Abstract
The reported effects of temperature on sweet taste in humans have generally been small and inconsistent. Here, we describe 3 experiments that follow up a recent finding that cooling from 37 to 21 °C does not reduce the initial sweetness of sucrose but increases sweet taste adaptation. In experiment 1, subjects rated the sweetness of sucrose, glucose, and fructose solutions at 5–41 °C by dipping the tongue tip into the solutions after 0-, 3-, or 10-s pre-exposures to the same solutions or to H2O; experiment 2 compared the effects of temperature on the sweetness of 3 artificial sweeteners (sucralose, aspartame, and saccharin); and experiment 3 employed a flow-controlled gustometer to rule out the possibility the effects of temperature in the preceding experiments were unique to dipping the tongue into a still taste solution. The results (i) confirmed that mild cooling does not attenuate sweetness but can increase sweet taste adaptation; (ii) demonstrated that cooling to 5–12 °C can directly reduce sweetness intensity; and (iii) showed that both effects vary across stimuli. These findings have implications for the TRPM5 hypothesis of thermal effects on sweet taste and raise the possibility that temperature also affects an earlier step in the T1R2–T1R3 transduction cascade.
Key words: human, psychophysics, sweetness, taste, temperature, TRPM5
Introduction
Temperature can influence human sweet taste perception (e.g., Stone et al. 1969; McBurney et al. 1973; Bartoshuk et al. 1982; Calviño 1986), and temperature-sensitive neurons have been found throughout the gustatory system of animals (Zotterman 1935; Yamashita and Sato 1965; Rolls 2004; Breza et al. 2006; Lu et al. 2012; Wilson and Lemon 2014) and in the chorda tympani nerve of humans (Oakley 1985). However, the measured effects of temperature on human sweet taste have generally been small and inconsistent. For example, effects on the sweetness of sucrose have been limited to low concentrations (<0.56M; Bartoshuk et al. 1982; Calviño 1986; Green and Frankmann 1987) or have not been found at all (Schiffman et al. 2000; Bajec et al. 2012), and a study that included 4 different sweeteners found significant effects of cooling for glucose, fructose, and aspartame but not for saccharin (Green and Frankmann 1988).
Findings from a recent study (Green and Nachtigal 2012) offer a possible explanation for the weak and inconsistent thermal effects on sucrose sweetness. First, 21 and 37 °C solutions sampled with the tongue tip were rated equally sweet when tasted for 3 s, but after 3-, 6-, or 12-s pre-exposures to the stimulus, the 21 °C solution tasted less sweet than the 37 °C solution. Cooling to 21 °C therefore failed to reduce initial sweetness but accelerated sweet taste adaptation. Second, it was also found that the increased adaptation to 21 °C could be counteracted by just a 3-s exposure to a 37 °C solution. These results imply that an effect of mild cooling on sucrose sweetness would be difficult to detect when solutions are sipped into the mouth: Initial sensitivity would not be reduced and adaptation would tend to be reversed as the solution warms in the mouth.
However, none of these findings ruled out the possibility that colder temperatures affect sweet taste independently of adaptation. In particular, lower temperatures might reduce the excitability of the calcium-dependent channel TRPM5, which is the final step in the T1R2–T1R3 sweet taste receptor transduction cascade that leads to receptor depolarization (Liman 2007). Talavera et al. (2005) reported that when expressed in HEK-293 cells, activation of TRPM5 by intracellular Ca2+ is strongly modulated by temperatures below 30 °C and proposed that TRPM5 is responsible for the temperature sensitivity of sweet taste. Because lower solution temperatures are likely to be necessary to produce equivalent cooling of TRPM5 in vivo, we hypothesized that temperatures below 21 °C might reduce human sweet taste sensitivity consistent with the effect of cold on sweet-specialist neurons in the geniculate ganglion of rats (Breza et al. 2006) and medulla of mice (Wilson and Lemon 2014).
We investigated this hypothesis and sought to replicate our earlier findings on sweet taste adaptation in 3 experiments. The first experiment confirmed the effect on sucrose adaptation at 21 °C and below, and further showed that at 5 and 10 °C, the initial sweetness of sucrose, glucose, and fructose was also reduced. The second experiment used the same procedure to compare the effects of temperature on the sweetness of 3 artificial sweeteners, and a final experiment employed a temperature and flow-controlled gustometer to rule out the possibility that the effects of temperature on sweetness in the first 2 experiments were unique to dipping the tongue into a still taste solution.
Materials and methods
Experiment 1: the effects of temperature on sweetness and adaptation for sugars
Subjects
A total of 27 adults (18 females and 9 males) between 18 and 45 years of age served as subjects in the experiment. Participants were recruited from public postings on the Yale Medical School and College campuses, were paid for their participation, and gave informed consent. The research protocol complies with the Declaration of Helsinki for Medical Research involving Human Subjects and was approved by the Human Investigations Committee of the Yale University Institutional Review Board. All subjects were self-reported healthy nonsmokers who had no known taste or smell disorders or deficiencies. The subjects were asked to refrain from eating or drinking foods or beverages for at least 1h prior to their scheduled session.
Stimuli
The stimuli were aqueous solutions of 0.42M sucrose, 1.0M fructose, and 1.4M glucose (Sigma-Aldrich). These concentrations were determined in pilot experiments to produce approximately equal sensations of sweetness. All stimuli were prepared weekly with deionized water in 250-mL volumes and stored in airtight flasks. The solution temperatures tested were 5, 10, 21, 30, 37, and 41 °C. The taste solutions were stored in 250-mL capped glass bottles that were placed in temperature-controlled circulated water baths at least 30min prior to each testing session to control the temperature of the solutions. The temperatures of the baths were monitored throughout each session via BAT-12 thermocouple thermometers (Physitemp Instruments). On each trial the stimuli were pipetted in 7.5-mL volumes into plastic weighing dishes (polystyrene square, 41×41×8mm, Fisher Scientific), and subjects sampled the stimuli by dipping the tongue tip into the solutions for intervals of 3 or 10 s that were timed by the experimenter. The stimuli were pipetted into the weighing dishes just prior to each trial and measurements showed that during this brief time solution temperature deviated from target temperature by less than ±0.5 °C.
Practice session procedure
Prior to the first data collection session, all subjects attended a short practice session in which they were instructed how to use the general version of the labeled magnitude scale (gLMS; Green et al. 1993; Green et al. 1996; Bartoshuk et al. 2003) to rate sensation intensity. The gLMS was displayed on a computer monitor and subjects used a mouse to move a cursor to appropriate locations on the scale to indicate perceived intensity. After the instructions were given, the subjects rated 15 remembered or imagined sensations (e.g., the sweetness of cotton candy, the weight of a feather in your hand, the pain of biting your tongue) to give them experience using the gLMS in the broad context of everyday experiences.
Experimental session procedure
The experiment had 2 conditions: A control condition in which pre-exposure to H2O alone for 3 or 10 s at one of the 6 temperatures preceded a 3-s exposure to the test stimulus at the same temperature; and an adaptation condition in which pre-exposure to the taste stimulus for 3 or 10 s at one of the 6 temperatures preceded a 3-s exposure to the test stimulus at the same temperature. In addition, to measure initial (baseline) taste sensitivity without pre-exposure to either H2O or the taste stimulus (i.e., pre-exposure = 0 s), each taste stimulus was sampled for 3 s at each of the 6 temperatures.
On pre-exposure trials, the subject was instructed to seal off her/his tongue with the lips and dip the tongue tip into a weighing dish containing the pre-exposure solution (taste stimulus in the adaptation condition; H2O in the control condition) for 3 or 10 s, which was timed by the experimenter. At the end of the interval, the subject immediately removed the tongue from the first weighing dish and dipped it into an adjacent dish containing the test stimulus at the same temperature. Exposure to the test stimulus was always for 3 s, which was also timed by the experimenter. At the experimenter’s instruction, the subject lifted the tongue from the solution and immediately rated the sweetness of the test solution, keeping the tongue extended outside the mouth. Instructions were given to ignore the temperature of the solution and focus only on sweetness.
On baseline trials subjects only dipped the tongue tip into a single weighing dish containing the test solution for 3 s, then rated sweetness immediately after lifting the tongue from the solution, also keeping the tongue extended outside the mouth.
Because the effects of adaptation and temperature could both reduce sweetness to undetectable levels on some trials, subjects were informed that they may or may not perceive a taste on a given trial and that the intensity of sweetness may change over time. There was a 1-min intertrial interval during which the subject rinsed 3 times with 37 °C deionized water to ensure there was no contamination from the previous stimulus and that the tongue had returned to normal oral temperature.
In each session, subjects received one of the 3 taste stimuli presented at 3 of the 6 solution temperatures: 5, 21, and 37 °C, or 10, 30, and 41 °C. The order of stimulus presentation was randomly assigned, and subjects were alternately assigned to one of the 2 temperature orders. Each session comprised 15 trials (2 conditions × 3 temperatures × 2 pre-exposure durations + 3 baseline sensitivity measurements) with the control and adaptation conditions pseudorandomly intermixed across trials. This procedure required 2 sessions to test each stimulus at every temperature, for a total of 6 sessions per subject.
Experiment 2: the effects of temperature on sweetness and adaptation for artificial sweeteners
Subjects
A total of 21 adults (14 females and 7 males) between 18 and 45 years of age participated in the experiment. The procedures for subject recruitment, human subjects protocol approval, and informed consent were the same as those of experiment 1. The subjects were also asked to refrain from eating or drinking foods or beverages for at least 1h prior to their scheduled session.
Stimuli
The taste stimuli used were aqueous solutions of 3.2mM saccharin, 5.6mM aspartame, and 1.5mM sucralose. These concentrations were determined in pilot testing to produce approximately the same initial sweetness as the 3 sugars of experiment 1. Stimulus preparation and delivery was the same as experiment 1.
Practice session procedure
Subjects who had not participated in previous experiments in the laboratory were given the same practice session described for experiment 1.
Experimental session procedure
To enable direct comparison of the effects of temperature on artificial sweeteners with the data collected on sugars in experiment 1, the same procedure was used.
Experiment 3: the effects of solution flow rate and temperature on sucrose sweetness and adaptation
Subjects
A total of 25 adults (14 females and 11 males) between 18 and 45 years of age participated in the experiment. The procedures for subject recruitment, human subjects protocol approval, and informed consent were the same as those of experiments 1 and 2. The subjects were again asked to refrain from eating or drinking foods or beverages for at least 1h prior to their scheduled session.
Stimuli
The taste stimulus was an aqueous solution of 0.50M sucrose that was prepared daily in 4-L volumes in deionized H2O. Using the custom-designed system described below, the solutions were cooled or heated to 12, 21, 32, or 38 °C and flowed over the tongue tip at 0, 1.5, 2.5, and 4.0mL/s.
Stimulus delivery system
The sucrose solutions were delivered to the tongue tip via a temperature-controlled, dual-channel gustometer that was designed and built in the John B. Pierce Laboratory electronics and machine shop. In this system, which is controlled by LabVIEW software, solutions are pumped from 4-L glass reservoir bottles through 2 inline Peltier heating and cooling chambers that control solution temperature before being delivered to the base of a custom machined Teflon tongue bath where the solution flows up through a mixing screen and into the bath (~4-mL volume). The mixing screen helps to produce a uniform (laminar) flow of taste solution over and around the tongue tip as it is submerged in the bath. The solution is allowed to flow over the rim of the bath and drain into a sink below, thereby keeping solution depth constant across flow rates. A LabVIEW VI displayed on a computer screen enables the experimenter to (i) select from 5 different taste stimuli and deionized H2O in the reservoir bottles via electronic pinch valves, (ii) set solution flow rate via variable speed peristaltic pumps, and (iii) set a steady-state solution temperature and/or target temperatures and ramp rates. Temperature control is via proportional-integral-derivative (PID) control of separate Peltier cold and heat exchangers in each of the 2 channels. Entering each channel the solution is split between the heating and cooling chambers and remixed at the output to arrive at the set temperature. Solution temperature delivered to the tongue bath is monitored by a thermocouple inserted into the delivery tube at the base of the bath. In the present study, the solution was flowed through only one channel at a time with solution temperature and flow rate set before each trial.
To parallel mode of exposure in the preceding experiments, the subject dipped the tongue into the bath for time intervals indicated by verbal signals from the experimenter. To mimic stimulus delivery in a weighing dish, in the flow = 0mL/s condition, the pumps were turned off when the desired solution temperature was reached and the experimenter signaled the subject to dip his or her tongue into the still solution.
Practice session procedure
Subjects who had not participated in previous experiments in the laboratory were given the same practice session as in experiment 1.
Experimental session procedure
There were 2 experimental sessions on separate days. A session consisted of delivering the stimulus at 2 of the 4 flow rates. The order in which the flow rates were tested was alternated across subjects, with half receiving 1.5 and 4.0mL/s during the first session and 0 and 2.5mL/s for the second session. Sessions were blocked by flow rate and consisted of 2 blocks of 12 trials. In each block, the sucrose stimulus was delivered at each of the 4 temperatures and sampled for 3, 6, or 15 s. Six pseudorandom orders of stimulus temperature were created to avoid instances of multiple back-to-back trials of the same temperature that could occur with complete randomization. The 6 orders were counterbalanced across subjects.
Subjects were instructed to dip their tongue tip into the tongue bath containing the solution for intervals of 3, 6, and 15 s timed by the experimenter. A trial began when the solution in the tongue bath reached the target temperature. The experimenter then cued the subject to place the tip of the tongue into the tongue bath. Otherwise, the instructions to subjects and procedure were the same as experiments 1 and 2.
Results
Experiment 1: differential effects of temperature on the initial sweetness of sugars
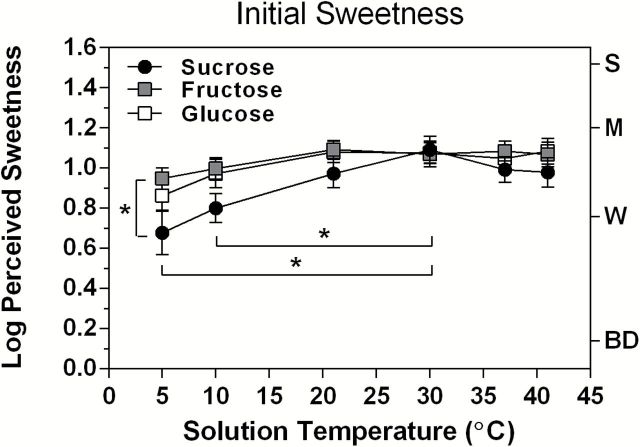
Figure 1 contains the log-mean ratings of initial perceived sweetness as a function of solution temperature for sucrose, glucose, and fructose. These data show the direct effect of solution temperature on sweetness at the 6 test temperatures without pre-exposures to H2O or to the taste stimuli (pre-exposure = 0 s). An analysis of variance (ANOVA) indicated that there was a significant main effect of temperature (F (5,130) = 9.30, P < 0.00001), which reflected lower sweetness ratings for all 3 stimuli at the coldest temperatures of 5 and 10 °C. At those temperatures, the sweetness of sucrose was more strongly affected on average than was the sweetness of the 2 monosaccharides, but the Stimulus × Temperature interaction fell just short of significance (F (10,260) = 1.87, P = 0.06). However, further analyses indicated that the main effect of temperature owed primarily to significant attenuation of the sweetness of sucrose and glucose. Tukey honestly significant difference (HSD) tests showed that the sweetness of sucrose was significantly less at both 5 and 10 °C than at 30 °C, whereas the sweetness of glucose was significantly lower only at 5 °C, and fructose sweetness was not significantly lower at either temperature. These statistical differences reflect substantial perceptual differences in the effects of temperature for the 3 sugars: Compared with the 30 °C solution, the difference in log-mean sweetness of sucrose was −0.43 log10 at 5 °C, which represents a 62.9% decline in sweetness compared with declines of only 39.2% for glucose and 22.4% for fructose. Tukey HSD tests further showed that a trend toward lower sweetness ratings for sucrose at the warm temperatures of 37 and 41 °C was not statistically significant when compared with ratings at 30 °C.
Figure 1.
Log10-mean perceived sweetness ratings as a function of solution temperature are shown for an initial 3-s exposure to the 3 saccharides tested in experiment 1. The vertical bar and asterisk next to the data for 5 °C indicate that sweetness was significantly lower for sucrose than for fructose at that temperature; horizontal bars and asterisks indicate significant differences between the sweetness of sucrose at both 5 and 10 °C relative to 30 °C, where mean sucrose sweetness was highest. Error bars represent standard errors of the means; letters on the right y axis refer to intensity descriptors on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong.
Experiment 1: effect of temperature on the sweet taste of sugars over time
Figure 2 displays log-mean sweetness ratings in response to the 3 saccharides following 0-, 3-, or 10-s pre-exposures to H2O (i.e., to temperature alone; Figure 2A) and to solutions of the same stimulus at each of the 6 temperatures (Figure 2B). The data for 0-s pre-exposure are the same as those in Figure 1. These results show that cooling affected sweetness over time both by attenuating initial sweet taste at 5 and 10 °C (Figure 2A) and by increasing the rate of sweet taste adaptation at 21 °C and below (Figure 2B).
Figure 2.
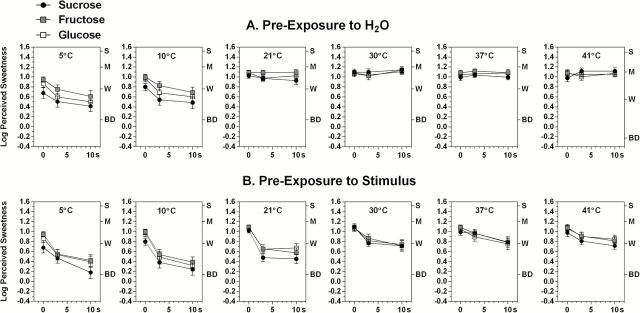
Shown are log10-mean perceived sweetness ratings for 3-s exposures to each of the 3 saccharides after 0-, 3-, or 10-s pre-exposures to (A) deionized H2O or (B) the same taste stimulus at 6 different solution temperatures. Solution temperature was the same for pre- and post-exposures. Note that (A) shows the effect of solution temperature alone on suprathreshold sensitivity, whereas (B) shows the combined effects of solution temperature and adaptation. Error bars represent standard errors of the means; letters on the right y axis refer to intensity descriptors on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong.
A 3-way repeated measures ANOVA on the data of Figure 2A showed that pre-exposure to cooling alone led to a significant main effect of Temperature (F (5,130) = 28.02, P < 0.000001), which Tukey HSD tests indicated was caused in part by lower sweetness ratings for all 3 stimuli at 5 and 10 °C compared with 37 °C (P < 0.05). A significant Temperature × Time interaction (F (10,260) = 8.64, P < 0.00001) confirmed that the effect of temperature increased as the tongue tip continued to cool in the solution, although much of the decline occurred over the first 3 s of exposure. A significant Stimulus × Temperature interaction (F (10,260) = 3.88, P < 0.0001) confirmed that, as was true for the initial 3-s exposure (Figure 1), the effect of temperature was not the same for all the 3 stimuli when exposure continued for up to 10 s. Tukey HSD tests showed that log-mean sweetness ratings over time for sucrose were significantly lower than for glucose at both 5 and 10 °C and lower than for fructose at 5 °C (P < 0.05), whereas glucose and fructose did not differ in sweetness from one another at either temperature.
Figure 2B shows that the rate and amount of sweet taste adaptation was temperature-dependent. A 3-way ANOVA confirmed there were main effects of Stimulus (F (2,52) = 9.10, P < 0.0005), Temperature (F (5,130) = 20.18, P < 0.000001), and Time (F (2,52) = 71.37, P < 0.000001) and a Temperature × Time interaction (F(10,260) = 6.28, P < 0.00001). To explore the source of the Temperature × Time interaction, an ANOVA was conducted to compare the data for 21 and 37 °C. Significant main effects of Temperature (F(2,26) = 33.68, P < 0.00001) and Time (F(2,52) = 39.31, P < 0.00001) were found as well as interactions between Stimulus and Temperature (F(2,52) = 4.26, P < 0.02) and Time and Temperature (F(2,52) = 21.70, P < 0.00001). Tukey HSD tests showed that the Stimulus × Temperature interaction was driven by lower mean sweetness ratings for sucrose compared with the monosaccharides at 21 °C (P < 0.05). However, the Stimulus × Temperature × Time interaction did not reach significance (F(4,104) = 1.24, P = 0.30), indicating that the rate of adaptation did not differ significantly among the 3 stimuli. An additional ANOVA limited to the data for 21, 10, and 5 °C also revealed a significant Stimulus × Temperature interaction (F(4,104) = 3.26, P < 0.02), which Tukey HSD tests showed was due to lower log-mean ratings of sucrose sweetness. Together, these data indicate that the effect of cooling to 21 °C (and below) on sweet taste adaptation is not limited to sucrose (Green et al. 2012) and that at colder temperatures, sweetness is further reduced by a direct thermal effect that is greatest for sucrose.
Experiment 2: effect of temperature on the initial sweetness of artificial sweeteners
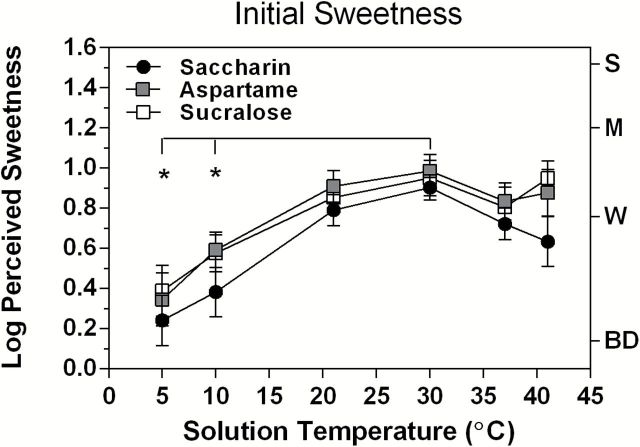
Figure 3 displays the log-mean ratings of perceived sweetness during 3-s exposures to the 3 artificial sweeteners as a function of solution temperature. Like Figure 1 for sugars, these data show a direct effect of temperature on sweetness without pre-exposure to H2O or to the artificial sweeteners. As was found for the sugars, there were main effects of both Temperature (F (5,100) = 22.14, P < 0.00001) and Stimulus (F (2,40) = 7.70, P < 0.002). The effect of stimulus was due to slightly lower mean sweetness ratings for saccharin at all temperatures. There was no Stimulus × Temperature interaction (F (10,200) = 0.63, P = 0.79). Tukey HSD tests showed that sweetness ratings at 5 and 10 °C were significantly lower than ratings at 30 °C, the temperature at which log10-mean sweetness ratings peaked for all 3 stimuli. In contrast, the tendency toward lower sweetness ratings above 30 °C, which was clearest for saccharin, did not reach statistical significance for any stimulus (Tukey HSD, P > 0.05).
Figure 3.
Same as Figure 1 but for the 3 artificial sweeteners tested in experiment 2. The horizontal line indicates that the perceived sweetness of all 3 artificial sweeteners at 5 and 10 °C was significantly lower than at 30 °C, where mean sweetness intensity was highest for all 3 stimuli. The trends toward lower sweetness ratings at 37 and 41 °C, particularly for saccharin, were not significant. Error bars represent standard errors of the means; the letters on the right y axis refer to intensity descriptors on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong.
Based on differences in log10-mean ratings, the effect of cooling on initial sweetness intensity was greater for the artificial sweeteners than for the sugars in experiment 1: Cooling from 30 to 5 °C led to reductions in perceived sweetness of 78.1% for saccharin, 70.5% for aspartame, and 72.5% for sucralose, compared with 62.9% for sucrose, 39.2% for glucose, and only 22.4% for fructose. Conversely, these differences mean that warming from 5 to 30 °C increased the sweetness of both saccharin and sucralose solutions by more than 4-fold, compared with 2.5- and 1.3-fold for sucrose and fructose, respectively.
Experiment 2: differential effects of temperature on the sweet taste of artificial sweeteners over time
Figure 4 displays log10-mean sweetness ratings after 0-, 3-, or 10-s exposures to pure H2O (temperature alone; Figure 4A) or to the same taste solutions at each temperature (Figure 4B). Temperature alone had the same general effect on the sweetness of artificial sweeteners that it had on the sweetness of sugars (main effect of temperature; F (5,100) = 51.54, P < 0.00001). However, the effect at cold temperatures was greater for the artificial sweeteners, with sweetness falling to just “barely detectable” after only a few seconds of exposure to 5 °C H2O (cf. Figures 2A,B). Significant Stimulus × Temperature (F (10,200) = 1.90, P < 0.05) and Stimulus × Time interactions appear to have been driven by a curious effect on saccharin at 10 °C, in which exposures resulted in smaller reductions in sweetness over time. This effect appeared to be due to a few subjects who rated sweetness as weak-to-moderate rather than nearly barely detectable, as they did for the other 2 stimuli. Otherwise, the effect of temperature was similar across sweeteners. As also occurred for the sugars, there was a significant Temperature × Time interaction (F (10,200) = 9.66, P < 0.00001), although unlike the sugars the interaction was driven in part by the tendency for sweetness to increase after exposure to 37 and 41 °C H2O.
Figure 4.
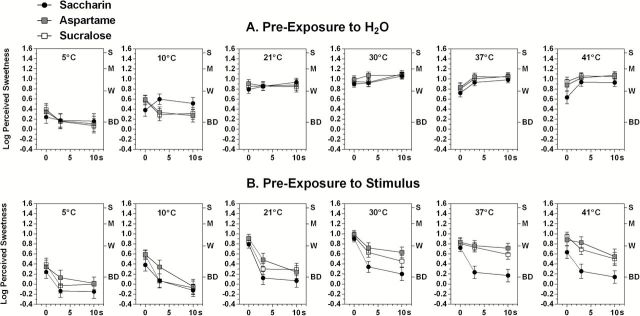
Same as Figure 2 but for the 3 artificial sweeteners tested in experiment 2. Note the overall stronger effect of solution temperature on perception of the artificial sweeteners compared with the sugars in Figure 2 and the rapid, temperature-independent adaptation of saccharin. Error bars represent standard errors of the means; letters on the right y axis refer to intensity descriptors on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong.
Figure 4B shows an effect of temperature on adaptation to aspartame and sucralose similar to the effect on adaptation to the 3 sugars, whereas adaptation to saccharin was rapid and independent of temperature. Consistent with the difference in the effect of temperature on adaptation across stimuli, an ANOVA conducted on the data for 30, 37, and 41 °C found a significant main effect of Stimulus (F (2,40) = 23.83, P < 0.00001), a Stimulus × Time interaction (F (4,80) = 10.14, P < 0.00001), but no main effect of Temperature (F (2,40) = 0.57, P = 0.57). In contrast, an ANOVA that focused on sweetness ratings after exposure to 21 or 37 °C found a significant main effect of Temperature (F (1,20) = 8.54, P < 0.01) and a Temperature × Time interaction (F (2,40) = 3.36, P < 0.05), reflecting enhanced adaption to aspartame and sucralose at 21 °C. An ANOVA that compared the data for the 3 cold temperatures of 5, 10, and 21 °C showed that the coldest temperatures further reduced perceived sweetness (main effect of Temperature; F (2,40) = 22.83, P < 0.00001) to a degree that limited the amount of measureable adaptation because sweetness became “barely detectable” after just 3 s of prior exposure to the stimulus (Temperature × Time interaction; F (4,80) = 3.79, P < 0.01).
Separate ANOVAs were also carried out on the data for aspartame and sucralose across all 6 temperatures to investigate what appeared to be a trend for adaptation to aspartame to be less temperature-dependent than adaptation to sucralose. A significant Stimulus × Time interaction (F (2,40) = 2.65, P < 0.02) indicated that the sweetness of aspartame did in fact tend to adapt less rapidly than the sweetness of sucralose, but the absence of a Stimulus × Time × Temperature interaction (F (10,200) = 0.84, P = 0.59) meant that this difference in rate for the 2 stimuli was not significantly different across temperatures.
Experiment 3: effects of temperature on sucrose sweetness for still versus flowing solutions
Both effects of temperature on sweet taste intensity and sweet taste adaptation were replicated when the tongue tip was submerged in a flowing sucrose solution. An ANOVA that included all of the data in Figure 5 showed that cooling significantly decreased the intensity of sucrose sweetness (main effect of temperature; F (3,72) = 24,24, P < 0.00001). Tukey HSD tests confirmed that cooling to both 12 and 21 °C led to significantly lower sweetness ratings overall compared with 32 and 38 °C and that baseline sweetness was significantly lower at 12 °C than at 32 and 38 °C. A significant Temperature × Time interaction confirmed that the rate of adaptation to sweetness was again strongly temperature-dependent (F (6,144) = 7.76, P < 0.00001), and Tukey HSD tests showed that it became significant at 21 °C. A significant Flow Rate × Temperature interaction (F (9,216) = 3.53, P < 0.0005) was consistent with the expectation that a flowing solution would more effectively cool the tongue tip than a still solution. To investigate whether the effect of flow rate differed across temperatures, an ANOVA limited to the data for 21 and 32 °C confirmed that the effect of flow rate was significant at 21 °C (Flow Rate × Temperature interaction; F (3,69) = 3.8, P < 0.02) but not 32 °C, and an ANOVA that included only the data for 12 and 21 °C confirmed there was a significant main effect of flow rate (F (3,72) = 6.18, P < 0.01) for both temperatures. However, Tukey HSD tests on these data showed that the effect of flow rate was driven primarily by large differences in sweetness for the zero flow-rate condition compared with all 3 non-zero flow rates (1.5, 2.5, and 4.0mL/s; all P’s < 0.05). This was an unexpected finding that suggests that flow rates faster than 1.5mL/s did not produce enough additional cooling of the lingual surface to further impair sweet taste transduction.
Figure 5.
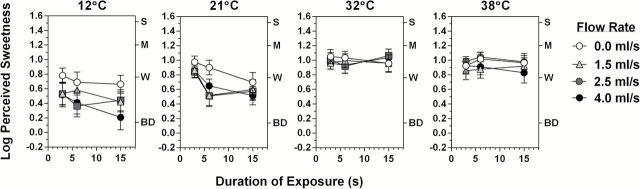
Log10-mean perceived sweetness ratings in response to a 0.5M sucrose solution as a function of the duration of exposure for 4 different temperature and 4 flow rates (0.0–4.0mL/s). Adaptation to sucrose sweetness became significant at 21 °C for all flow rates, confirming that the effect of mild cooling on sweetness adaptation in the prior experiments was not an artifact of dipping the tongue tip into a still taste solution. Error bars represent standard errors of the means; letters on the right y axis refer to intensity descriptors on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong.
Discussion
The present results confirm the recent finding that mild cooling can reduce sweetness by increasing the rate of sweet taste adaptation (Green and Nachtigal 2012) and provide new evidence that solution temperatures below 21 °C can reduce sweetness directly. Attenuation of sweetness at 5 and 10 °C but not 21 °C is consistent with the evidence from Breza et al. (2006) that the response of sucrose-specialist geniculate ganglion neurons in rats is reduced at 10 °C but not at 25 °C. Similarly, Wilson and Lemon (2014) found that the response to 0.56M sucrose in taste neurons in the medulla of mice was nearly identical at 37 and 22 °C but was lower for 18 °C solutions. In the present study, both thermal effects were to some extent stimulus-dependent: Cooling to 21 °C did not alter the rate of sweet taste adaptation for saccharin, whereas it did affect adaptation to the sugars, and the suppression of sweetness at colder temperatures varied across the sugars and was both stronger and more consistent for the artificial sweeteners.
Temperature and sweet taste sensitivity
The stimulus-dependence of the effect of temperature on sweetness brings into question the hypothesis that thermal modulation of the sensitivity of TRPM5 is the sole source of temperature effects on sweet taste (Talavera et al. 2005). Because TRPM5 is a downstream step in the T1R2–T1R3 transduction cascade, its modulation by temperature should affect the sensitivity to all sweet-tasting stimuli equally. The present results imply either that temperature modulates the sensitivity of the T1R2–T1R3 receptor through more than one mechanism or that sweet taste transduction occurs via more than one pathway.
The possibility of multiple sweet taste receptors in humans was raised first by psychophysical studies that found asymmetries in cross-adaptation between sucrose and artificial sweeteners in which the sweeteners were more strongly adapted by sucrose than the reverse (e.g., Schiffman and Cahn 1981; Lawless and Stevens 1983). Biological evidence of multiple sweet taste transduction pathways later came from a study by Bernhardt et al. (1996) in which it was found that sweet-sensitive taste cells in rat taste papillae responded to sucrose with an increase in cAMP and Ca2+ uptake, whereas artificial sweeteners produced an increase in inositol trisphosphate (IP3) and Ca2+. Relevant to the present findings is the fact that saccharin was one of the sweeteners Bernhardt et al. reported increased production of IP3. However, there is no evidence at present for more than one transduction cascade within the T1R2–T1R3 receptor (Kinnamon 2012). The possibility of multiple sweet taste transduction pathways has been raised more recently by Ohkuri et al. (2009) based on studies of the effects of temperature on sweet taste responses in T1R3 or TRPM5 KO mice and by Yee et al. (2011) based on evidence that a glucose transporter (GLUT4) and metabolic sensors (SGLT1, SUR1) are expressed in mouse taste buds. The sensing of sugars via transporters or metabolic sensors could explain the lesser effects of cooling on sugars compared with artificial sweeteners if these other modes of transduction were less temperature-sensitive. On the other hand, it is unclear why sucrose sweetness should be more dependent on the T1R2–T1R3 pathway than fructose sweetness (Figures 1 and 2A). Fructose sweetness was least affected by temperature in the present study, a result that is supported by previous evidence that the response to sucrose in the canine chorda tympani nerve was suppressed more strongly by cooling than was the response to fructose (Nakamura and Kurihara 1991). However, we have found no reports that the fructose transporter GLUT5 (Burant et al. 1992; Wright et al. 2003) is expressed in gustatory tissue, which makes that route of transduction unlikely. Further complicating the transporter explanation of the resistance of fructose sweetness to cooling is evidence from Talavera et al. (2005) that in TRPM5 KO mice, glucose (not fructose) was the only sugar tested for which the chorda tympani response was not completely eliminated.
Alternatively, the differential effects across sweet-tasting stimuli might be explained if temperature affects an early step in the T1R2–T1R3 transduction cascade in addition to TRPM5. The most logical place to exert selective effects is in the Venus fly trap (VFT) or transmembrane (TM) domains, where agonist binding occurs and the chemical selectivity of the T1R2–T1R3 receptor is determined. For example, cold temperatures might affect the conformation of the VFT such that the affinity of some ligands is reduced and/or their ability to change the conformation of the VFT to the closed and active state is impaired (Kunishima et al. 2000; Pin et al. 2003). Despite the fundamental role that conformational changes play in transduction in T1R2–T1R3 and all G-protein-coupled receptors (GPCRs; Kobilka and Deupi 2007; Vaidehi 2010), we could find no published studies of thermal effects on receptor conformation and/or ligand binding in any class of GPCR.
Temperature and sweet taste adaptation
The fact that temperature did not affect the rate of adaptation equally for all sweet ligands further implies that a downstream step in the transduction cascade alone cannot fully explain the thermal effects we observed. The absence of known or hypothesized mechanisms of sweet taste adaptation makes speculation about an upstream effect difficult to evaluate. However, the possibility that temperature affects ligand binding and receptor conformation should also be considered here. For example, if cooler temperatures lower ligand-binding kinetics in the VFT or TM domains, changes in the rate of ligand association and/or disassociation could affect ongoing receptor activity.
It must also be considered that the absence of a temperature effect on adaptation for saccharin might owe to its rapid rate of adaptation even at warm temperatures. Augmentation of this fast rate might not be detectable with the present psychophysical method. However, even independent of temperature, the rapid adaptation again points to a mechanism early in transduction: The slower adaptation of the other stimuli that produce similar sweet taste intensities demonstrates that downstream steps in the cascade remain viable as the sensitivity to saccharin steeply declines. The ability of saccharin to block the T1R2–T1R3 receptor at higher concentrations (>4–6mM) by binding to a hypothesized low-affinity allosteric site in the TM domain (Galindo-Cuspinera et al. 2006) suggests a possible peripheral mechanism for the rapid adaptation. Low-level binding of saccharin at this site might decrease the sensitivity of the receptor without blocking transduction. We are investigating this possibility in a study of self- and cross-adaptation using high and low concentrations of saccharin and sucrose.
Implications for the effect of temperature on sweet taste during normal tasting
It is possible that different results would be obtained if the gustatory regions in the back of the mouth were stimulated instead of or in addition to the fungiform region. However, there is no evidence to date that taste papillae in the foliate, circumvallate, and palatal regions contain candidate sweet taste receptors that are not found in fungiform papillae, whereas there is evidence the opposite may be true. Ninomiya et al. (1993) reported higher sensitivity to sugars in the chorda tympani nerve than in the glossopharyngeal nerve in mice and that the sweet taste inhibitor gurmarin suppressed activity in the chorda tympani nerve but not in the glossopharyngeal nerve (Ninomiya et al. 1997). In humans, thermal induction of sweet taste (Thermal Taste; Cruz and Green 2000), which the temperature sensitivity of TRPM5 has also been proposed to explain (Talavera et al. 2005), was much more frequently reported on the front of the tongue than on the back of the tongue.
Limiting stimulation to the tongue tip provided a level of spatial and temporal control over the effects of solution temperature on chemical taste that is simply not possible with sip-and-spit procedures. When a solution is sipped, it begins to warm as it contacts the anterior surfaces of the mouth and flows posteriorly, and expectoration does not fully clear the mouth of taste stimulus. After expectoration, the residual stimulus continues to warm and a sweet “aftertaste” persists while subjects rate taste intensity. These biophysical factors have very likely contributed to the inconsistent results among psychophysical studies of temperature and sweet taste. For example, in a study that reported no effect of temperature on sucrose sweetness (but did find effects for some other sweeteners), a trained panel swirled small volumes of solution throughout the mouth for 10 s before expectorating, then completed “a full flavor profile” that included 8 or more ratings of different taste and mouth-feel qualities (Schiffman et al. 2000). The long periods of tasting and rating may have contributed to the null effect of temperature on sucrose sweetness.
The temporal effects of whole-mouth tasting may also explain why the effect of mild cooling on sweet taste adaptation (Green and Nachtigal 2012) was not reported in previous studies. Faster adaptation to cool solutions would tend to be counteracted by the spread of solution to other gustatory areas, where sweet taste stimulation would be delivered anew as warming of the solution continued. Support for this view comes from a study mentioned earlier in which cooling reduced the sweetness of glucose, fructose, and aspartame but not the sweetness of saccharin (Green and Frankmann 1988). In that study, 20 °C solutions of glucose or fructose were rated significantly less sweet only after the tongue’s surface had also been cooled to 20 °C by repeated ice water rinses. Based on the present evidence that adaptation increases at 21 °C for all stimuli except saccharin, and that colder temperatures are required to reduce initial sweetness, we believe the results from previous whole-mouth studies primarily reflect the cooling effect on sweet taste adaptation rather than on sweet taste sensitivity per se.
Thus, although the present findings provide new information about the temperature sensitivity of sweet taste transduction and adaptation in humans, they also indicate that under most conditions, the perception of sweetness is effectively independent of temperature. Only when the gustatory surfaces are exposed to temperatures significantly below 20 °C is initial sweetness reduced, and in the modern built environment such cooling occurs only during consumption of refrigerated beverages or foods. In the natural environment, this independence may serve the adaptive function of preventing fluctuations in ambient temperature from impairing the ability to judge the sugar content of foods by their sweetness.
Funding
National Institute on Deafness and other Communicative Disorders of the National Institutes of Health (RO1-DC05002).
References
- Bajec M, Pickering G, DeCourville N. 2012. Influence of stimulus temperature on orosensory perception and variation with taste phenotype. Chem Percept. 5:243–265. [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. 2003. Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Prefer. 14:125–138. [Google Scholar]
- Bartoshuk LM, Rennert K, Rodin J, Stevens JC. 1982. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav. 28:905–910. [DOI] [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B. 1996. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 490(Pt 2):325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. 2006. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol. 95:674–685. [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. 1992. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 267:14523–14526. [PubMed] [Google Scholar]
- Calviño AM. 1986. Perception of sweetness: the effects of concentration and temperature. Physiol Behav. 36:1021–1028. [DOI] [PubMed] [Google Scholar]
- Cruz A, Green BG. 2000. Thermal stimulation of taste. Nature. 403:889–892. [DOI] [PubMed] [Google Scholar]
- Galindo-Cuspinera V, Winnig M, Bufe B, Meyerhof W, Breslin PA. 2006. A TAS1R receptor-based explanation of sweet ‘water-taste’. Nature. 441:354–357. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. 1996. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 21:323–334. [DOI] [PubMed] [Google Scholar]
- Green BG, Frankmann SP. 1987. The effect of cooling the tongue on the perceived intensity of taste. Chem Senses. 12:609–619. [Google Scholar]
- Green BG, Frankmann SP. 1988. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav. 43:515–519. [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D. 2012. Orosensory factors in taste perception: effects of active tasting and solution temperature. Physiol Behav. 107:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Nachtigal D, Hammond S, Lim J. 2012. Enhancement of retronasal odors by taste. Chem Senses. 37:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. 1993. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 18:683–702. [Google Scholar]
- Kinnamon SC. 2012. Taste receptor signalling - from tongues to lungs. Acta Physiol (Oxf). 204:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK, Deupi X. 2007. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 28:397–406. [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. 2000. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 407:971–977. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Stevens DA. 1983. Cross Adaptation of sucrose and intensive sweeteners. Chem Senses. 7:309–315. [Google Scholar]
- Liman ER. 2007. TRPM5 and taste transduction. Handb Exp Pharmacol. 179:287–298. [DOI] [PubMed] [Google Scholar]
- Lu B, Breza JM, Nikonov AA, Paedae AB, Contreras RJ. 2012. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol Behav. 107:533–539. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Collings VB, Glanz LM. 1973. Temperature dependence of human taste response. Physiol Behav. 11:89–94. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kurihara K. 1991. Canine taste nerve responses to monosodium glutamate and disodium guanylate: differentiation between umami and salt components with amiloride. Brain Res. 541:21–28. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Inoue M, Imoto T, Nakashima K. 1997. Lack of gurmarin sensitivity of sweet taste receptors innervated by the glossopharyngeal nerve in C57BL mice. Am J Physiol. 272(3 Pt 2):R1002–R1006. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kajiura H, Mochizuki K. 1993. Differential taste responses of mouse chorda tympani and glossopharyngeal nerves to sugars and amino acids. Neurosci Lett. 163:197–200. [DOI] [PubMed] [Google Scholar]
- Oakley B. 1985. Taste responses of human chorda tympani nerve. Chem Senses. 10:469–481. [Google Scholar]
- Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. 2009. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 296:R960–R971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Galvez T, Prézeau L. 2003. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 98:325–354. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2004. Smell, taste, texture, and temperature multimodal representations in the brain, and their relevance to the control of appetite. Nutr Rev. 62(11 Pt 2):S193–204. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Cahn H, Lindley MG. 1981. Multiple receptor sites mediate sweetness: evidence from cross adaptation. Pharmacol Biochem Behav. 15:377–388. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Sattely-Miller EA, Graham BG, Bennett JL, Booth BJ, Desai N, Bishay I. 2000. Effect of temperature, pH, and ions on sweet taste. Physiol Behav. 68:469–481. [DOI] [PubMed] [Google Scholar]
- Stone H, Oliver S, Kloehn J. 1969. Temperature and ph effects on the relative sweetness of suprathreshold mixtures of dextrose fructose. Percept Psychophys. 5:257–260. [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. 2005. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 438:1022–1025. [DOI] [PubMed] [Google Scholar]
- Vaidehi N. 2010. Dynamics and flexibility of G-protein-coupled receptor conformations and their relevance to drug design. Drug Discov Today. 15:951–957. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Lemon CH. 2014. Temperature systematically modifies neural activity for sweet taste. J Neurophysiol. 112:1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM, Martín MG, Turk E. 2003. Intestinal absorption in health and disease–sugars. Best Pract Res Clin Gastroenterol. 17:943–956. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Sato M. 1965. The effects of temperature on gustatory response of rats. J Cell Physiol. 66:1–17. [DOI] [PubMed] [Google Scholar]
- Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. 2011. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 108:5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotterman Y. 1935. Action potentials in the glossopharyngeal nerve and in the chorda tympani. Skand Arch Physiol. 72:73–77. [Google Scholar]