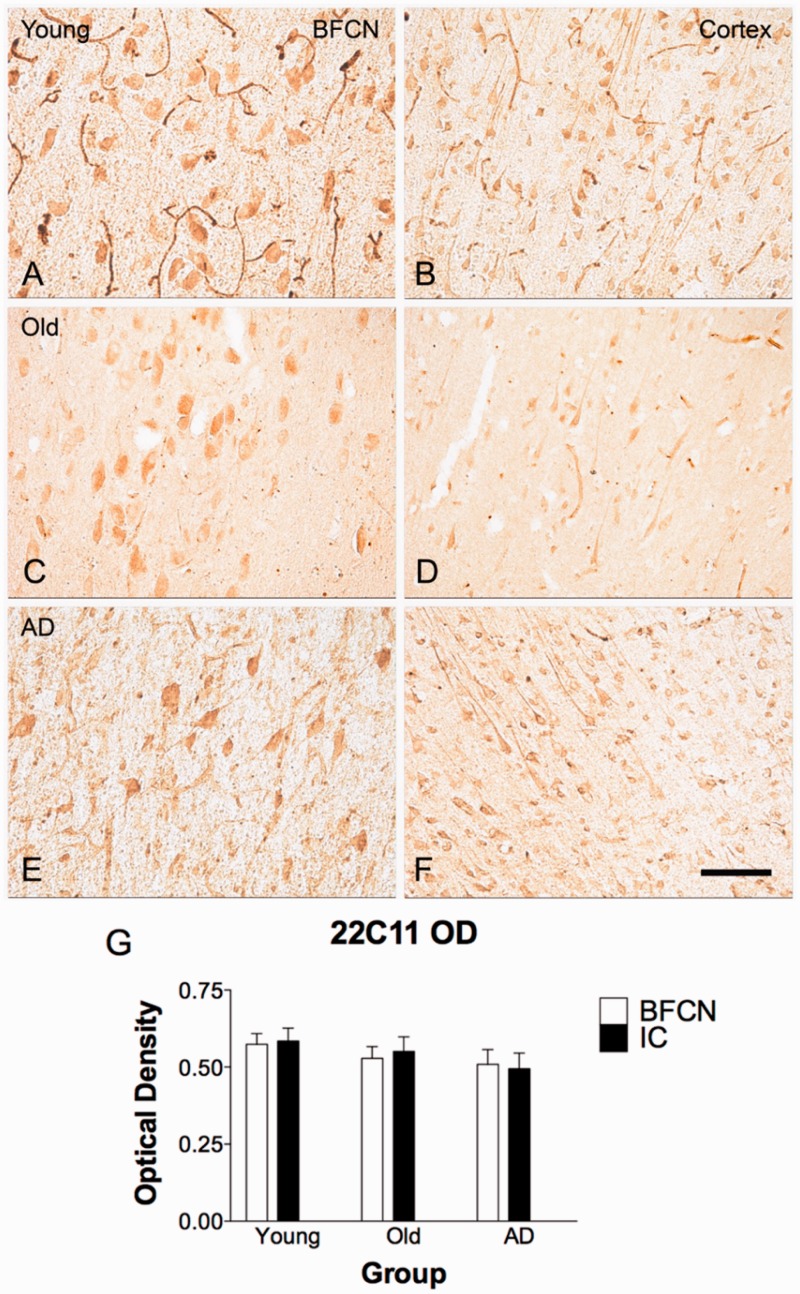
To determine the potential contribution of APP expression to amyloid-β accumulation, immunohistochemistry was performed using the 22C11 monoclonal antibody against APP. No qualitative difference was observed in intensity of staining in BFCNs compared to neurons in the insular cortex (Fig. 3). Quantification of optical density confirmed this result by one-way non-parametric ANOVA [H(2) = 2.026, P = 0.84], indicating that differences in intracellular amyloid-β observed in BFCN and cortical neurons with anti-amyloid-β42 antibodies was not due to differences in APP content (Fig. 3G).
Figure 3.
APP immunostaining in BFCNs and cortex. APP is not differentially expressed in BFCNs compared to cortical pyramidal neurons, as indicated by immunoreactivity for 22C11. Pyramidal neurons of the insular cortex from the same section of tissue as BFCNs exhibit equivalent neuronal APP staining as in BFCNs in young (A and B), old (C and D), and Alzheimer (E and F) cases. Quantification of optical density is represented in G. One-way Kruskal-Wallis non-parametric ANOVA (InStat) revealed no differences for 22C11 optical density between groups nor by region; H(2) = 2.841, P = 0.7245, ns. n = 3 young, n = 6 old, and n = 6 Alzheimer’s disease cases. Scale bar = 100 µm. IC = insular cortex.
Western blot and dot blot analyses
Holo-amyloid-β in basal forebrain
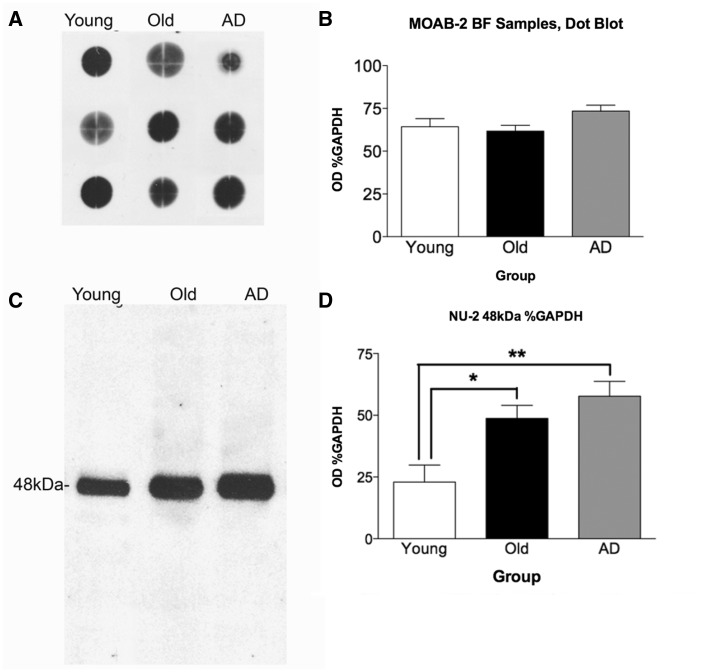
Immunoblot of tissue homogenates from basal forebrain or inferoparietal cortex regions of young, normal old, and Alzheimer’s disease cases were used to validate immunohistochemistry results, and to assess differences in assembly states of amyloid-β between groups. We used antibody MOAB-2 to assess total amyloid-β protein levels in the three groups of subjects. This antibody was not amenable to immunohistochemistry staining or western blot in homogenate samples of human tissue, therefore dot blot was performed on basal forebrain homogenate samples to assess total amyloid-β per case. As with immunohistochemistry using other amyloid-β antibodies, no significant differences were found between groups (P > 0.05; Fig. 4A and B).
Figure 4.
MOAB-2 dot blot and quantitation and NU-2 48 kDa amyloid-β oligomer western blot and quantitation of basal forebrain homogenates. (A) Representative MOAB-2 dot blot of basal forebrain (BF) samples; n = 6 young, n = 8 old, n = 7 Alzheimer’s disease cases. As observed in immunohistochemistry, there is no difference apparent between groups in total amyloid-β in basal forebrain. Quantitation of optical density of dots (B) confirmed absence of statistically significant difference between groups. The NU-2 antibody is highly specific, revealing a single band of amyloid-β oligomer at 48 kDa (C). One-way ANOVA with Student-Newman-Keuls post hoc test revealed significant difference between young cases compared to old (*P < 0.05) and Alzheimer cases [**P < 0.001; F(2,11) = 8.287, P = 0.0064]. n = 6 young, n = 8 old, and n = 7 Alzheimer’s disease cases (D).
Large oligomeric species in basal forebrain
An oligomer-specific antibody, NU-2, demonstrated a single band at 48 kDa in western blot of basal forebrain homogenates. This species exemplifies a high-molecular-weight soluble oligomer, and was found to be significantly higher in basal forebrain of old brains compared to young (P < 0.05) and in Alzheimer’s disease basal forebrain samples compared to young cases (P < 0.01; Fig. 4C and D).
Small and intermediate oligomeric species in basal forebrain
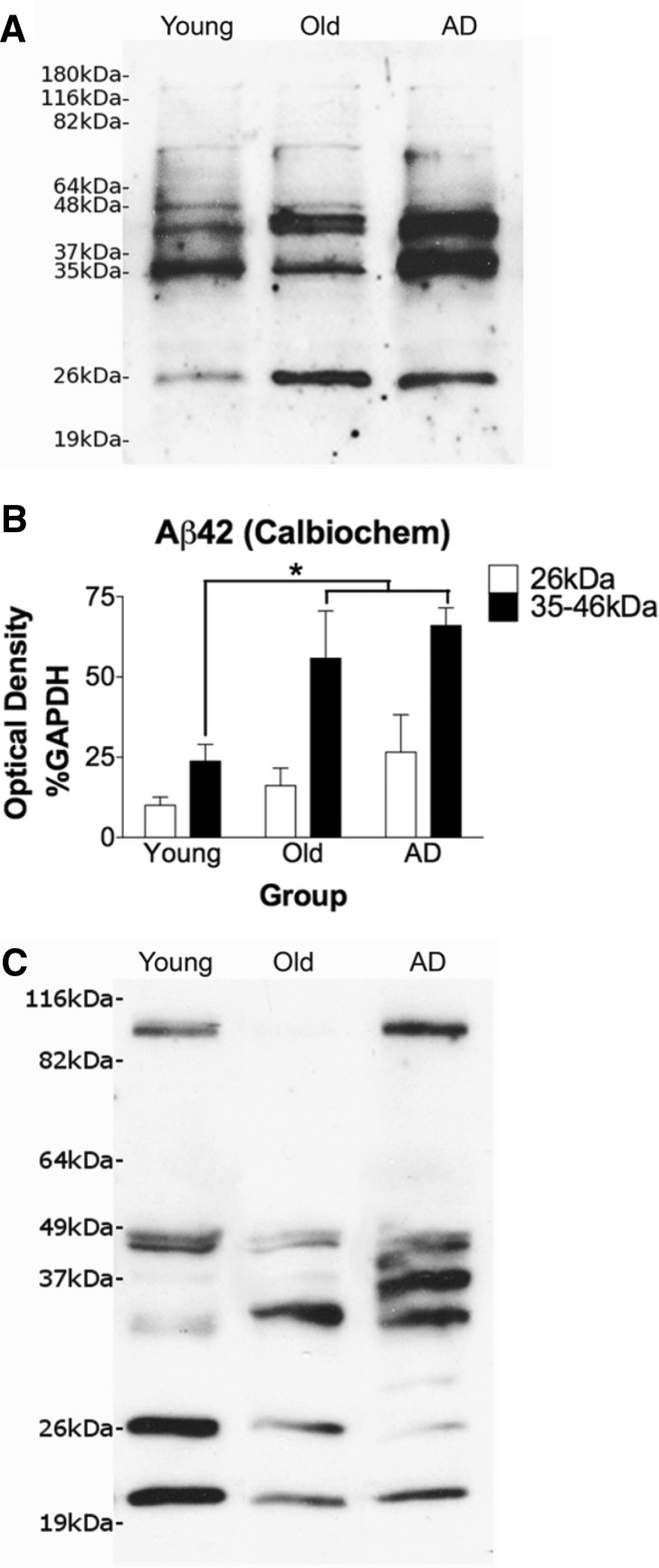
In electrophoretic blot applications, most amyloid-β antibodies reveal many discrete bands and may differ greatly in banding pattern depending on the epitope recognition of the antibody, in part because the multiple assembly states of oligomeric species may differentially present the target for antibody binding (Pryor et al., 2012). Comparisons of banding patterns between the two amyloid-β42-specific antibodies revealed a number of shared bands that, because of recognition by both antibodies, are likely to represent specific amyloid-β oligomers of varying sizes. These bands were selected for analysis, including a 26 kDa band (low molecular weight) and a series of sequential bands between 35–46 kDa (intermediate molecular weight). A relatively faint band at 48 kDa (the epitope recognized by the NU-2 antibody) was also observed.
Amyloid-β42-specific immunoreactivity exhibited a significant increase in ageing and Alzheimer’s disease at the intermediate molecular weight band when compared with young cases [F(2,15) = 5.299, P = 0.0182; P < 0.05; Fig. 5]. With the Invitrogen antibody, the low molecular weight band showed the inverse with a trend towards decrease in ageing and Alzheimer’s disease; however, quantitation of the 26 kD band with the Calbiochem antibody did not reveal a significant difference between groups.
Figure 5.
Calbiochem and Invitrogen amyloid-β42 western blots with quantitation of the 26 kDa and 35-46 kDa banding regions. Sample blot for Calbiochem is shown in A with quantitation in B. For the 35–46 kDa band region, one-way ANOVA with Student-Newman-Keuls post hoc tests revealed a significant increase in both old cases and Alzheimer’s disease patients (AD) basal forebrain compared to young cases [P < 0.05 for each comparison; F(2,15) = 5.299, P = 0.0182]. n = 6 young, n = 6 old, and n = 6 Alzheimer cases. No significant differences were seen for the 26 kDa band. A faint band at 48 kDa was too light to assess quantitatively. All quantification was normalized to GAPDH standard. Sample blot of Invitrogen amyloid-β42 (C) shows the same pattern as the Calbiochem amyloid-β42 antibody at the intermediate molecular weight oligomer 35 kDa band region, with an apparent increase in old and Alzheimer cases when compared to the young. However, with this antibody the 26 kDa low molecular weight oligomer band trends toward a decrease.
Specificity of changes in amyloid-β oligomeric species in basal forebrain
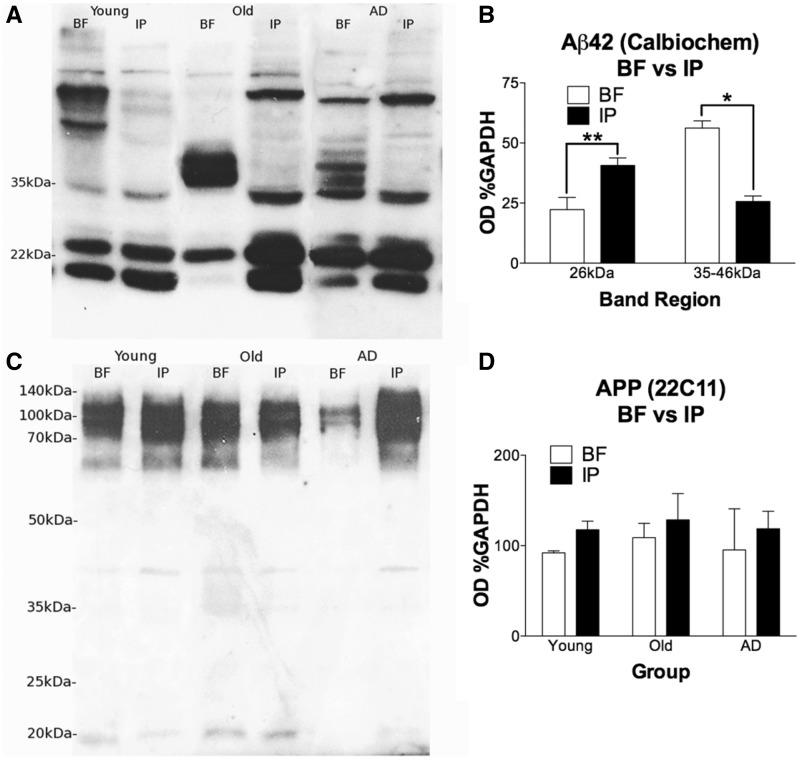
A comparison blot between inferoparietal cortical homogenates and basal forebrain samples using Calbiochem anti-amyloid-β42 was performed to examine the regional specificity of the observed banding pattern across all cases, including the low, intermediate, and high molecular weight banding regions. Student’s t-test revealed that the 26 kDa lower molecular weight band was significantly lower in the basal forebrain compared to cortex [t(28) = 3.480, P = 0.0017, two-tailed], whereas the 35–46 kDa band region was significantly higher in basal forebrain when compared with cortical levels [t(27) = 2.648, P = 0.0134, two tailed with Welch’s correction; Fig. 6A and B] when data from the three groups were combined. No differences between regions were observed for the faint 48 kDa band.
Figure 6.
Basal forebrain and inferoparietal cortex samples in western blot for amyloid-β42 (Calbiochem; 26 kDa and 35–46 kDa) and APP (22C11; 100 kDa). Banding regions of interest with Calbiochem amyloid-β42 were compared between basal forebrain (BF) and inferoparietal cortex (IP) samples (A). Samples across groups were pooled by region for quantitative comparison (B). T-tests revealed that the 26 kDa band was significantly lower in basal forebrain than inferoparietal cortex, t(28) = 3.480, P = 0.0017, two-tailed. The 35-46 kDa collective region was significantly higher in basal forebrain when compared with inferoparietal cortex, t(27) = 2.648, P = 0.0134, two-tailed with Welch’s correction. At 48 kDa no differences were observed (not shown). Basal forebrain samples included n = 6 young, n = 7 old, and n = 6 Alzheimer’s disease (n = 19), whereas inferoparietal cortex samples from the same cases as basal forebrain included n = 5 young, n = 3 old, and n = 3 Alzheimer cases (n = 11). APP expression in basal forebrain is not significantly different between groups or compared to cortex (C). A slight trend towards lower APP expression in basal forebrain compared to cortex is present, but likewise not statistically significant by unpaired Student’s t-test (D). These results demonstrate that APP levels are unchanged across groups in either structure, indicating that observed differences are not due to alterations in APP expression. Basal forebrain samples for n = 4 young, n = 4 old, and n = 3 Alzheimer’s disease cases were compared to inferior parietal cortex homogenate from paired cases (n = 3 young, n = 3 old, and n = 2 Alzheimer’s disease).
Amyloid precursor protein in basal forebrain and cortex
Full-length APP was visualized at ∼100–115 kDa using antibody 22C11, which was not significantly different between basal forebrain and inferoparietal cortex by ANOVA. There was a slight and non-significant trend for higher cortical APP, consistent with immunohistochemistry observations. Similarly, APP expression was not different between groups for basal forebrain samples (Fig. 6C and D). This indicates that observed changes in amyloid-β species with ageing, namely the conversion of lower molecular weight amyloid-β species to higher species, does not appear to be due to differential expression of APP in BFCNs over the lifespan.
Discussion
Immunohistochemical detection of intracellular amyloid-β in BFCNs
Previous work in our laboratory first demonstrated intracellular amyloid-β accumulation in BFCNs in non-human primates (rhesus macaque; unpublished results). In the non-human primate brain, the primary amyloid species is amyloid-β40 rather than amyloid-β42 (Selkoe et al., 1987; Podlisny et al., 1991; Gearing et al., 1996; Geula et al., 2002), the latter being the predominant species in humans. Immunohistochemistry with an amyloid-β40-specific antibody demonstrated strong intracellular staining in BFCN in rhesus monkeys with a significant age-related increase in staining intensity.
In contrast to our findings in the rhesus monkey, all human cases examined in the present studies displayed similar immunoreactivity for amyloid-β in BFCNs regardless of age and disease state. Both polyclonal (B7) and monoclonal (6E10) amyloid-β antibodies that recognize amyloid plaques also visualized BFCN amyloid-β immunopositivity even in young cases. However, these antibodies are not isoform-specific and may also exhibit cross-reaction with APP. Thus, C-terminal specific antibodies were used to discriminate between amyloid-β isoforms. Unlike the rhesus brain, human BFCNs did not demonstrate strong positive staining with amyloid-β40-specific antibodies even at high concentrations. Rather, they exhibited significant intracellular accumulation of amyloid-β42, the species prevalent in humans and particularly implicated in Alzheimer’s disease plaque formation.
Qualitatively, amyloid-β42 staining was often granular in appearance, although this aspect was variable between cases and sections. Others have attributed this characteristic phenomenon to the endosomal-lysosomal vesicular packaging of intracellular amyloid-β, which results in compartmentalized staining within the cytosol (Knauer et al., 1992). Instances of smooth-appearing amyloid-β42 staining may depend on differential fixation and lot of antibody.
We did not observe age or Alzheimer’s disease related differences in the intensity of intracellular amyloid-β immunoreactivity in BFCNs. All cases, even the youngest at age 20, exhibited robustly amyloid-β42-positive BFCNs. Moreover, amyloid-β42 positivity was a common, possibly universal phenomenon in BFCNs, as evidenced by the nearly complete numerical overlap between counts of amyloid-β42-positive neurons with counts of cells positive for the cholinergic marker CHAT from matched sections. The observed staining is not due to non-specific magnocellular affinity for chromagen, as demonstrated by control staining with non-specific IgG, nor is it attributable to cross-reactivity to APP. Indeed, APP antibody demonstrated equivalent and non-selective staining in both BFCN and cortical pyramidal neurons while staining with amyloid-β42 antibodies demonstrated significantly higher immunopositivity in BFCN compared to the cortical regions examined.
The region containing nbM-Ch4 neurons is adjacent to globus pallidus, with the anterior commisure passing between the two regions. Large cells of globus pallidus are a population of magnocellular neurons (Difiglia et al., 1982) that send projections to the subthalamic nucleus, thalamus, and midbrain tegmentum (Kemp and Powell, 1971; Knauer et al., 1992). In contrast to BFCNs, large globus pallidus neurons are not selectively vulnerable to pathological changes (particularly tau accumulation) in Alzheimer’s disease, nor are they vulnerable to degeneration. The quality of staining with amyloid-β42 antibodies was similar between the two regions (usually granular, sometimes smooth) while qualitative rating assessment indicated that the intensity of globus pallidus immunoreactivity may be slightly lower than BFCNs. However, quantitation of the proportion of large globus pallidus neurons that are immunoreactive for amyloid-β42 revealed that 52–76% of these cells accumulate amyloid-β, compared to nearly 100% of BFCNs. Thus, BFCN amyloid-β42 staining may be partly due to regional characteristics shared by local magnocellular neurons. However, neuronal vulnerability may be associated with how extensively and at what levels a population exhibits amyloid-β accumulation.
Immunoblot of tissue homogenates
Optical density of immunohistochemically stained tissue is a rough estimate of protein load, so immunoblot methods are required to quantify amyloid-β more accurately. A number of antibodies were selected for western blot analysis, primarily as a confirmation and means of quantifying further the immunohistochemistry results.
MOAB-2 is a monoclonal antibody that measures total amyloid-β, including all isoforms and multiple aggregation states, without exhibiting cross-reactivity with APP or amyloid-β C-terminal fragments. Consistent with immunohistochemistry results using other antibodies, dot blot analysis using MOAB-2 in basal forebrain samples did not detect significant differences in amyloid-β protein levels between groups.
Western blot with a highly specific oligomer-targeting antibody, NU-2, resulted in a single band at 48 kDa. This oligomeric species was shown to be significantly enriched in Alzheimer and aged control basal forebrain compared to young cases. It is notable that amyloid-β42-specific antibodies also revealed a faint band at 48 kDa, similar to that visualized by the oligomer-specific NU-2 antibody, but the signal strength at this molecular weight was not sufficient for quantitation. This may be due to obstruction of recognition sites specific to the conformation of the 48 kDa oligomer. However, the specificity of the 48 kDa band and its appearance, even to a lesser extent, in blots with other amyloid-β-specific antibodies support the notion that NU-2 is identifying a high molecular weight amyloid-β oligomer. We were unable to obtain satisfactory immunohistochemical staining with MOAB-2 and NU-2 antibodies for a direct comparison with immunoblot studies.
Amyloid-β42-specific antibodies from Invitrogen and Calbiochem revealed a number of shared quantifiable bands at small and intermediate oligomeric sizes. Interestingly, both the intermediate band group (35–46 kDa) and the high molecular weight species observed with NU-2 exhibited a pattern of increasing intensity in ageing and in Alzheimer’s disease. The low molecular weight band (26 kDa) was less intense than the intermediate bands and either demonstrated no difference or a trend towards decreasing in ageing and Alzheimer’s disease with the amyloid-β42-specific antibodies.
Consistently, we observed strong intracellular amyloid-β42 even in the youngest cases assessed. The potential implications of the alterations in oligomeric band pattern include an age- and disease-related assembly-state change in amyloid-β42 in BFCN that is directional: while small oligomeric assemblies either stay in equilibrium or decrease, large species become increasingly prevalent. Immunostaining alone is not sufficient to observe these differences because the total levels of amyloid-β appear to remain the same, and indeed, the results with MOAB-2 indicate that holo-amyloid-β in particular is consistently the same between groups.
Regional differences were also apparent; the low molecular weight oligomer is enriched in cortex compared to basal forebrain across groups, whereas the intermediate molecular weight bands are greatly enriched in basal forebrain across groups.
Care must be exercised in drawing equivalencies between regions, in particular because samples used in immunoblot analysis are dissected whole-tissue homogenates. Extracellular amyloid-β deposits are not excluded from the sample, so particularly in Alzheimer brains (and to a lesser extent in normal aged brains) the presence of plaque material may influence these results. However, consistent with our past observations (Wu et al., 2005; Riascos et al., 2011), we found that basal forebrain is relatively protected from plaque formation compared to cortex. Significantly, the intermediate amyloid-β oligomer size is higher in basal forebrain than cortical samples despite the potential effect of greater plaque density in cortex. In fact, the molecular weights of bands we detected in basal forebrain homogenates are consistent with soluble amyloid-β oligomers, precluding inclusion of the very large oligomers that aggregate in plaques. Because of its insoluble nature, the aggregated amyloid-β in plaques is not amenable to electrophoretic separation, precluding its contribution to the western blot results.
Another issue of concern given the nature of our tissue samples is equating results between basal forebrain samples in various groups given the fact that BFCN loss is often extensive in Alzheimer’s disease. Thus, protein concentration measures of intracellular amyloid-β would be expected to decrease in samples containing a smaller cell population. It should be noted that the remaining BFCN in the disease state appeared to maintain their high content of amyloid-β immunoreactivity in immunohistochemistry processed sections. Moreover, the intermediate and high molecular-weight bands were increased in Alzheimer compared to young brains, suggesting that the difference is likely more pronounced per remaining BFCN, given the loss of these neurons in Alzheimer’s disease. At present, there are no refined methods for quantitation of protein content per cell using western blot or similar analyses.
It is well known that amyloid-β aggregation state, like other proteins that oligomerize, is sensitive to storage conditions and the denaturing treatments of SDS-PAGE and similar techniques. However, because our comparisons are between samples that received similar treatment and were stored under identical conditions, our immunoblot results are likely to be valid insofar as differences between groups or regions reflect actual aggregation-state differences between samples. This conclusion is further validated by the fact that in immunohistochemistry and immunoblot studies, high levels of amyloid-β immunoreactivity were observed in all groups, including young brains. Therefore, differences in amyloid-β oligomer size between groups is highly likely to be a reflection of age and disease-related alterations in amyloid-β assembly rather than a result of experimental conditions, which would be expected to affect all groups similarly, resulting in no differences in amyloid-β assembly given the initially high levels of amyloid-β in each group.
Notably, the APP blot results, along with our APP immunohistochemistry findings, definitively demonstrate that group and regional differences in intracellular amyloid-β are due to mechanisms other than upregulation of APP expression. In fact, the trend revealed by the 22C11 blots indicates the possibility for slightly lower levels of APP in basal forebrain compared to cortex. There remain numerous other possible routes by which BFCNs could accumulate amyloid-β intracellularly, including a cell-specific increase in activation of the β-secretase amyloidogenic pathway, the downregulation of amyloid-β clearance molecules such as ADAM10 and insulin-degrading enzyme, or uptake of released amyloid-β that originated in other cells. Moreover, evidence suggests that amyloid-β uptake is variable between cell types (Knauer et al., 1992; Bahr et al., 1998), and in particular the presence of CHRNA7 (previously α7nAChR), which is expressed in BFCN and cortical areas where projecting BFCN axons terminate, is thought to mediate endocytosis of soluble amyloid-β42 and lead to intracellular accumulation (Difiglia et al., 1982; Nagele et al., 2002).
Pathophysiological implications
The ultimate destination of amyloid-β that is sequestered in BFCN, particularly during and after the activation of neurodegenerative pathways in Alzheimer’s disease, is unknown. As previously mentioned, local plaque pathology in basal forebrain in our sample was less than cortex, with virtually no compact plaques and minimal diffuse plaques. One possibility is that intracellular amyloid-β42 is trafficked via anterograde axoplasmic transport from damaged or dying BFCNs to cortex, where it may be released and contribute to cortical plaque burden. Indeed, extensive plaque formation is common in regions that receive innervation from BFCNs, including the entire cortical mantle, and the endosomes in which amyloid-β is sequestered intracellularly have been shown to be trafficked to exosomes for release into the extracellular space (Rajendran et al., 2006).
The universal amyloid-β accumulation observed in young BFCNs raises the possibility of normal physiological function(s) of this peptide. Several reports indicate potential functional roles for amyloid-β including antioxidant action (Nunomura et al., 2010), promotion of synaptic plasticity (Puzzo et al., 2012), neurotrophic effects (Yankner et al., 1990) and involvement in memory formation (Garcia-Osta and Alberini, 2009). The current consensus in the literature is that these functions of amyloid-β are optimally evident at low (picomolar) concentrations, and that the peptide loses function and becomes primarily toxic as concentration increases (Atwood et al., 2003; Tampellini and Gouras, 2010; Puzzo and Arancio, 2013). The role of amyloid-β at synapses, in particular, is of great interest. Oligomeric species have been shown to bind synapses preferentially, exhibiting pathogenic action (Lacor et al., 2004; Klein, 2006). Intraneuronal amyloid-β has been shown to be associated with degenerative synaptic abnormalities (Takahashi et al., 2002). Physiologically normal synaptic activity has been shown to increase amyloid-β secretion at the expense of intracellular accumulation, which may be associated with a neuroprotective effect (Tampellini et al., 2009).
We observed increased intermediate and large oligomeric amyloid-β in basal forebrain from normal aged and Alzheimer brains. Recent work has demonstrated that oligomeric assemblies may be differentially prevalent in early versus late-onset Alzheimer’s disease in frontal cortex (Bao et al., 2012), which may implicate differential contributions of various assembly states to distinct disease pathways. Selectively vulnerable neuronal populations, such as BFCNs, may likewise exhibit specific sensitivity to the neurotoxic effects of particular oligomeric assembly states of amyloid-β. Indeed, Bao et al. (2012) have reported an association between increased amyloid-β decamer prevalence (∼45 kDa), reduced CHAT activity, and loss of CHRNA7 in frontal cortex in late-onset Alzheimer’s disease, supporting a link between this species and cholinergic dysfunction.
The case with the highest measured optical density of amyloid-β staining in BFCNs was an aged control, confirming previous reports indicating that intraneuronal amyloid-β may accumulate with age (Gouras et al., 2005). Furthermore, the average optical density among Alzheimer cases was lower than normal controls at any age; two Alzheimer’s disease cases fell below all control cases excepting one SuperAged case. It should be noted that the population of BFCNs in Alzheimer’s disease represents a subset of surviving cells, which may have the benefit of protective factors such as increased calcium buffering capacity (Riascos et al., 2011) or differences in amyloid-β production, trafficking, or degradation. These differences could be reflected in decreased intracellular amyloid-β. Although the number of SuperAged cases available for the current studies precludes statistical validation, the lowest optical density recorded occurred in one SuperAged case, whereas the optical density measure for the second SuperAged case was lower than the mean optical density for the cognitively normal controls. Future characterization of pathology from SuperAged individuals who are now enrolled in cognitive studies will be necessary to resolve this potential relationship between relatively low intracellular amyloid-β and preserved cognitive ability, and warrants further study.
Conclusion
These combined immunoblot and immunohistochemistry findings demonstrate that accumulation of amyloid-β42 within BFCNs occurs early in young brains and continues in the course of ageing and disease. Furthermore, early accumulation of amyloid-β42 seems to be a selective feature of BFCNs when compared with cortex and is not due to differences or changes in APP expression. The accumulation of amyloid-β may be a characteristic of the region of the brain within which these cells are located, as a subpopulation of magnocellular neurons of globus pallidus exhibits amyloid-β positivity but in substantially lower proportion of total neurons when compared with the BFCN. It is likely that early accumulation of amyloid-β in the BFCN has deleterious effects on neuronal (synaptic) function, possibly via disruption of resting cytosolic calcium. The lifelong accumulation of intraneuronal amyloid-β and increases in size of amyloid-β oligomers in ageing and Alzheimer’s disease may be responsible, at least in part, for the selective vulnerability of these neurons to pathology and degeneration.
Acknowledgements
We are grateful to Katherine Gasho and Nicholas Nagykery for expert technical assistance.
Glossary
Abbreviation
- BFCN
basal forebrain cholinergic neuron
Funding
This work was supported in part by a Zenith Fellows Award (C.G.) from the Alzheimer’s Association, and by grants from the National Institute on Ageing (AG014706 and AG027141 to C.G. and AG20506 T32 to A.B.N). A portion of the tissue used in these studies was received from the Northwestern University Alzheimer’s Disease Center (AG013854).
Supplementary material
Supplementary material is available at Brain online.
References
- Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, et al. Amyloid-β: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-β. Brain Res Rev. 2003;43:1–16. doi: 10.1016/s0165-0173(03)00174-7. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G. Amyloid β protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. J Comp Neurol. 1998;397:139–47. [PubMed] [Google Scholar]
- Bao F, Wicklund L, Lacor PN, Klein WL, Nordberg A, Marutle A. Different β-amyloid oligomer assemblies in alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol Aging. 2012;33:825–e1–13. doi: 10.1016/j.neurobiolaging.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, et al. Self-assembly of Abeta(1-42) into globular neurotoxins. Biochemistry. 2003;42:12749–60. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Difiglia M, Pasik P, Pasik T. A Golgi and ultrastructural study of the monkey globus pallidus. J Comp Neurol. 1982;212:53–75. doi: 10.1002/cne.902120105. [DOI] [PubMed] [Google Scholar]
- Emre M, Geula C, Ransil BJ, Mesulam MM. The acute neurotoxicity and effects upon cholinergic axons of intracerebrally injected β-amyloid in the rat brain. Neurobiol Aging. 1992;13:553–9. doi: 10.1016/0197-4580(92)90055-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Osta A, Alberini CM. Amyloid beta mediates memory formation. Learn Mem. 2009;16:267–72. doi: 10.1101/lm.1310209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra SS. Aβ40 is a major form of β-amyloid in nonhuman primates. Neurobiol Aging. 1996;17:903–8. doi: 10.1016/s0197-4580(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Geula C, Bu J, Nagykery N, Scinto LFM, Chan J, Joseph J, et al. Loss of Calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003;455:249–59. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam M-M, Saroff DM, Wu C-K. Relationship between plaques, tangles, and loss of cortical cholinergic fibers in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:63–75. doi: 10.1097/00005072-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam M-M. Alzheimer disease. 2nd edn. Philadelphia: Lippincott Williams and Wilkins; 1999. Cholinergic system in Alzheimer's disease. [Google Scholar]
- Geula C, Nagykery N, Nicholas A, Wu C-K. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:309–18. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Nagykery N, Wu C-K. Amyloid-β deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): incidence and chemical composition. Acta Neuropathol. 2002;103:48–58. doi: 10.1007/s004010100429. [DOI] [PubMed] [Google Scholar]
- Geula C, Wu C-K, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid-β protein neurotoxicity. Nat Med. 1998;4:827–31. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- Goedert M. Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer's disease. EMBO J. 1987;6:3627–32. doi: 10.1002/j.1460-2075.1987.tb02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Aβ accumulation and origin of plaques in Alzheimer's disease. Neurobiol Aging. 2005;26:1235–44. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, et al. Intraneuronal Aβ42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, LaFerla FM. Linking calcium to Aβ and Alzheimer's disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Green KN, Smith IF, LaFerla FM. Role of calcium in the pathogenesis of Alzheimer's disease and transgenic models. In: Carafoli E, Brini M. editors. Calcium Signalling and Disease. Subcell Biochem; 2007; 45: 507–21. [DOI] [PubMed]
- Guo Q, Christakos S, Robinson N, Mattson M. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci. 1998;95:3227–32. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Ábrahám I, Timmerman W, Laskay G, Tóth B, Sasvári M, et al. β-Amyloid neurotoxicity is mediated by a glutamate-triggered excitotoxic cascade in rat nucleus basalis. Eur J Neurosci. 2000;12:2735–45. doi: 10.1046/j.1460-9568.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National institute on aging-Alzheimer's association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JM, Powell TPS. The connections of the striatum and globus pallidus: synthesis and speculation. Philos Trans R Soc B: Biol Sci. 1971;262:441–57. doi: 10.1098/rstb.1971.0106. [DOI] [PubMed] [Google Scholar]
- Klein WL. Synaptic targeting by Aβ oligomers (ADDLS) as a basis for memory loss in early Alzheimer's disease. Alzheimer‘s & Dement. 2006;2:43–55. doi: 10.1016/j.jalz.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Knauer MF, Soreghan B, Burdick DA, Kosmoski J, Glabe CG. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/β protein. Proc Natl Acad Sci USA. 1992;89:7437–41. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, et al. Monoclonal antibodies that target pathological assemblies of Aβ. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985;4:2757–63. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Rychlik B, Chu C, Christakos S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–50. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzhei Dem. 2011;7:263–69. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney M. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem Pharmacol. 2005;70:1115–24. doi: 10.1016/j.bcp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55:815–28. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). part ii. standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer‘s disease (CERAD): I. clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–58. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele RG, D'Andrea MR, Anderson WJ, Wang H-Y. Intracellular accumulation of β-amyloid1-42 in neurons is facilitated by the α7 nicotinic acetylcholine receptor in Alzheimer's disease. Neuroscience. 2002;110:199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the national institute on aging (NIA)-reagan institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1147–55. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Tamaoki T, Tanaka K, Motohashi N, Nakamura M, Hayashi T, et al. Intraneuronal amyloid β accumulation and oxidative damage to nucleic acids in Alzheimer disease. Neurobiol Dis. 2010;37:731–37. doi: 10.1016/j.nbd.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Perez SE, He B, Muhammad N, Oh K-J, Fahnestock M, Ikonomovic MD, et al. Cholinotrophic basal forebrain system alterations in 3xTg-AD transgenic mice. Neurobiol Dis. 2011;41:338–52. doi: 10.1016/j.nbd.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny M, Tolan DR, Selkoe DJ. Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer's disease. Am J Pathol. 1991;138:1423–35. [PMC free article] [PubMed] [Google Scholar]
- Pryor NE, Moss MA, Hestekin CN. Unraveling the early events of amyloid-β protein (Aβ) aggregation: techniques for the determination of Aβ aggregate size. Int J Mol Sci. 2012;13:3038–72. doi: 10.3390/ijms13033038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Arancio O. Amyloid-β peptide: Dr. Jekyll or Mr. Hyde? J Alzheimers Dis. 2013;33:S111–S120. doi: 10.3233/JAD-2012-129033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Palmeri A. Hormetic effect of amyloid-beta peptide in synaptic plasticity and memory. Neurobiol Aging. 2012;33:1484–e15–24. doi: 10.1016/j.neurobiolaging.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci. 2006;103:11172–77. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, et al. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–54. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riascos D, de Leon D, Baker-Nigh A, Nicholas A, Yukhananov R, Bu J, et al. Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer's disease. Acta Neuropathol. 2011;122:565–76. doi: 10.1007/s00401-011-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, et al. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cog Neurosci. 2013;25:29–36. doi: 10.1162/jocn_a_00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D, Bell D, Podlisny M, Price D, Cork L. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987;235:873–77. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Shah P, Lal N, Leung E, Traul DE, Gonzalo-Ruiz A, Geula C. Neuronal and axonal loss are selectively linked to fibrillar amyloid-β within plaques of the aged primate cerebral cortex. Am J Pathol. 2010;177:325–33. doi: 10.2353/ajpath.2010.090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, et al. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–79. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Gouras GK. Synapses, synaptic activity and intraneuronal Aβ in Alzheimer's disease. Front Aging Neurosci. 2010;2:1–5. doi: 10.3389/fnagi.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, et al. Synaptic activity reduces intraneuronal Aβ, promotes APP transport to synapses, and protects against Aβ-related synaptic alterations. J Neurosci. 2009;29:9704–13. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, et al. A mouse model of amyloid beta oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–56. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-K, Nagykery N, Hersh LB, Scinto LFM, Geula C. Selective age-related loss of calbindin-D28k from basal forebrain cholinergic neurons in the common marmoset (Callithrix jacchus) J. Neurosci. 2003;120:249–59. doi: 10.1016/s0306-4522(03)00248-3. [DOI] [PubMed] [Google Scholar]
- Wu C-K, Thal L, Pizzo D, Hansen L, Masliah E, Geula C. Apoptotic signals within the basal forebrain cholinergic neurons in Alzheimer's disease. Exp Neurol. 2005;195:484–96. doi: 10.1016/j.expneurol.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–82. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Youmans KL, Tai LM, Kanekiyo T, Stine WB, Jr, Michon S-C, Nwabuisi-Heath E, et al. Intraneuronal Aβ detection in 5xFAD mice by a new Aβ-specific antibody. Mol Neurodegener. 2012;7:8. doi: 10.1186/1750-1326-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]