Abstract
Escherichia coli is the leading cause of urinary tract infections (UTIs), one of the most common infections in humans. P fimbria was arguably the first proposed virulence factor for uropathogenic E. coli, based on the capacity of E. coli isolated from UTIs to adhere to exfoliated epithelial cells in higher numbers than fecal strains of E. coli. Overwhelming epidemiologic evidence has been presented for involvement of P fimbriae in colonization. It has been difficult, however, to demonstrate this requirement for uropathogenic strains in animal models of infections or in humans. In this study, a signature-tagged mutagenesis screen identified a P-fimbrial gene (papC) and 18 other genes as being among those required for full fitness of cystitis isolate E. coli F11. A P-fimbrial mutant was outcompeted by the wild-type strain in cochallenge in the murine model of ascending UTI, and this colonization defect could be complemented with the cloned pap operon. To our knowledge, this study is the first to fulfill molecular Koch's postulates in which a pathogenic strain was attenuated by mutation of pap genes and then complemented to restore fitness, confirming P fimbria as a virulence factor in a pathogenic clinical isolate.
Keywords: UPEC, UTI, transposon mutagenesis, F11, uropathogenic
Our study confirms for the first time papC as a virulence factor by molecular Koch's postulates in a uropathogenic Escherichia coli clinical isolate.
Graphical Abstract Figure.
Our study confirms for the first time papC as a virulence factor by molecular Koch's postulates in a uropathogenic Escherichia coli clinical isolate.
INTRODUCTION
Extraintestinal pathogenic Escherichia coli (ExPEC) strains are capable of colonizing niches distinct from the gut environment and can cause severe infections in humans, including neonatal meningitis, urinary tract infections (UTIs) and sepsis. UTIs are among the most prevalent bacterial infections in humans and in some cases can require extensive medical attention. Each year, as many as 1–2 million people suffering from UTIs visit the emergency department and almost 400 000 infections are severe enough to require hospitalization (Litwin et al. 2005). Antimicrobial drug resistance among ExPEC, steadily on the rise, adds further to the difficulty of treating these infections (DeBusscher et al. 2009; Schito et al. 2009). Total costs to the healthcare system exceed 3 billion dollars annually, making UTIs also a substantial economic burden (Litwin et al. 2005). The majority of UTIs progress stepwise from initial colonization by microorganisms of the periurethral area, followed by entrance into the urethra, and ascension into the bladder, resulting in inflammation or cystitis. More severe complications of UTIs can occur when bacteria travel through the ureters into the kidneys, producing pyelonephritis and, in some cases, later bypass the kidney parenchyma to reach the bloodstream or lymphatic system, resulting in bacteremia and potentially sepsis.
Adhesion organelles, known as fimbriae, mediate colonization by ExPEC of the urinary tract, a necessary step in the establishment of UTIs (Eden and Hansson 1978; Johnson 1991; Mobley et al. 1993; Hung et al. 2002). Epidemiologic studies have shown a positive correlation between ExPEC strains and the presence of genes encoding for type 1, P, S, F1C, Dr and Auf fimbriae, as well as other non-fimbrial adhesins (O'Hanley et al. 1985; Ott et al. 1986; Nowicki et al. 1989; Mobley et al. 1993; Connell et al. 1996 Spurbeck et al. 2011). Among these fimbriae, only type 1 fimbriae, which are encoded by the fim operon, have been proven in animal models as a virulence factor according to the conditions of molecular Koch's postulates (Connell et al. 1996). It was demonstrated in the murine model of UTI using clinical isolates that mutations within the fimH adhesin gene or associated with its promoter resulted in reduced virulence. Virulence was restored following complementation with a plasmid encoding a functional fim operon (Connell et al. 1996).
Although P fimbria was arguably the first virulence determinant associated with ExPEC strains (Kallenius et al. 1981), it has been difficult to directly demonstrate a requirement for P fimbria as a colonization factor in UTI. Clinical studies have shown a positive correlation between ExPEC strains that cause pyelonephritis and the presence of the genes-encoding P fimbriae (Kallenius et al. 1981; Johnson 1998, 2003). However, in a murine model of ascending UTI, no differences could be detected in colonization or histological findings between pyelonephritis ExPEC strain CFT073 and its isogenic mutant strain deleted for genes in both copies of the pap operon and unable to produce fully assembled P fimbria (Mobley et al. 1993). Conversely, in a primate model in which bacteria were inoculated directly into the ureter, pyelonephritis ExPEC strain DS17 persisted significantly longer in the urinary tract than its isogenic papG mutant, which was unable to cause acute pyelonephritis (Roberts et al. 1994). However, the papG mutant was still capable of colonizing the bladder, resulting in cystitis, similar to what was observed with the wild-type strain. Complementation studies were not performed in either study and therefore molecular Koch's postulates were not fulfilled (Falkow 1988). A volunteer study using E. coli 83972, which was isolated from a case of asymptomatic bacteriuria and also does not express functional P fimbriae, showed that following transformation with the pap operon, bacteriuria was established faster and to a higher concentration than wild-type E. coli 83972 (Wullt et al. 2000). However, this study was not conducted using a virulent isolate, and by using a plasmid-based operon, was not a comparison of isogenic mutants. Furthermore, the data suggest that P and type 1 fimbriae were not required for persistence of E. coli 83972 in the human urinary tract, since both the isogenic papG and papGfimH mutants of this avirulent strain were still able to colonize the human bladder up to 3 months following infection (Hull et al. 2002).
The lack of a defined set of virulence factors in ExPEC strains suggests that a combination of known and unidentified virulence factors may dictate fitness during infection. Signature-tagged mutagenesis (STM) is a useful and unbiased technique to identify genes involved with bacterial survival in vivo by screening a pool of mutants simultaneously within a limited number of animals (Hensel et al. 1995). This method has been successfully used to both discover and confirm virulence and fitness genes in other pathogens (Mei et al. 1997; Chiang and Mekalanos 1998; Sanschagrin, Kukavica-Ibrulj and Levesque 2008). Previously, we applied STM to the pyelonephritis ExPEC strain CFT073 using a mouse model of ascending UTI to identify 19 mutants with reduced fitness from a non-saturating library of 2049 mutants (Bahrani-Mougeot et al. 2002). Of these 19 mutants, 8 had mutations in six different sites within the type 1 fimbrial locus. Several other survival-defective mutants had disruptions in genes responsible for the production of extracellular polysaccharides, metabolic pathways as well as within genes of unknown function. However, that study had a number of limitations. It was performed using a single strain of a particular serotype (O6:K2:H1), originally isolated from a case of pyelonephritis. Furthermore, in the murine model of UTI, this strain regularly colonizes the kidneys, and therefore may not be fully representative of other ExPEC.
In this study, we continue to take advantage of STM and the CBA murine model of ascending UTI to identify virulence or fitness genes in the cystitis isolate ExPEC strain F11. Escherichia coli F11 is a member of a clonal group commonly found in human UTI that includes the prototypic serotype O6:K15:H31 isolate 536. F11 is highly pathogenic in the murine model of ascending UTI, as it is capable of colonizing the bladder to a higher concentration and more rapidly than ExPEC pyelonephritis isolate CFT073 (Johnson et al. 1998). We tested the hypothesis that some virulence or fitness factors are unique to cystitis and pyelonephritis strains and can be identified using techniques such as STM. In the original STM study (Bahrani-Mougeot et al. 2002), we did not identify a P-fimbrial mutant. However, we recognized that doing so was unlikely given that there are two complete and functional pap operons in strain CFT073 (Mobley et al. 1993; Welch et al. 2002). Here we report that an STM screen of a cystitis strain that carries only a single pap operon did yield an attenuated papC mutant deficient in synthesis of P fimbriae. In cochallenges of the murine model, pap-negative mutants were outcompeted by the wild-type strain, and the loss of fitness could be complemented with the pap operon in trans. Thus, we have satisfied molecular Koch's postulates and conclude that P fimbriae contribute to the full colonization potential of ExPEC strains.
MATERIALS AND METHODS
Bacterial strains, growth conditions and plasmids. Table 1 lists the bacterial strains, growth conditions and the plasmids used for this study. Escherichia coli strain F11, originally cultured from a case of cystitis, served as the recipient strain during transposon mutagenesis (Stapleton, Moseley and Stamm 1991; Johnson et al. 1998). Donor strains consisted of E. coli S17 λpir strains transformed with pUT/mini-Tn5km2 (AmpRKanR) plasmid carrying a unique pUT/mini-Tn5 sequence. All pUT/mini-Tn5 signature tags were tested for non-cross-reactivity (de Lorenzo et al. 1990; Herrero, de Lorenzo and Timmis 1990). Bacteria were cultured at 37°C in Luria-Bertani (LB) medium or on Luria agar with the addition of the appropriate antibiotics to provide selective pressure at the following concentration: ampicillin 50 μg mL−1; chloramphenicol, 50 μg mL−1; nalidixic acid, 50 μg mL−1; and rifampin 50 μg mL−1. Strains were stored at –70°C in a 1:1 ratio of glycerol and LB medium. For RNA preparation, E. coli was cultured on Luria agar for 18 h at 37°C with biological replicates being inoculated on independent plates to induce pap gene expression (Blyn et al. 1989). Bacteria were resuspended in phosphate-buffered saline (PBS) to a final OD600 of 1.0. Bacteria were treated with a stop solution (5% phenol, 95% EtOH), harvested by centrifugation (13 000 rpm, 10 min, 4°C), and the pellet was stored at –20°C prior to RNA extraction.
Table 1.
Bacterial strains and plasmids used in this study.
| Strains | Description | Reference |
|---|---|---|
| E. coli | ||
| F11 | UPEC strain (cystitis isolate, O6:K2:H31); NalR | Stapleton et al. (1991) |
| F11 L-ON | F11 with fim invertible element phase L-ON | Snyder et al. (2006) |
| F11 L-OFF | F11 with fim invertible element phase locked off | Snyder et al. (2006) |
| S17 λpir | Conjugative donor strain | Miller and Mekalanos (1988) |
| CFT073 | UPEC strain (pyelonephritis isolate, O6:K2:H1) | Welch et al. (2002) |
| UPEC76 | Deletion derivative of E. coli CFT073 with both copies of P fimbrial operon disrupted | Mobley et al. (1993) |
| DH5α | Donor strain for cloning | Hanahan (1985) |
| Plasmids | ||
| pUT/mini-Tn5km2 | Suicide vector for transposon delivery (AmpRKanR) | de Lorenzo et al. (1990) |
| Cosmid 1-B10 | Encodes for CFT073 genes papIAHCDJKEFG–2 | Mobley et al. (1993) |
| Cosmid 1-E8 | Encodes for CFT073 genes papIAHCDJKEFG–2 | Mobley et al. (1993) |
| pWSK29 | Empty cloning vector | Wang and Kushner (1991) |
| pXLW34 | pWSK29 containing papIAHCDJKEFG–2 and into BamHI-and SalI digested plasmid transformed | This study |
Construction of E. coli F11 signature-tagged mutant library
For conjugation, overnight cultures of S17 λpir donor strains, each being transformed with a unique signature tag carried on the pUT/mini-Tn5km2 (AmpRKanR) plasmid (Hensel et al. 1995), and E. coli F11 (NalR) were mixed 1:1 in the wells of a 96-well microtiter plate. Aliquots of each mixture were plated on LB agar (Hensel et al. 1995; Mei et al. 1997). To confirm transposon insertion, isolated colonies were cultured overnight at 37°C on LB supplemented with nalidixic acid and kanamycin. Additional screening for ampicillin sensitivity confirmed the loss of the pUT plasmid among the transconjugants. This process was repeated to generate 1334 transposon mutants each carrying 1 of the 46 unique sequence tags. Transposon mutants were grouped and placed into individual wells of a 96-well microtiter plate, resulting in 29 pools with each pool having one representative of each of the uniquely tagged sequences. Each pool also contained two controls for identification of cross-reactivity with the blotting membrane. Mutants were stored in LB containing 20% glycerol at –70°C.
Mouse model of ascending UTIs
Infections were carried out in a previously described CBA mouse model of ascending UTI (Hagberg et al. 1983; Johnson et al. 1987). For the mutant screen, 6 to 8-week-old female mice (Harlan Sprague-Dawley) were anesthetized with pentobarbital and 50 μL suspensions of overnight bacterial cultures containing 108 CFU of a pool of 48 uniquely tagged mutants were inoculated transurethrally. The input pool was standardized by calculating the optical density at 600 nm of a 1 mL sample of the initial inoculum and stored as a centrifuged pellet at –20°C. Mice were sacrificed at 48 h by the administration of an overdose of isoflurane. The bladder and kidneys were collected aseptically and homogenized in 1 mL aliquots of PBS. Homogenizations were plated on LB agar containing kanamycin and nalidixic acid. Following incubation, bacteria, now representing the output pools, were collected by washes with PBS (pH 7.2). The samples were standardized at an optical density of 600 nm, centrifuged and stored at –20°C.
Cochallenge infections were used to confirm attenuation of STM mutants. A total of 108 CFU per inoculum comprised of a 1:1 mixture of independent overnight cultures of wild-type E. coli F11 (5 × 107 CFU) and a single STM mutant (5 × 107 CFU) was inoculated into five mice. The inoculum was quantified by plating dilutions onto LB agar containing the appropriate antibiotics to provide selective pressure. For studies of P fimbriae in UTI, CFT073 containing the plasmid pWSK29, representing the control empty vector, was tested and compared to double pap mutant UPEC76 (Mobley et al. 1993) transformed with either the complementing pXLW34 carrying papIAHCDJKEFG–2 under control of their native promoter or the control empty vector. At 48 h, urine samples were collected and the bladder and kidneys were isolated, weighed and homogenized. A spiral plater was used to plate dilutions of the collected samples onto selective medium containing the appropriate antibiotics. Following overnight incubation, viable counts were represented as CFU mL−1 urine or CFU g−1 tissue with 102 CFU mL−1 urine or CFU g−1 tissue acting as the lower limit of detection for samples lacking colonies. A competitive index (CI) for each mutant from each sample site was calculated by dividing the ratio of mutant to wild-type strains in the output pool by the input pool, or inoculum, values of the same ratio.
Statistical analysis
For the independent infections, comparisons of the CFU mL−1 or CFU g−1 distributions were analyzed using the Mann–Whitney test. In contrast, the cochallenge data were analyzed using a repeated measure of analysis of variance with rank order data (STATA software) (Bahrani-Mougeot et al. 2002). P values less than 0.05 were considered significant. For quantitative real-time PCR (qPCR) experiments, an unpaired Student's t-test was used to analyze differences in gene expression between F11 and F11 L-ON (Prism; GraphPad Software).
Screening of STM mutants
To obtain DNA template for sequencing, more than 5000 CFUs were scraped from the plated output samples of the collected bladder or kidneys and resuspended in PBS. Samples were normalized to the same OD600 (Hensel et al. 1995). Proteinase K was used to lyse a fraction of each sample. Chromosomal DNA was used to generate labeled PCR products of the input and output pools using primers targeting the transposon insertion site (Table 2). The PCR products were labeled with digoxigenin-dUTP (Roche) before hybridization to a prepared DNA dot-blot membrane carrying the original uniquely tagged plasmids. Two controls to evaluate cross-hybridization were included with one tag being absent on the blot but common to the probes, while the other tag was only present in the blot. For the primary screen, mutants were considered to be attenuated in vivo and were further pursued if the output hybridization signal was weaker than the input hybridization signal in all three blots derived from the same organ or in at least four of the possible six blots. Confirmation of weak hybridization signals were conducted by testing the potentially attenuated mutants in single competition assays with wild-type strain F11. If found to be attenuated, DNA adjacent to the transposon junctions was amplified and sequenced. After several attenuated mutants with insertions in known fitness factors were identified in this manner, specifically colanic acid biosynthesis genes and the fim operon, the order of the screen was changed to sequence all mutants with confirmed weak hybridization signals, reserving confirmatory co-infection studies for mutants with insertions in previously unconfirmed genes.
Table 2.
Oligonucleotide primers used in this study.
| Primer | Direction of primer | Sequence (5′→3′) | Purpose |
|---|---|---|---|
| Donne340 | Forward | GGCCACGCGTCGACTAGTCANNNNNNNNNNACGCC | Amplification of variable region of DNA signature tags |
| Donne341 | Reverse | GGCCACGCGTCGACTAGTCANNNNNNNNNNGATAT | |
| Donne298 | Forward | AAAGCTTGCTCAATCAATC | Amplification of DNA adjacent to transposon junctions for sequencing |
| Donne299 | Reverse | AGCATAAAGCTTGCTCAATC | |
| Donne646 | Forward | CGGTAGCTATGGCAGTGGTGTCTTTTG | PCR screening for presence of pap operon |
| Donne792 | Reverse | CCCAGATATCCACAACACTCTATCC |
Identification of pap cosmid clones and subcloning of the pap operon
PCR primers, Donne646 and Donne792, specific to sequences located 7 bp upstream of the papA–2 start codon and 42 bp downstream of the papG–2, were used to locate the pap operon from a previously generated chromosomal DNA library of CFT073 (Mobley et al. 1993). The CFT073 library is arranged across 19 microtiter plates and is comprised of approximately 1834 clones. Initial PCR, using the eLongase enzyme system, involved isolating the plasmid DNA from clones pooled from each microtiter plate. A total of 14 of the 19 pooled samples contained the pap cosmid clones. From a selected positive plate, pooled plasmid DNA from each row and column was PCR amplified leading to the identification of two cosmid clones, 1-B10 and 1-E8, carrying the pap genes. Escherichia coli DH5α was transformed with cosmid 1-B10 or cosmid 1-E8 and passaged on LB agar plates with antibiotic selection. The presence of functional P fimbriae was confirmed using a previously described hemagglutination assay using human and sheep erythrocytes in the presence or absence of 50 mM α-methyl D-mannoside (Bahrani-Mougeot et al. 2002). The cosmid 1-E8 was selected for subcloning of the pap operon after confirming the presence of P fimbriae by positive agglutination.
Cosmid clone 1-E8 was digested using the restriction enzymes BamHI and SalI to yield a fragment approximately 10.7 kb containing the papIAHCDJKEFG–2 genes (the second of two pap operons in strain CFT073). The 10.7 kb BamHI/SalI fragment was isolated from an agarose gel and ligated into BamHI and SalI digested pWSK29 (Wang and Kushner 1991). The modified plasmid was transformed into E. coli DH5α to generate the recombinant clone, pXLW34, containing the papIAHCDJKEFG–2 genes.
RNA extraction and cDNA synthesis
Total RNA was isolated using the RNeasy Kit (Qiagen) according to the manufacturer's protocol. To remove contaminating DNA, RNA samples were treated with DNase (Ambion). The absence of chromosomal DNA was confirmed by PCR using Taq DNA polymerase. For cDNA synthesis, the RNA samples were reverse transcribed using the SuperScript II system for first-strand cDNA synthesis (Invitrogen). Briefly, a total of 1.5 μg of RNA was mixed with 50 ng random hexamers and 10 mM dNTPs based on the manufacturer's protocol. cDNA was purified using a PCR Purification Kit (Qiagen) and stored at –20°C.
Quantitative real-time PCR
RNA was harvested from E. coli F11 strains that were cultured for 18 h at 37°C on LB agar plates. Isogenic mutants of F11 were constructed by disrupting the invertible element that regulates fim expression so that expression of fim genes is no longer phase variable but is either locked on (L-ON) or locked off (L-OFF) (Snyder et al. 2006). Primers were generated for F11 fimA (forward—5′TGCACAAACAACCCTGAATAAC3′ and reverse—′AAGGTCGCATCCGCATTAG5′) and for F11 papA (forward—5′ GGGACGCTAATCTCCTGAAAG3′ and reverse—3′ AGGTTGCGACTGCAGAAA5′). Expression of gapA was used for normalization and was measured using previously published primers (Lane, Simms and Mobley 2007). To measure gene expression, 30 ng of cDNA was combined with Brilliant III Ultra Fast SYBR green QPCR mix (Agilent), 300 nM of forward and reverse primers, and Rox, which acted as a reference dye. Comparative quantitation was performed by the 2−ΔΔCt method (Livak and Schmittgen 2001) with F11 L-OFF serving as the calibrator for F11 and F11 L-ON. Experiments were performed using three biological replicates.
RESULTS
Screening of E. coli F11 signature-tagged mutants in a CBA mouse model of ascending UTI
A library of 1334 signature-tagged mutants of E. coli F11 was constructed (see the section ‘Materials and Methods’). A total of 48 uniquely tagged transposon mutants, including two controls for cross-hybridization, were assembled into 29 screening pools. All mutants were kanamycin-resistant and ampicillin-sensitive, indicating a legitimate transposition event.
For screening in the CBA mouse model of UTI, an input pool (108 CFU) of 48 mutants (∼2 × 106 CFU of each mutant) was transurethrally inoculated into the bladders of three female CBA mice. Two days post-inoculation, bacteria were cultured from urine, bladder and kidneys, and represent the output pools. Mutants were identified by PCR amplification of the unique sequence tags from both the input and output pools and hybridization of these identifiers with dot blots containing plasmids carrying the original sequence tags. Mutants from the initial screens with reduced hybridization signals in the output pools were regrouped and assayed by a secondary screen of single competition assays in five mice. The secondary screening yielded a total of 23 (1.7% of the total bank) putatively attenuated mutants (Table 3).
Table 3.
Confirmed attenuated STM mutants.
| Mutant | Gene/homologuea | Accession no. | Function | K-12b | CFT073c |
|---|---|---|---|---|---|
| 5-A3 | fimA | EDV68690 | Type-1 fimbrial subunit | + | + |
| 20-A4 | fimD | EDV68628 | Type-1 fimbrial usher | + | + |
| 29-G4 | fimD | EDV68628 | Type-1 fimbrial usher | + | + |
| 3-H2 | EcF11–0984 | EDV69299 | Colanic acid biosynthesis | – | + |
| 21-E6 | galE | EDV69028 | Colanic acid biosynthesis | – | + |
| 22-B3 | EcF11–0986 | EDV69113 | Colanic acid biosynthesis | – | + |
| 25-B3 | cpsB/EcF11–0983d | EDV69166/EDV69210 | Colanic acid biosynthesis | +/– | + / + |
| 5-C3 | papC | EDV68631 | P fimbrial usher | – | + |
| 2-H1 | kdpA | EDV65279 | Potassium transport | + | + |
| 5-E3 | narK | EDV68420 | Nitrate/nitrite transport | + | + |
| 12-B3 | ycjV | AAN80256 | Putative ABC transporter ATP-binding protein | + | + |
| 5-C5 | uidR | EDV65917 | Repressor of β-glucuronidase (uid) | + | + |
| 11-E6 | yeeP | EDV67436 | Putative GTPase | + | + |
| 6-B3 | evgA | EDV65560 | Positive transcription regulator of EvgSA two-component system | + | + |
| 24-E2 | cpxA | EDV66646 | Sensor histidine kinase | + | + |
| 18-C3 | nadB | EDV65323 | L-aspartate oxidase | + | + |
| 25-E2 | wzy | EDV69037 | Enterobacterial common antigen biosynthesis | – | + |
| 29-A4 | None | AAN80576 | Hypothetical protein | + | + |
| 2-F1 | EcF11–3365 | EDV65997 | Hypothetical protein | – | + |
| 19-E5 | yegP | EDV69027 | Hypothetical protein | + | + |
| 28-G6 | Nonee | ||||
| 7-E3 | Nonee | ||||
| 28-H2 | Nonee |
aGenetic locus with the closest match to the sequence interrupted by the transposon in each mutant.
b +, present in the genome of E. coli K-12 strain MG1655; –, absent from the MG1655 genome.
c +, present in the genome of E. coli strain CFT073; –, absent from the CFT073 genome.
dSequence data unable to differentiate between homologous genes
eGenetic locus was not sequenced.
Confirmation of attenuation in E. coli F11 by competitive cochallenge assays
Following identification by repeat screening of mutants putatively attenuated for colonization of the murine urinary tract, we performed competition colonization experiments by coinoculating the wild-type strain F11 and each mutant in a 1:1 ratio. After 48 h, the urine, bladder and kidneys were collected, homogenized and CFU were calculated by plating onto selective medium. A CI, as described in the section ‘Materials and Methods’, was calculated for each mutant, and statistical analysis was performed to quantify the differences between the recovered ratios of mutant to wild type. A total of 23 mutants (1.7% of the pool) were confirmed that exhibited statistically significant attenuation in cochallenge or had transposon insertions in genes that had already been identified as virulence factors (Table 3). In some cases, we observed multiple independent transposon events affecting the same gene, resulting in a final tally of 19 candidate genes. These genes can be described initially as ‘fitness factors’ until mutants are assessed in independent challenge and subjected to complementation.
Among these mutants, three carried insertions disrupting fim operon genes encoding type 1 fimbria, four mutants had insertions in colanic acid biosynthesis genes and one had an insertion in wzy encoding enterobacterial common antigen. All of these loci had previously been identified in an STM screen using pyelonephritis isolate CFT073 (Bahrani-Mougeot et al. 2002).
Additionally, a number of genes involved in transport across membranes were also identified including kdpA, an ATP-dependent P-Type ATPase potassium transporter, narK, a nitrate/nitrite antiporter and ycjV, a putative ABC transporter. Mutants carrying insertions in genes encoding transcriptional regulators uidR and evgA were also attenuated. As well, yeeP, a predicted GTP-binding protein, and nadB, an L-aspartate oxidase involved in nicotinamide adenine dinucleotide (NAD) biosynthesis, were identified as fitness genes from our screen. Finally, we observed that insertion in papC, encoding the usher for P-fimbria biogenesis, resulted in attenuation during colonization of the murine model.
Measurement of crosstalk between type 1 and P-Fimbriae gene expression in E. coli F11
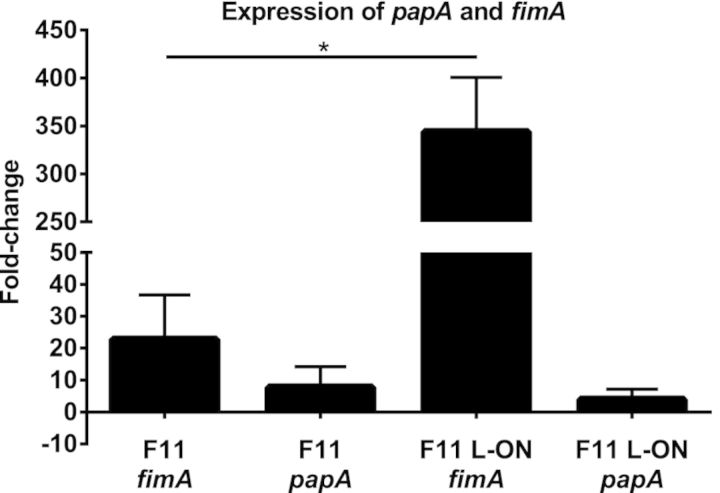
qPCR was used to measure cross regulation of type 1 and P-fimbrial genes in E. coli F11. Expression of fimA and papA was quantified in F11 and in two isogenic strains that have been mutated such that the invertible element that controls fim gene expression is phase locked to either constitutively express fimA (F11 L-ON) or to repress expression of fimA (F11 L-OFF) (Snyder et al. 2006). All strains were cultured independently on LB agar for 18 h at 37°C to favor production of P fimbriae (Blyn et al. 1989). F11 L-OFF was set as the baseline ‘calibrator’, and expression of gapA acted as the normalizing internal control. As expected, qPCR demonstrated that fimA expression was 22.7-fold higher in F11 and 344-fold higher in F11 L-ON compared to F11 L-OFF (Fig. 1). Expression of fimA was significantly higher in F11 L-ON compared to F11 (P < 0.01). However, papA expression was only 7.77- fold higher and 3.93-fold higher in F11 L-ON compared to F11 L-OFF, and the expression of papA in F11 and F11 L-ON was not statistically different (P = 0.403). Thus, expression of type 1 fimbriae does not influence expression of P fimbriae in F11.
Figure 1.
qPCR analysis of fimA and papA expression in E. coli F11 and type 1 fimbrial phase-locked mutants. The black bars represent the change in gene expression (n-fold) of fimA and papA between wild-type F11 and F11 L-ON. F11 L-OFF was used as the calibrator for calculating the fold-change in expression of fimA and papA. Results represent three independent experiments, and the error bars show standard deviation. Significant differences in gene expression were determined using an unpaired Student's t-test. *, P < 0.01.
P fimbriae enhance urinary tract colonization by CFT073
In prior studies, no difference in urinary tract colonization was detected in independent challenges using a wide range of inoculum doses (105–109 CFU) between strain CFT073 and mutant construct UPEC76, which had deletions in both of its pap operons (Mobley et al. 1993). However, the identification and confirmed attenuation of an E. coli F11 papC mutant in the current screen prompted us to re-examine the contribution of P fimbriae in the murine model of UTI by a more sensitive co-infection method. Accordingly, we cloned one of the pap operons from strain CFT073 into a low-copy-number plasmid and transformed the plasmid, designated pXLW34, into UPEC76 (the double pap mutant).
Consistent with expression of functional P fimbriae, clones were capable of mannose- resistant hemagglutination of human and sheep erythrocytes (data not shown). Therefore, plasmid pXLW34 restored the ability of UPEC76 to produce functional P fimbriae. To control for the effect of the plasmid, we transformed the empty vector into both CFT073 and UPEC76. Then, we compared CFT073 containing the vector to either UPEC76 with the vector or UPEC76 with the cloned pap operon in co-infection studies.
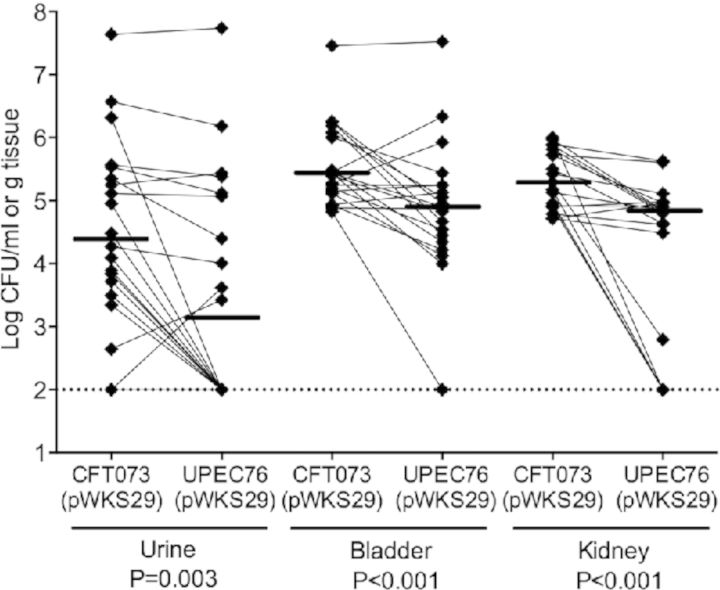
In 20 mice, CFT073 significantly outcompeted UPEC76 in cultures from urine (median 2.4 × 104 versus 1.4 × 103 CFU mL−1, respectively, P = 0.003), bladder (median 2.6 × 105 versus 7.8 × 104 CFU g−1, respectively, P < 0.001) and kidneys (median 1.9 × 105 versus 6.7 × 104 CFU g−1, respectively, P < 0.001), when both strains had the control vector plasmid (Fig. 2A). These results suggest that P fimbriae are important during colonization throughout the urinary tract.
Figure 2.

Cochallenges of mice with E. coli CFT073, pap mutant and complemented mutant. (A) Cochallenges of mice with E. coli CFT073 and pap operon mutant UPEC76. A total of 20 mice were transurethrally inoculated with a 1:1 mixture of the wide-type and mutant strains. After 48 h, the mice were sacrificed and urine, bladder and kidneys were collected. Each data point represents the CFU per milliliter of urine or per gram of tissue collected, and a solid line indicates the median. Paired results from individual mice are connected. pWSK29 acts as the empty vector. (B) Cochallenges of mice with E. coli CFT073 and pap operon mutant UPEC76 transformed with pXLW34 encoding for papIAHCDJKEFG–2. A total of 20 mice were transurethrally inoculated with a 1:1 mixture of the wide-type and mutant strains. The mice were sacrificed after 48 h, and urine, bladder and kidneys were collected. Each data point represents the CFU per milliliter of urine or per gram of tissue collected, and a solid line indicates the median. Paired results from individual mice are connected.
To exclude the possibility that the difference in colonization between CFT073 and UPEC76 was due to an inadvertent mutation other than the deliberate deletion of both copies of the papG adhesin genes, we compared CFT073 containing the control plasmid vector to UPEC76 containing the pap operon plasmid in co-infection of 20 mice (Fig. 2B). In contrast to the results in the absence of complementation, we found no significant difference between CFT073 containing the control plasmid and UPEC76 containing the complementing plasmid in ability to colonize the urine (median 1.2 × 105 versus 4.3 × 104 CFU mL−1, respectively, P = 0.325) or bladder (median 5.7 × 105 versus 6.4 × 105 CFU g−1, respectively, P = 0.118). However in the kidneys, the wild-type strain outcompeted the complemented mutant (median 9.6 × 104 versus 5.9 × 104 CFU g−1, respectively, P = 0.035).
DISCUSSION
Compared to commensal E. coli, ExPEC strains are more likely to carry additional virulence or fitness factors that enable more effective colonization and extended persistence within the urinary tract (Welch et al. 1981; O'Hanley et al. 1985; Mobley et al. 1993; Johnson 1998; Spurbeck et al. 2011; Spurbeck et al. 2012). However, efforts to identify a defined set of requisite virulence and fitness factors remain incomplete. The aim of our study was to identify novel genes in ExPEC that are required for fitness during infection. In this study, we describe screening of signature-tagged mutants of E. coli F11, a cystitis strain originally isolated from a human case of uncomplicated UTI, in the murine model of UTI and the identification of 19 candidate fitness genes. STM is an effective technique to screen an array of transposon mutants in parallel in an animal model of infection. Indeed, STM has been used previously on ExPEC pyelonephritis strain CFT073 (Bahrani-Mougeot et al. 2002) and Proteus mirabilis (Zhao et al. 1999; Burall et al. 2004; Himpsl et al. 2008) in the murine model of UTI. This work identified novel fitness factors in cystitis isolate E. coli F11 and provides support for previously identified virulence or fitness factors, including fimD, wzy and colanic acid biosynthesis genes (Bahrani-Mougeot et al. 2002). In addition, our study has for the first time successfully fulfilled molecular Koch's postulates for P fimbriae (papC) as a virulence factor.
To conduct this trial, mutants in E. coli F11 were generated by transposon mutagenesis using unique sequence tags (Hensel et al. 1995) that allowed for detection of individual mutants following passage in the CBA murine model of UTI. The final library consisted of 1334 transposon mutants. Pools of 48 mutants were transurethrally inoculated into 6 to 8-week-old CBA mice. Urine, bladder and kidneys were collected at 48 h and suspensions were analyzed by dot-blot hybridization to identify absent or attenuated mutants. We initially identified 27 candidate fitness genes necessary for bladder or kidney colonization (2% of the bank). Attenuation of the mutants was confirmed by co-infection of each mutant with wild-type strain F11 in the CBA murine model, confirming a subset of 19 fitness genes (Table 3).
Importantly, one gene identified was papC, which encodes the chaperone required for P-fimbriae biogenesis and, like the rest of the pap operon, is found more frequently among UPEC strains than in fecal E. coli strains (O'Hanley et al. 1985; Mobley et al. 1993; Johnson 1998). P fimbriae are extracellular adhesion organelles that bind to the P blood group antigen found on erythrocytes as well as to the Galα(1-4)βGal moieties of glycosphingolipids found on renal epithelial cells (Kallenius et al. 1980). While overwhelming epidemiologic evidence supports a role for P fimbriae during the development of UTI, heretofore, no specific studies have satisfied molecular Koch's postulates, demonstrating P fimbria as a complementable virulence determinant.
P fimbriae have been shown to participate in coordinate regulation with type 1 fimbriae in an inverse manner, and deletion of Pap-related fimbrial clusters in the pyelonephritis strain E. coli 536 resulted in increased fim expression (Snyder et al. 2005; Holden et al. 2006). Transcription of the fim operon is dependent on the orientation of a σ70 consensus promoter located on an invertible element (Abraham et al. 1985). PapB has been shown to block expression of fim by inhibiting the site-specific recombinase FimB, which mediates switching of the invertible element to either on or off orientation, while concurrently promoting expression of FimE, which can only mediate switching from on to off (Gally, Leathart and Blomfield 1996; Xia et al. 2000; Holden et al. 2006). Conversely, microarray analysis in CFT073 demonstrated that when fim expression was phase L-ON, and thereby constitutively expressed, there was a subsequent decrease in expression of both pap gene clusters with the pheU-associated pap–2 being more strongly repressed than the pheV-associated pap–1 (Snyder et al. 2005). Additionally, inhibition of fim expression in the L-OFF phase resulted in an increase in the expression of both pap operons, further supporting the presence of crosstalk. This coordinated regulation has also been verified by qRT-PCR in CFT073 where expression in the wild type and L-ON backgrounds led to repression of papA–2 expression compared to L-OFF (Snyder et al. 2005).
Unlike CFT073, crosstalk between type 1 and P fimbriae has not been well characterized in the F11 strain. To characterize coordinated regulation between type 1 and P fimbriae in F11, we cultured wild-type, L-ON and L-OFF strains on LB agar for 18 h at 37°C, which are conditions that have been shown to favor pap expression (Blyn et al. 1989). We performed qPCR to quantify fold-change gene expression in fimA and papA using F11 L-OFF as the relative measure for comparison. As we expected, fimA expression was elevated in wild type compared to L-OFF and dramatically increased in L-ON. Despite this elevated fimA expression, there was only a subtle decrease in papA gene expression in the L-ON background compared to wild type, which was not statistically significant. Our results therefore indicate that fimA expression does not impact pap expression when F11 is cultured under these conditions. F11 carries a different subset of fimbrial types including only one copy of pap, compared to the two copies found in CFT073, which may influence the degree of crosstalk between type 1 and P fimbriae. Additionally, F11 has been shown to have a higher percentage of phase-on fim switches than CFT073 at 24 h in the CBA murine model of UTI (Gunther et al. 2001) demonstrating that fimbrial expression can vary between UPEC strains.
The E. coli F11 genome has only one pap operon, which may explain why our current study was able to identify papC as a candidate fitness gene in contrast to previous work with pyelonephritis isolate CFT073, carrying two copies of the pap operon (Mobley et al. 1993). Since our competition data already gave us a strong indication that papC is important for in vivo fitness of cystitis strain F11, we chose to further validate our screen and extend our study to multiple strains by pursuing molecular Koch's postulates in the pyelonephritis strain, CFT073.
Previous studies revealed no differences in colonization during murine UTI between wild-type CFT073 and an isogenic mutant UPEC76, lacking both pap operons. However, this initial work compared colonization by independent infections. It is now understood that cochallenge experiments are more sensitive for detecting attenuation during infection (Beuzon and Holden 2001). To this end, we were able to observe attenuation of UPEC76 when cochallenged with CFT073. We were also able to complement expression of the pap operon in UPEC76 (double pap mutant), which restored its ability to colonize the urinary tract. This is the first time that a gene within the pap operon (papC in this case) has been identified using STM.
In addition to papC, we identified three mutants with transposons within fim genes, which encode for type 1 fimbriae. Type 1 fimbria is one of the most common fimbrial types carried by E. coli strains, found in nearly all strains and multiple studies have shown that type 1 fimbriae are critical for colonization of mice by both cystitis and pyelonephritis strains (Hultgren et al. 1985; Keith et al. 1986; Connell et al. 1996; Gunther et al. 2002). Therefore, we did not pursue cochallenge experiments with these mutants. We applied the same reasoning to genes involved with the biosynthesis of colanic acid, a group I capsule-associated extracellular polysaccharide and another previously identified virulence factor of ExPEC (Bahrani-Mougeot et al. 2002). Nevertheless, the current study adds weight to the conclusion that surface polysaccharides and type 1 fimbriae are preeminent urovirulence factors.
Bacteria must overcome a unique combination of environmental stresses for successful colonization of the urinary tract, including high osmolarity, high urea concentration, variable pH and limited nutrient availability (Brooks and Keevil 1997; Snyder et al. 2004). Previous data suggest that ExPEC strains are able to take advantage of the carbon and nitrogen sources within the urinary tract more effectively than commensal E. coli strains (Anfora et al. 2007; Jones et al. 2008; Alteri, Smith and Mobley 2009). Our screen identified a number genes in E. coli F11 associated with metabolism that when disrupted resulted in a reduction of in vivo fitness during UTI. One mutant was identified in uidR, a negative regulator of the uidAB operon involved with the transport and hydrolysis of glucuronides (Novel and Novel 1976). Imported glucuronides are catabolized into 2-keto-3-deoxy-6-phosphogluconate (KDPG) for utilization in the Entner–Doudoroff (ED) pathway, an alternative pathway to classic glycolysis (Rodionov et al. 2000). KDPG can also be generated by Edd using gluconate as the input carbon source, and disruption of edd in commensal E. coli reduces gut colonization (Chang et al. 2004). However, despite genes involved with the ED pathway being upregulated (2–5-fold) in strain CFT073 during ascending UTI (Snyder et al. 2004), an edd mutant does not show a loss of fitness during UTI infection (Alteri, Smith and Mobley 2009). Further complementation and knockout studies are needed to ascertain if disruption of uidR in our study results in a polar mutation that affects regulation of the uidAB operon. However, our data suggest that upon entering the urinary tract, maintaining regulation of glucuronide import into the ED pathway is important for fitness.
Another fitness factor identified was nadB, encoding for a flavoenzyme involved with de novo biosynthesis of NAD. NAD plays a central role in metabolism (Silverman 2000), yet the role of NAD during ascending murine UTI model is unclear. Nicotinamide auxotrophy did not show an influence on bladder colonization by strains CFT073 or UTI89 during UTI (Li et al. 2012). Our identification of nadB in strain F11 suggests that further investigation is needed into the role of NAD during UTI. Another mutant identified was in narK, one of two nitrate transport genes in E. coli (Stewart 1993). narK is involved with anaerobic nitrate respiration and encodes for a nitrate/nitrite antiporter that couples nitrate uptake to nitrite excretion (Clegg et al. 2002). narK is upregulated in CFT073 during in vitro growth in human urine in comparison to growth in LB broth (Snyder et al. 2004). Our data support a role for narK as a fitness factor during UTI and suggests an advantageous contribution of increased anaerobic respiration within the urinary tract.
Our screen also identified ycjV, which is an uncharacterized member of the ABC superfamily of transporters and may be involved in sugar transport. It was shown to be upregulated in an antibiotic-resistant strain of Klebsiella pneumoniae compared to a susceptible isolate, suggesting that it could act as a novel active efflux mechanism (Domenech-Sanchez et al. 2006). However, its role during UTI in E. coli has not been fully investigated.
ExPEC strains have adapted various strategies to maintain cellular homeostasis despite variable osmolality and elevated urea levels within the urinary tract (Culham et al. 2001; Schwan 2009). Our screen identified two genes that were critical during ascending infection of the urinary tract and are also involved with limiting the effects of osmotic stress. One mutant that we identified was kdpA, which is part of the kdpFABC operon that encodes for a high-affinity ATP-driven potassium transporter (Laimins et al. 1978). KdpA is the K+-translocating subunit and is essential for Kdp-ATPase function (Gassel et al. 1998). In prokaryotes, potassium is vital in regulating intracellular pH levels and maintaining turgor pressure in response to stress-inducing environments (Booth 1985; Epstein 1986). In response to low external K+ levels or osmotic upshock, expression of the kdpFABC operon is upregulated, which differs from the only other K+ transporters in E. coli, Trk and Kup, that are constitutively expressed (Laimins, Rhoads and Epstein 1981). Recent RNA-seq experiments done by the Mobley lab using clinical samples showed that kdpA was selectively expressed during UTI (Subashchandrabose et al. 2014). Our work supports the supposition that the Kdp system is critical during infection of the urinary tract. Our screen also identified evgA, which encodes one subunit of a two-component signal transduction system, EvgAS (Utsumi et al. 1992). EvgA regulates the expression of multiple genes including those related to osmotic stress, acid resistance and drug tolerance (Nishino Inazumi and Yamaguchi 2003; Eguchi et al. 2004; Ma, Masuda and Foster 2004; Eguchi and Utsumi 2014). Further studies are needed to investigate the exact role of EvgA in the context of a UTI. However, we hypothesize that given the high osmolality of the urinary tract, EvgA is critical for regulating expression of additional genes needed to maintain osmotic homeostasis during UTI. We also identified another mutant in cpxA, the sensor kinase of the CpxRA two-component signal transduction system triggered by changes in pH, envelope stress, interaction with hydrophobic surfaces or high osmolarity (Cosma et al. 1995; Danese and Silhavy 1998; Otto and Silhavy 2002; Jubelin et al. 2005). In response to these triggers, CpxA–CpxR modulates porin expression as well as activates proteases and folding proteins (Duguay and Silhavy 2004; Batchelor et al. 2005). Deletion of cpxRA operon reduced fitness and virulence in both UTI89 during colonization of the murine bladder and in CFT073 during localized and systemic infections in zebrafish embryos (Debnath et al. 2013). Our results support these findings and advocate that CpxA is also important in F11 during colonization of the murine urinary tract.
In contrast to P fimbriae, which we show here to be required for full urovirulence, the identification of these other putative urovirulence factors remains provisional until complementation studies confirm their importance. Another limitation of this current study is the limited number of mutants screened. Thus, additional genes required for UTI likely remain to be identified.
This study demonstrates the advantages of cochallenge experiments and the use of transposon mutagenesis to identify novel fitness factors. We were able to confirm, after decades of study, that P fimbriae are important during UTI as well as support previously identified virulence factors. At this time, there has been no identification of a defined group of virulence and fitness factors in ExPEC suggesting that ExPEC strains may express combinations of virulence and fitness genes resulting in different strategies for survival within the urinary tract.
FUNDING
Funding for this work was supported in part by the National Institutes of Health Program Project Grant 2PO1 DK49720, the United Negro College Fund/Merck Fellowship (to E.L.B.), and Public Health Service Grant AI059722 from the National Institutes of Health.
Conflict of interest statement. None declared.
REFERENCES
- Abraham JM, Freitag CS, Clements JR, et al. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. P Natl Acad Sci USA. 1985;82:5724–7. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alteri CJ, Smith SN, Mobley HL. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009;5:e1000448. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfora AT, Haugen BJ, Roesch P, et al. Roles of serine accumulation and catabolism in the colonization of the murine urinary tract by Escherichia coli CFT073. Infect Immun. 2007;75:5298–304. doi: 10.1128/IAI.00652-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani-Mougeot FK, Buckles EL, Lockatell CV, et al. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol. 2002;45:1079–93. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- Batchelor E, Walthers D, Kenney LJ, et al. The Escherichia coli CpxA-CpxR envelope stress response system regulates expression of the porins ompF and ompC. J Bacteriol. 2005;187:5723–31. doi: 10.1128/JB.187.16.5723-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzon CR, Holden DW. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 2001;3:1345–52. doi: 10.1016/s1286-4579(01)01496-4. [DOI] [PubMed] [Google Scholar]
- Blyn LB, Braaten BA, White-Ziegler CA, et al. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 1989;8:613–20. doi: 10.1002/j.1460-2075.1989.tb03416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–78. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks T, Keevil CW. A simple artificial urine for the growth of urinary pathogens. Lett Appl Microbiol. 1997;24:203–6. doi: 10.1046/j.1472-765x.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- Burall LS, Harro JM, Li X, et al. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect Immun. 2004;72:2922–38. doi: 10.1128/IAI.72.5.2922-2938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DE, Smalley DJ, Tucker DL, et al. Carbon nutrition of Escherichia coli in the mouse intestine. P Natl Acad Sci USA. 2004;101:7427–32. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SL, Mekalanos JJ. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- Clegg S, Yu F, Griffiths L, et al. The roles of the polytopic membrane proteins NarK, NarU and NirC in Escherichia coli K-12: two nitrate and three nitrite transporters. Mol Microbiol. 2002;44:143–55. doi: 10.1046/j.1365-2958.2002.02858.x. [DOI] [PubMed] [Google Scholar]
- Connell I, Agace W, Klemm P, et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. P Natl Acad Sci USA. 1996;93:9827–32. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma CL, Danese PN, Carlson JH, et al. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol Microbiol. 1995;18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- Culham DE, Lu A, Jishage M, et al. The osmotic stress response and virulence in pyelonephritis isolates of Escherichia coli: contributions of RpoS, ProP, ProU and other systems. Microbiology. 2001;147:1657–70. doi: 10.1099/00221287-147-6-1657. [DOI] [PubMed] [Google Scholar]
- Danese PN, Silhavy TJ. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol. 1998;180:831–9. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V, Herrero M, Jakubzik U, et al. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–72. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath I, Norton JP, Barber AE, et al. The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli. Infect Immun. 2013;81:1450–9. doi: 10.1128/IAI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusscher J, Zhang L, Buxton M, et al. Persistent extended-spectrum beta-lactamase urinary tract infection. Emerg Infect Dis. 2009;15:1862–4. doi: 10.3201/eid1511.081501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech-Sanchez A, Benedi VJ, Martinez-Martinez L, et al. Evaluation of differential gene expression in susceptible and resistant clinical isolates of Klebsiella pneumoniae by DNA microarray analysis. Clin Microbiol Infec. 2006;12:936–40. doi: 10.1111/j.1469-0691..01470.x. [DOI] [PubMed] [Google Scholar]
- Duguay AR, Silhavy TJ. Quality control in the bacterial periplasm. Biochim Biophys Acta. 2004;1694:121–34. doi: 10.1016/j.bbamcr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Eden CS, Hansson HA. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978;21:229–37. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Okada T, Minagawa S, et al. Signal transduction cascade between EvgA/EvgS and PhoP/PhoQ two-component systems of Escherichia coli. J Bacteriol. 2004;186:3006–14. doi: 10.1128/JB.186.10.3006-3014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Utsumi R. Alkali metals in addition to acidic pH activate the EvgS histidine kinase sensor in Escherichia coli. J Bacteriol. 2014;196:3140–9. doi: 10.1128/JB.01742-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. Osmoregulation by potassium transport in Escherichia coli. FEMS Microbiol Rev. 1986;39:73–8. [Google Scholar]
- Falkow S. Molecular Koch's postulates applied to microbial pathogenicity. Rev Infect Dis. 1988;10(Suppl 2):S274–6. doi: 10.1093/cid/10.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- Gally DL, Leathart J, Blomfield IC. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol Microbiol. 1996;21:725–38. doi: 10.1046/j.1365-2958.1996.311388.x. [DOI] [PubMed] [Google Scholar]
- Gassel M, Siebers A, Epstein W, et al. Assembly of the Kdp complex, the multi-subunit K+-transport ATPase of Escherichia coli. Biochim Biophys Acta. 1998;1415:77–84. doi: 10.1016/s0005-2736(98)00179-5. [DOI] [PubMed] [Google Scholar]
- Gunther NW, IV, Lockatell V, Johnson DE, et al. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect Immun. 2001;69:2838–46. doi: 10.1128/IAI.69.5.2838-2846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther NW, IV, Snyder JA, Lockatell V, et al. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect Immun. 2002;70:3344–54. doi: 10.1128/IAI.70.7.3344-3354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L, Engberg I, Freter R, et al. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–83. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Techniques for transformation of E. coli. In: Glover DM, editor. DNA cloning: A practical approach. Washington DC: Oxford University Press; 1985. pp. 109–135. [Google Scholar]
- Hensel M, Shea JE, Gleeson C, et al. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–3. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Herrero M, de Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–67. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpsl SD, Lockatell CV, Hebel JR, et al. Identification of virulence determinants in uropathogenic Proteus mirabilis using signature-tagged mutagenesis. J Med Microbiol. 2008;57:1068–78. doi: 10.1099/jmm.0.2008/002071-0. [DOI] [PubMed] [Google Scholar]
- Holden NJ, Totsika M, Mahler E, et al. Demonstration of regulatory cross-talk between P fimbriae and type 1 fimbriae in uropathogenic Escherichia coli. Microbiology. 2006;152:1143–53. doi: 10.1099/mic.0.28677-0. [DOI] [PubMed] [Google Scholar]
- Hull RA, Donovan WH, Del Terzo M, et al. Role of type 1 fimbria- and P fimbria-specific adherence in colonization of the neurogenic human bladder by Escherichia coli. Infect Immun. 2002;70:6481–4. doi: 10.1128/IAI.70.11.6481-6484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren SJ, Porter TN, Schaeffer AJ, et al. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985;50:370–7. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CS, Bouckaert J, Hung D, et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol Microbiol. 2002;44:903–15. doi: 10.1046/j.1365-2958.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Lockatell CV, Hall-Craigs M, et al. Uropathogenicity in rats and mice of Providencia stuartii from long-term catheterized patients. J Urology. 1987;138:632–5. doi: 10.1016/s0022-5347(17)43287-3. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Lockatell CV, Russell RG, et al. Comparison of Escherichia coli strains recovered from human cystitis and pyelonephritis infections in transurethrally challenged mice. Infect Immun. 1998;66:3059–65. doi: 10.1128/iai.66.7.3059-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR. papG alleles among Escherichia coli strains causing urosepsis: associations with other bacterial characteristics and host compromise. Infect Immun. 1998;66:4568–71. doi: 10.1128/iai.66.9.4568-4571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR. Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect Dis Clin N Am. 2003;17:261–78. doi: 10.1016/s0891-5520(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Jones SA, Jorgensen M, Chowdhury FZ, et al. Glycogen and maltose utilization by Escherichia coli O157:H7 in the mouse intestine. Infect Immun. 2008;76:2531–40. doi: 10.1128/IAI.00096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelin G, Vianney A, Beloin C, et al. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J Bacteriol. 2005;187:2038–49. doi: 10.1128/JB.187.6.2038-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenius G, Mollby R, Svenson SB, et al. Identification of a carbohydrate receptor recognized by uropathogenic Escherichia coli. Infection. 1980;8:288–93. doi: 10.1007/BF01639597. [DOI] [PubMed] [Google Scholar]
- Kallenius G, Mollby R, Svenson SB, et al. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981;2:1369–72. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Keith BR, Maurer L, Spears PA, et al. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun. 1986;53:693–6. doi: 10.1128/iai.53.3.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins LA, Rhoads DB, Altendorf K, et al. Identification of the structural proteins of an ATP-driven potassium transport system in Escherichia coli. P Natl Acad Sci USA. 1978;75:3216–9. doi: 10.1073/pnas.75.7.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins LA, Rhoads DB, Epstein W. Osmotic control of kdp operon expression in Escherichia coli. P Natl Acad Sci USA. 1981;78:464–8. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MC, Simms AN, Mobley HL. Complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J Bacteriol. 2007;189:5523–33. doi: 10.1128/JB.00434-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bouckaert J, Deboeck F, et al. Nicotinamide dependence of uropathogenic Escherichia coli UTI89 and application of nadB as a neutral insertion site. Microbiology. 2012;158:736–45. doi: 10.1099/mic.0.052043-0. [DOI] [PubMed] [Google Scholar]
- Litwin MS, Saigal CS, Yano EM, et al. Urologic diseases in America Project: analytical methods and principal findings. J Urology. 2005;173:933–7. doi: 10.1097/01.ju.0000152365.43125.3b. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma Z, Masuda N, Foster JW. Characterization of EvgAS-YdeO-GadE branched regulatory circuit governing glutamate-dependent acid resistance in Escherichia coli. J Bacteriol. 2004;186:7378–89. doi: 10.1128/JB.186.21.7378-7389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei JM, Nourbakhsh F, Ford CW, et al. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol. 1997;26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–83. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley HL, Jarvis KG, Elwood JP, et al. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10:143–55. doi: 10.1111/j.1365-2958.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Nishino K, Inazumi Y, Yamaguchi A. Global analysis of genes regulated by EvgA of the two-component regulatory system in Escherichia coli. J Bacteriol. 2003;185:2667–72. doi: 10.1128/JB.185.8.2667-2672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel M, Novel G. Regulation of beta-glucuronidase synthesis in Escherichia coli K-12: pleiotropic constitutive mutations affecting uxu and uidA expression. J Bacteriol. 1976;127:418–32. doi: 10.1128/jb.127.1.418-432.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B, Svanborg-Eden C, Hull R, et al. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect Immun. 1989;57:446–51. doi: 10.1128/iai.57.2.446-451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P, Low D, Romero I, et al. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. New Engl J Med. 1985;313:414–20. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Ott M, Hacker J, Schmoll T, et al. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect Immun. 1986;54:646–53. doi: 10.1128/iai.54.3.646-653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto K, Silhavy TJ. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. P Natl Acad Sci USA. 2002;99:2287–92. doi: 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Marklund BI, Ilver D, et al. The Gal(alpha 1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. P Natl Acad Sci USA. 1994;91:11889–93. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Mironov AA, Rakhmaninova AB, et al. Transcriptional regulation of transport and utilization systems for hexuronides, hexuronates and hexonates in gamma purple bacteria. Mol Microbiol. 2000;38:673–83. doi: 10.1046/j.1365-2958.2000.02115.x. [DOI] [PubMed] [Google Scholar]
- Sanschagrin F, Kukavica-Ibrulj I, Levesque RC. Essential genes in the infection model of Pseudomonas aeruginosa PCR-based signature-tagged mutagenesis. Methods Mol Biol. 2008;416:61–82. doi: 10.1007/978-1-59745-321-9_5. [DOI] [PubMed] [Google Scholar]
- Schito GC, Naber KG, Botto H, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Ag. 2009;34:407–13. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Schwan WR. Survival of uropathogenic Escherichia coli in the murine urinary tract is dependent on OmpR. Microbiology. 2009;155:1832–9. doi: 10.1099/mic.0.026187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. The Organic Chemistry of Enzyme-catalyzed Reactions. San Diego: Academic Press; 2000. [Google Scholar]
- Snyder JA, Haugen BJ, Buckles EL, et al. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun. 2004;72:6373–81. doi: 10.1128/IAI.72.11.6373-6381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JA, Haugen BJ, Lockatell CV, et al. Coordinate expression of fimbriae in uropathogenic Escherichia coli. Infect Immun. 2005;73:7588–96. doi: 10.1128/IAI.73.11.7588-7596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JA, Lloyd AL, Lockatell CV, et al. Role of phase variation of type 1 fimbriae in a uropathogenic Escherichia coli cystitis isolate during urinary tract infection. Infect Immun. 2006;74:1387–93. doi: 10.1128/IAI.74.2.1387-1393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck RR, Dinh PC, Walk ST, Jr, et al. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect Immun. 2012;80:4115–22. doi: 10.1128/IAI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck RR, Stapleton AE, Johnson JR, et al. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect Immun. 2011;79:4753–63. doi: 10.1128/IAI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton A, Moseley S, Stamm WE. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991;163:773–9. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- Stewart V. Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli. Mol Microbiol. 1993;9:425–34. doi: 10.1111/j.1365-2958.1993.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Subashchandrabose S, Hazen TH, Brumbaugh AR, et al. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. P Natl Acad Sci USA. 2014;111:18327–32. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R, Katayama S, Ikeda M, et al. Cloning and sequence analysis of the evgAS genes involved in signal transduction of Escherichia coli K-12. Nucl Acid S. 1992;27:149–50. [PubMed] [Google Scholar]
- Wang RF, Kushner SR. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–9. [PubMed] [Google Scholar]
- Welch RA, Burland V, Plunkett G, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. P Natl Acad Sci USA. 2002;99:17020–4. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RA, Dellinger EP, Minshew B, et al. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981;294:665–7. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Wullt B, Bergsten G, Connell H, et al. P fimbriae enhance the early establishment of Escherichia coli in the human urinary tract. Mol Microbiol. 2000;38:456–64. doi: 10.1046/j.1365-2958.2000.02165.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Gally D, Forsman-Semb K, et al. Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB protein. EMBO J. 2000;9:1450–7. doi: 10.1093/emboj/19.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Li X, Johnson DE, et al. Identification of protease and rpoN-associated genes of uropathogenic Proteus mirabilis by negative selection in a mouse model of ascending urinary tract infection. Microbiology. 1999;145:185–95. doi: 10.1099/13500872-145-1-185. [DOI] [PubMed] [Google Scholar]