Abstract
The mechanisms that regulate the efficacy of thymic selection remain ill-defined. The method presented here allows in vivo analyses of the development and selection of T cells specific for self and foreign antigens. The approach entails implantation of thymic grafts derived from various aged mice into immunodeficient scid recipients. Over a relatively short period of time the recipients are fully reconstituted with T cells derived from the implanted thymus graft. Only thymocytes seeding the thymus at the time of isolation undergo selection and develop into mature T cells. As such, changes in the nature and specificity of the engrafted T cells as a function of age-dependent thymic events can be assessed. Although technical expertise is required for successful thymic transplantation, this method provides a unique strategy to study in vivo a wide range of pathologies that are due to or a result of aberrant thymic function and/or homeostasis.
Keywords: Immunology, Issue 99, Immunology, thymus, transplantation, tolerance, negative selection, T cell development
Introduction
The thymus is an organ in which critical events in T cell development occur1. Resident thymocytes, upon rearrangement of the T cell receptor (TCR) α and β genes, undergo a series of interactions with lymphostromal and antigen presenting cells (APC) in the cortical and medullary regions of the thymus2. Thymic positive selection is mediated by cortical thymic epithelial cells (TEC) to produce thymocytes that recognize antigenic peptides in the context of host major histocompatibility complex (MHC) class I and II molecules2-3. Subsequent thymic negative selection entails purging of autoreactive thymocytes, driven by an interaction with medullary TEC or dendritic cells (DC) that present peptides derived from self-proteins bound by MHC class I and II molecules3. The end result of these processes is the establishment of a pool of mature CD4+ and CD8+ T cells able to respond to a broad spectrum of foreign antigens while exhibiting minimal reactivity to self-proteins4.
The efficiency of thymic selection events is influenced by a host of factors, including thymic maturation, frequency of medullary and cortical TEC, subset composition of thymic DC, and the source of thymic precursors3. Notably, aberrant thymic selection can result in autoimmune5 or immunodeficient pathologies, which arise from impaired negative or positive selection, respectively. The molecular events regulating thymic selection, however, are poorly understood. In vitro approaches such as reaggregate thymic organ cultures (RTOC)6, have proven to be useful for analyzing basic events associated with thymic selection, but fail to fully recapitulate the dynamics of ongoing in vivo events. As a result, this thymic transplantation-based approach was established to better study thymocyte selection events in vivo7.
This protocol describes transplanting thymi from newborn and adult donor mice into immunodeficient scid recipient mice. This technique permits the study of mechanisms that regulate positive and negative thymic selection, as well as thymic output of various T cell subsets during ontogeny. Most recently, this approach has been used to demonstrate that the efficiency of thymic selection is limited early after birth in mice leading to increased development of autoreactive T cells, and a reduced T cell repertoire specific for foreign antigens7.
Protocol
The murine studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina Chapel Hill and all animal care was in accordance with the IACUC guidelines.
1. Preparation of Newborn and Adult Thymi
Prepare all reagents and equipment prior to euthanizing donor mice.
Sterilize surgical instruments by autoclaving or other appropriate methods. All surgical procedures must be performed under a laminar flow hood to maintain sterile conditions and avoid contamination. Assemble the tools needed for extraction of thymi from donor mice.
Fill a 60 mm dish with sterile 1x PBS (pH 7.4) and place on ice inside the hood. This will be used to briefly store thymi excised from donor mice prior to transplantation.
As per ethical guidelines, euthanize newborn donor mice by decapitation and adult donor mice by CO2 asphyxiation followed by cervical dislocation.
For removal of the thymus: Lay the mouse in a dorsal recumbent position on a sterile absorbent paper towel and spray with 70% ethanol prior to making an incision.
Expose the abdominal and thoracic cavity by making a midline incision through the skin. Fold the skin over the chest and forelimbs to reveal the thoracic cavity.
Make two lateral incisions through the diaphragm and ribcage to expose the superior mediastinum and anterior thoracic cavity. The thymus should be visible as two white lobes immediately above and adjacent to the heart.
Tease apart the connective tissue surrounding the thymus with fine forceps, making certain not to disrupt the capsule. While holding back the ribcage with forceps, use a second pair of forceps to extract the two lobes of the thymus by positioning curved forceps underneath the organ and pulling vertically. This can be done using a dissecting scope for extracting thymi from newborn mice.
Place the thymus in the 60 mm dish containing sterile 1x PBS (pH 7.4) on ice and separate thymic lobes by cutting through the connective isthmus. Remove any debris from the thymic lobes making certain not to damage the capsule, and cut the thymus into the appropriate number of sections for transplantation.
Do not manipulate the thymi obtained from newborn mice. NOTE: Used for a maximum of two recipient mice (1 lobe per recipient).
Transplant the thymi obtained from adult mice into a maximum of 4-6 recipient mice. Using a pair of forceps, carefully grasp one adult thymic lobe, as shown in Figure 1, cut the thymus into three equal sections using surgical scissors.
Repeat steps 1.3-1.4 for each donor mouse. In order to limit the time thymi are exposed, prepare 1 donor thymus at a time.
2. Thymus Implantation Under the Kidney Capsule
Assemble the pre-sterilized equipment listed in Table 1. Proper aseptic technique should be utilized during the course of the procedure to prevent exposing transplant-recipients to contaminated tools or reagents.
Prior to transplantation, weigh and tag each recipient mouse.
Set up the dissecting microscope and anesthesia circuit in the laminar flow hood.
Using an electric razor, shave the left side of the recipient mouse and ensure no hair remains around the area used to make the incision.
Turn on isofluorane vaporizer and anesthetize the mouse using a dose of between 1-2%. Ascertain proper anesthetization prior to starting the surgical procedure by checking for an absence of reflex following toe pinch.
After the mouse is properly anesthetized, apply veterinary ointment to the eyes of the mouse to prevent dryness and prepare the mouse for transplantation as follows:
Position the mouse under the dissecting microscope in a right lateral recumbent position so that the shaved side is facing up. Starting in the center of the surgical area, dispense in a circular motion using a disposable transfer pipette 70% ethanol, followed by povidone-iodine (betadine). Repeat the ethanol/betadine treatment 3 times prior to making an incision.
Using dissecting scissors make a 1-2 cm flank incision directly above the kidney.
Using medium forceps grasp connective tissue adjacent to the kidney and gently lift the kidney so that it lies atop the musculature. To keep the kidney exposed and in place over the musculature, insert one arm of medium forceps underneath the kidney, paying special attention not to disrupt the renal ilium.
In order to prevent tissue desiccation, irrigate the kidney with sterile 1x PBS (pH 7.4) during each step until manipulation of the kidney and kidney capsule is complete.
Using fine forceps pinch and lift the capsule near the edge of the kidney most distal to the adrenal glands to separate it from the kidney.
Use an 18 gauge needle to make an incision large enough to insert the prepared piece of donor thymus (Figure 2A). Make certain to keep the capsular incision as small as possible to prevent dislocation of the graft over time.
While keeping the capsule pulled away from the kidney, use a second pair of fine forceps to insert the graft underneath the capsule and push the thymus as far forward from the capsular incision as possible (Figure 2B).
Remove the forceps from underneath the kidney, and gently return the kidney back into place through the incision.
Close the musculature by suturing the peritoneal wall and applying betadine once closed. After suturing is complete, apply wound clips to close the dermis and apply betadine to the surrounding area.
Return the mouse to a cage that has been warmed using a heat lamp.
Monitor each post-operative mouse until it regains full consciousness and mobility and do not place mice that have not recovered following anesthesia into a cage with other animals.
Treat mice with post-operative pain management drugs as needed. Provide mice with post-operative analgesics such as acetaminophen in the drinking water at a concentration of 1.6 mg/ml.
Repeat steps 2.3-2.9 for each recipient mouse.
Ensure that mice return to normal activity within 1 hr post-surgery. Monitor post-operative weight, motor skills, as well as the drinking and feeding activity of each mouse to determine complications arising as a result of the transplant procedure. NOTE: Post-operative weight loss, when compared to pre-operative weight, as well as altered mobility or failure to eat or drink may indicate complications.
Monitor T cell reconstitution in peripheral blood by bleeding mice via tail nick followed by separation of lymphocytes using Lympholyte cell separation media according to the manufacturer’s specifications. Accomplish T cell characterization by staining lymphocytes with fluorescently conjugated antibodies specific for T cell markers CD3, CD4, and CD8, as well as a live dead discriminator such as a fluorescently activated succinimidyl ester8. Analysis as shown in Figure 3 can be accomplished via gating on singlets, live cells, and CD3 positive cells.
Representative Results
The success of this procedure is dependent on minimal surgical trauma as well as the accurate positioning of the graft underneath the kidney capsule. The thymic graft should be cut to ensure appropriately sized thymic sections for subsequent transplantation as shown in Figure 1. Following the schematic in Figure 1, thymi from newborn or adult mice can be used for successful subcapsular transplantation with consistent and reproducible T cell engraftment results. As mentioned, appropriate positioning of the graft under the kidney capsule is important in maintaining long-term survival, function, and vascularization of the graft. As seen in Figure 2, the thymic graft should be placed atop the kidney, closest to the adrenal glands (anterior of kidney), on the opposite end from the capsular incision (posterior of kidney). A successful thymic transplant, when coupled with an appropriate hematopoietic environment, can remain productive in excess of 30 weeks7.
Following transplantation, engraftment is assessed by flow cytometric analyses of T cells obtained from peripheral blood. Figure 3 and Table 2 demonstrate the typical level of T cell engraftment observed in organs and peripheral blood in a transplant recipient 6 weeks post-transplantation. Using this technique, our group has previously shown the kinetics of CD4+ and CD8+ T cell reconstitution in peripheral blood over time in transplant recipients of newborn and adult thymi. Briefly, circulating T cells can be observed in the periphery within one week post-surgery and CD4+ and CD8+ T cell numbers continue to increase until the peripheral T cell compartment is fully reconstituted at 5-6 weeks post transplantation7.
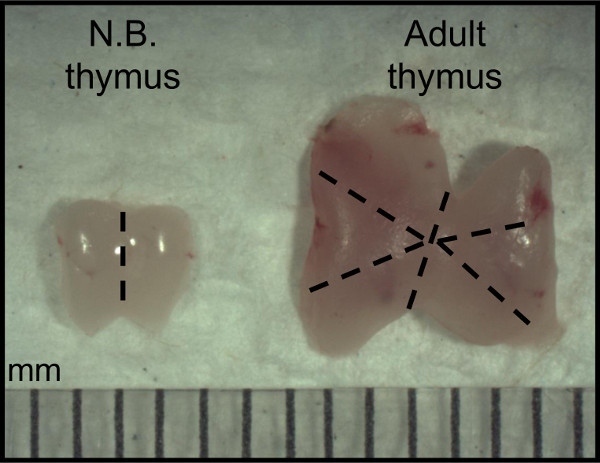 Figure 1: Preparation of thymic lobes and sections from donor newborn and adult mice. Donor thymi are excised from 1 day-old (N.B.) and 4 week-old (Adult). Dashed lines are presented as a guide for preparing the maximum number of sections from one donor organ for subsequent transplantation.
Figure 1: Preparation of thymic lobes and sections from donor newborn and adult mice. Donor thymi are excised from 1 day-old (N.B.) and 4 week-old (Adult). Dashed lines are presented as a guide for preparing the maximum number of sections from one donor organ for subsequent transplantation.
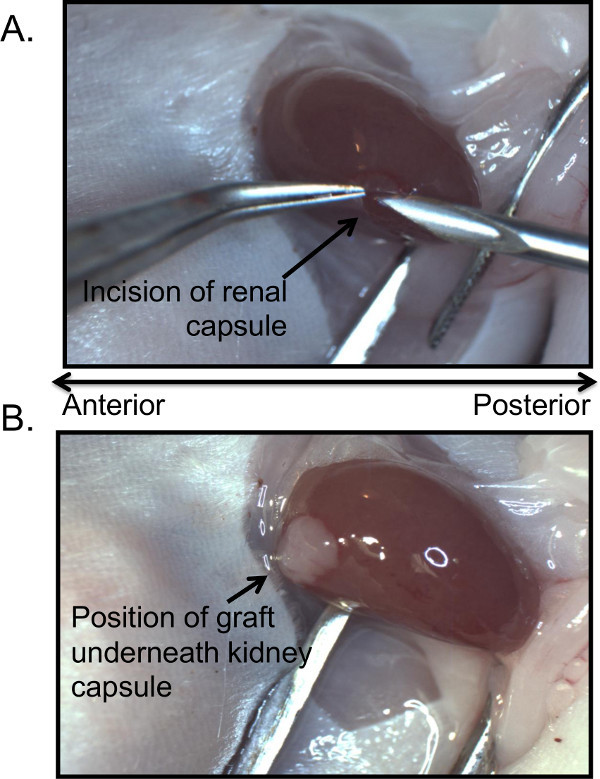 Figure 2: Position of capsular incision and placement of thymic graft. An appropriately sized section prepared from the donor thymus is inserted into the subcapsular space created by a small incision in the renal capsule at the posterior end of the kidney (A). Anterior to the kidney, closest to the adrenal glands (not pictured) and at the opposite end from the capsular incision, is the optimal position for the graft that is inserted underneath the capsule as shown in (B).
Figure 2: Position of capsular incision and placement of thymic graft. An appropriately sized section prepared from the donor thymus is inserted into the subcapsular space created by a small incision in the renal capsule at the posterior end of the kidney (A). Anterior to the kidney, closest to the adrenal glands (not pictured) and at the opposite end from the capsular incision, is the optimal position for the graft that is inserted underneath the capsule as shown in (B).
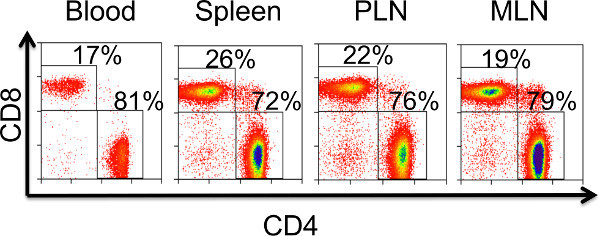 Figure 3. Determining successful engraftment via T cell reconstitution in peripheral blood and lymphoid organs. Flow cytometric data are representative of T cell reconstitution observed 6 weeks post-transplantation in a scid recipient of a N.B. thymic graft. Similar levels of T cell engraftment are observed in peripheral blood, spleen, pancreatic lymph node (PLN), and the mesenteric lymph node (MLN) following successful transplantation of a thymic graft.
Figure 3. Determining successful engraftment via T cell reconstitution in peripheral blood and lymphoid organs. Flow cytometric data are representative of T cell reconstitution observed 6 weeks post-transplantation in a scid recipient of a N.B. thymic graft. Similar levels of T cell engraftment are observed in peripheral blood, spleen, pancreatic lymph node (PLN), and the mesenteric lymph node (MLN) following successful transplantation of a thymic graft.
| Fine Dissecting forceps |
| Medium Dissecting forceps (2) |
| Fine dissecting forceps (curved tip) |
| Betadine Solution |
| 70% Ethanol |
| 18 gauge needle |
| Dissecting microscope w/light source |
| Disposable transfer pipettes (2) |
| Drugs for pain management: Acetaminophen |
| Sterile wipes |
| Heat lamp |
| Electric razor |
| Isoflurane and isoflurane vaporizer |
| Sutures (fast absorbing, plain gut) |
| Dissecting Scissors |
| 9 mm Stainless Steel Wound clips |
| Wound clip removal tool |
| Sterile 1x PBS |
| 60 mm Dish |
Table 1: Materials needed during surgical thymic transplantation. List of necessary materials used to implant the thymus underneath the kidney capsule of the recipient mouse. The quantity of specified materials is listed in parenthesis.
| Total Number of CD4 T cells | Total Number of CD8 T cells | |
| Blood | 1,652 cells/υl | 351 cells/υl |
| Spleen | 11,546,617 | 4,203,299 |
| PLN | 1,241,789 | 364,918 |
| MLN | 2,182,266 | 532,059 |
Table 2. Total number of T cells present in peripheral blood and lymphoid organs of a thymic transplant recipient. Total numbers of CD4+ and CD8+ T cells recovered from the organs as well as peripheral blood of a newborn thymic transplant recipient 6 weeks post-transplantation. These numbers are representative of CD4+ and CD8+ T cell cellularity that is typically observed in successful newborn and adult thymic transplant recipients.
Discussion
Events regulating thymic selection are poorly understood. Recent studies have demonstrated ontogenic changes in the efficiency of selection. Coupling this approach with different transgenic thymus donor and recipient mice will further facilitate studies to identify the events and parameters that regulate thymic selection. Notably, thymic transplantation is used for the treatment and management of T cell and thymic disorders seen for instance in athymic infants lacking functional T cells and in patients with DiGeorge syndrome, characterized by thymic hypoplasia or thymic aplasia9-10. Thymus transplantation, like most organ transplantation, is not free of complications, which are mostly due to transplant tolerance-related complications12 and development of autoimmune pathologies11. These issues, coupled with the need to better understand and characterize mechanisms involved in thymocyte tolerance induction led to the development of this method. The protocol described herein experimentally models thymic transplantation and demonstrates that efficient T cell reconstitution is obtained in recipients.
There are several key points to note in the protocol. It is necessary to be adept at surgery and aseptic technique prior to performing the transplantation surgery. Adherence to this will help minimize trauma, the time the mouse is anesthetized, and recovery time so that 100% post-surgery survival rate can be readily obtained. When preparing the donor thymus, it is imperative that the organ is kept on wet ice to minimize cell death. In addition the thymic lobes need to be cut to appropriate sized fragments to permit implantation without tearing the kidney capsule. Similarly, care is required to prevent any injury to the kidney upon making the incision of the renal capsule. Placement of the thymic graft underneath the kidney capsule is another important step in the process. Injury to the kidney or tearing of the renal capsule can be avoided with gentle handling, using appropriate fine-tipped surgical tools, and sufficient practice. One possibility to note is that successful T cell engraftment may also be achieved by implanting thymic lobes at sites other than under the kidney capsule, for instance at a subcutaneous site. The kidney however provides an ideal environment to receive thymic grafts due to a high level of vascularization, which promotes both graft survival as well as efficient T cell trafficking following thymic selection.
A distinct advantage of the above thymus transplant protocol is the technical ease in determining T cell engraftment. Using flow cytometry, success of the procedure is qualitatively and quantitatively assessed by measuring the frequency of T cells in peripheral blood. In addition to observing mature T cells in the blood, T cell engraftment is detected in various lymphoid organs. The latter permits further study of the expansion, activation, and differentiation of peripheral T cells in various host genetic environments under physiological conditions. One caveat that investigators may consider when interpreting results is that following thymic engraftment into an immunodeficient recipient, newly selected T cells will undergo lymphopenic expansion in the periphery. Although this rapid expansion will not alter thymic selection events, it may lead to preferential expansion of distinct T cell subsets in peripheral tissues.
This protocol is widely applicable for studying mechanisms regulating T cell development and homeostasis in a variety of pathologies. For example, this approach can be used to study FoxP3 regulatory T cell generation and development7,13-14, as well as T cell renewal and export from the thymus in in vivo models of persistent viral, fungal, or bacterial infection15. In summary, this article describes a method of thymus transplantation that entails minimal manipulation of donor tissue, and a high success rate once the technique has been mastered.
Disclosures
The authors do not have any competing financial interests to disclose.
Acknowledgments
This work was supported by funding received from the National Institutes of Health (1R01AI083269).
References
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat. Rev. Immunol. 2014;14(6):377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RH. Acquisition of immunologic self-tolerance. Cell. 1989;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat. Immunol. 2001;2(11):1025–1031. doi: 10.1038/ni726. [DOI] [PubMed] [Google Scholar]
- Anderson G, Jenkinson EJ. Investigating central tolerance with reaggregate thymus organ cultures. Methods. Mol. Biol. 2007;380:185–196. doi: 10.1007/978-1-59745-395-0_11. [DOI] [PubMed] [Google Scholar]
- He Q, et al. Thymic development of autoreactive T cells in NOD mice is regulated in an age-dependent manner. J. Immunol. 2013;191(12):5858–5866. doi: 10.4049/jimmunol.1302273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CC, Stewart SJ. Current Protocols in Cytometry. John Wiley & Sons, Inc; 2001. Chapter 6. Immunophenotyping. [DOI] [PubMed] [Google Scholar]
- Rice HE, et al. Thymic transplantation for complete DiGeorge syndrome: medical and surgical considerations. J. Pediatr. Surg. 2004;39(11):1607–1615. doi: 10.1016/j.jpedsurg.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Markert ML, et al. Transplantation of thymus tissue in complete DiGeorge Syndrome. N. Engl. J. Med. 1999;341:1180–1189. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- Market ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin. Immunol. 2010;135(2):236–246. doi: 10.1016/j.clim.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AD, Li X, Strom TB, Turka LA. The role of peripheral T-cell depletion in transplantation tolerance. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356(1409):617–623. doi: 10.1098/rstb.2001.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque AS, et al. Human FOXN1-Deficiency is associated with ab double-negative and FoxP3+ T-cell expansions that are distinctly modulated upon thymic transplantation. PLoS One. 2012;7(5):e37042. doi: 10.1371/journal.pone.0037042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn IK, Milner JD, Scheinberg P, Douek DC, Markert ML. Thymus transplantation restores the repertoires of forkhead box protein 3 (FoxP3)+ and FoxP3- T cells in complete DiGeorge anomaly. Clin Exp Immunol. 2013;173(1):140–149. doi: 10.1111/cei.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006;2(6):e62. doi: 10.1371/journal.ppat.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]


