Abstract
Virtual reality training for complex tasks has been shown to be of benefit in fields involving highly technical and demanding skill sets. The use of a stereoscopic three-dimensional (3D) virtual reality environment to teach a patient-specific analysis of the microsurgical treatment modalities of a complex basilar aneurysm is presented. Three different surgical approaches were evaluated in a virtual environment and then compared to elucidate the best surgical approach. These approaches were assessed with regard to the line-of-sight, skull base anatomy and visualisation of the relevant anatomy at the level of the basilar artery and surrounding structures. Overall, the stereoscopic 3D virtual reality environment with fusion of multimodality imaging affords an excellent teaching tool for residents and medical students to learn surgical approaches to vascular lesions. Future studies will assess the educational benefits of this modality and develop a series of metrics for student assessments.
Background
Virtual reality environments have historically been utilised to simulate hazardous environments for military training exercises or high-risk repair tasks by technicians. Simulators were developed, which immersed the user in a particular environment, as in the case with flight simulators. By the late 1980s, the term ‘virtual reality’ (VR) had been coined1 and immersion technology had been developed.
Research conducted at the University of North Carolina in the early 1990s began to adapt VR technology to stimulate surgery, bolstering the rapid development of such devices in the mid- to late 20th century.2 The integration of multiple imaging modalities—CT, MRI and magnetic resonance angiography—along with increasingly complex haptic interfaces has facilitated the application of VR simulators in laparoscopic surgeries.3
Advances in image-guided surgery via improved diagnostic imaging techniques, three-dimensional (3D) angiography and STEALTH technology have helped in making the use of 3D models very common within the perioperative environment. Thus, VR interfaces have continued to emerge as a preoperative planning modality and an adjuvant training tool for residents and students analysing neurosurgical approaches.1 4 We present an initial evaluation of the ability of a stereoscopic 3D virtual reality environment to provide a patient-specific analysis of various microsurgical approaches for a complex basilar tip aneurysm. To our knowledge, this is the first time that this technology is used in this manner for student education during preoperative planning.
Case presentation
We describe the case of a 12-year-old girl with a history of cerebral palsy who presented to the emergency department (ED) with new-onset seizure. She was subsequently transferred to the neurosurgery service at the University Hospital when a CT scan demonstrated a large extra-axial mass directly anterior to the mesencephalon with a substantial mass effect (figure 1A). The patient was awake, alert and non-verbal with a physical examination significant for hypertonia, contracture and spasticity in all extremities. T1-weighted MRI of the brain revealed a large, partially enhancing mass with a notable pulsation artefact (figure 1B). CT angiography was performed using a GE Systems LightSpeed 16-slice CT scanner. A total of 150 cc contrast was injected intravenously via the antecubital vein at a rate of 4 cc/s. Images were then obtained at 0.625 mm slice thickness with no overlap following an 18-s acquisition delay. Source images were then transferred to the GE Advantix 3D workstation where maximal intensity projections and 3D reconstructions were generated. Imaging confirmed a 2.2 cm × 1.8 cm basilar tip aneurysm with the incorporation of the left P1 segment of the posterior cerebral artery.
Figure 1.
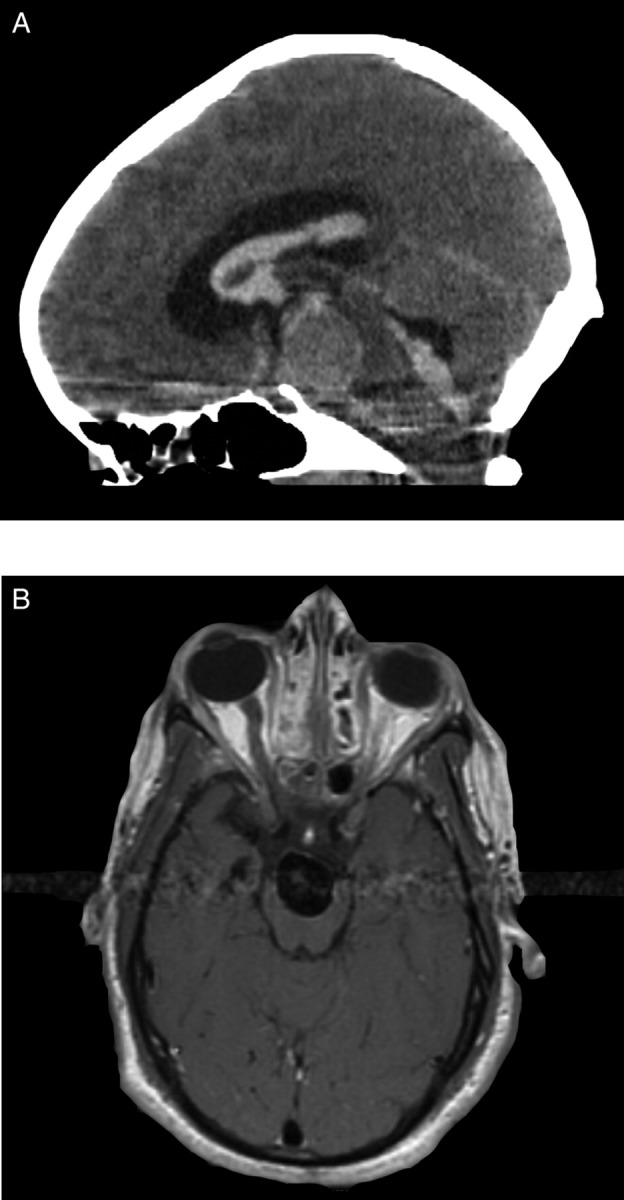
(A) CT scan demonstrated a large extra-axial mass directly anterior to the mesencephalon with a substantial mass effect. (B) T1-weighted MRI of the brain revealed a large, partially enhancing mass with a notable pulsation artefact.
Investigations
CT angiogram (CTA) and MRI scans were then imported into the Dextroscope system (Bracco Diagnostics Inc., Princeton, New Jersey, USA). The Dextroscope is a 3D stereoscopic VR system that allows the user to create and manipulate patient-specific anatomic models generated from the diagnostic imaging. Designed in Singapore in 1999, the first large case series describing its use and applications was published in 2000. The Dextroscope is a two-part system: a computer where image data are initially imported as well as compiled and a VR system where the resultant 3D models can be analysed in stereoscopic vision. The user interacts with the VR system via the use of a dual-handed control system: a six-dimensional controller and a pointing device. The combination of these devices allows maximal degrees of freedom for rotation and the use of various crop and drill tools to reveal anatomy and simulate surgical approaches. The technology also offers the user the ability to fuse various imaging modalities and to segment and highlight relevant structures.
The patient's CTA and T1-weighted MRI images (sagittal, coronal and axial views) were imported in Digital Imaging and Communications in Medicine (DICOM) format into the Dextroscope system utilising the system's native image retrieval environment. Once imported, the 16-bit DICOM images were converted into 8-bit volume objects. The two-dimensional (2D) series for each object was enhanced for best viewing via brightness and contrast controls.
Once loaded into the VR environment, the MRI and CTA volumes were co-registered using Dextroscope's proprietary software in combination with bony landmarks and vascular anatomy as delineated by the investigator. Proper co-registration was confirmed visually by cropping each MRI volume in all three planes sequentially and verifying against bony, parenchymal and vascular landmarks. Following co-registration, each volume was rendered and coloured for maximal anatomical visualisation and accuracy. The CTA volumetric images were windowed for viewing the vasculature and skull anatomy, while the MRI volumes were windowed for an optimum view of parenchymal anatomy, cranial nerves and cisternal spaces (figure 2A,B).
Figure 2.
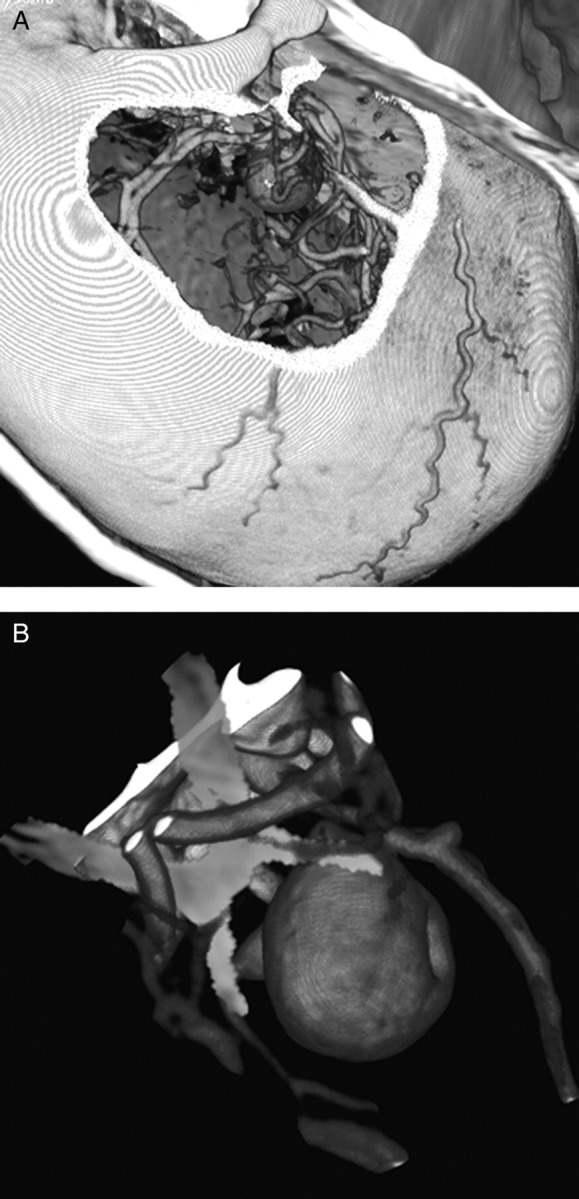
(A). The CTA volumetric images were windowed for viewing the vasculature and skull anatomy. (B) MRI volumes were windowed for an optimum view of parenchymal anatomy, cranial nerves and cisternal spaces, here allowing for the rendering of the optic chiasm (in yellow).
The CTA volumetric image was segmented to isolate the aneurysm and the surrounding vasculature and assigned specific colours for ease of identification. The optic and oculomotor nerves were identified on the MRI volume and then segmented and re-coloured for ease of identification. The merged images were then used by members of the department in various stages of training to position the ‘patient’ for standard approaches to the basilar tip and perform the craniotomy and ‘retraction’ of the parenchyma by carefully using the drill tool to remove the bony and parenchymal portion to provide accurate line-of-sight and simulate retraction in the virtual interface.
Outcome and follow-up
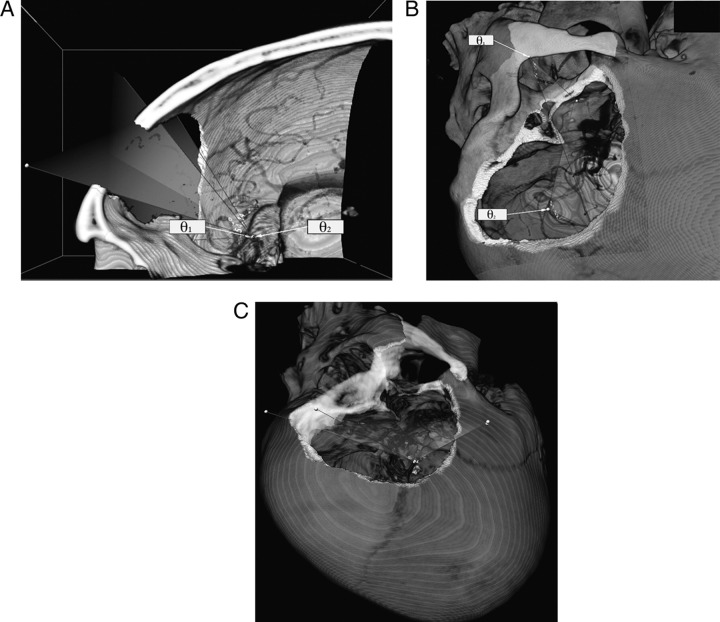
Several approaches were modelled using the Dextroscope VR interface. A subtemporal and a modified pterional approach with an orbitozygomatic craniotomy were assessed as possible surgical approaches for this aneurysm. Specific aspects of the approach with regard to the line-of-sight, skull base anatomy and visualisation of relevant anatomy at the basilar artery and surrounding structures were qualitatively and quantitatively assessed as denoted by the maximum angles of approach and the effective working area. Fusion of different image modalities (MRI and CTA) was important for determining vessel/aneurysm relations to parenchymal anatomy and cisternal spaces. Qualitative differences were noted in the three basic approaches to the skull base. Orbitozygomatic osteotomy (figure 3A) increased the exposure to the superior and posterior parts of the skull base and parenchymal anatomy as compared with the pterional craniotomy (figure 3B). By minimising the approach to include a zygomatic osteotomy, a substantial increase in exposure to the middle fossa was achieved (figure 3C). Advantages of this technique compared with cadaveric studies include the ability to render patient-specific and pathology-specific analyses of approaches to a specific lesion by ‘practicing’ each exposure and assessing its efficacy in a virtual reality environment.
Figure 3.
(A) The angle of attack provided by the pterional approach (θ1) is notably smaller than that allowed by performing an orbitozygomatic craniotomy. (B) Another view of the zygoma limiting the angle of attack when accessing the superior parts of the skull base (θ1), although the presence of the zygoma appears less important for visualising the posterio-inferior anatomy (θ2). (C) Increase in middle fossa exposure provided by the zygomatic osteotomy.
The subtemporal approach was conducted in the VR system via the use of the drill tool. A 4 cm×4 cm craniotomy was created in the temporal bone, following which the drill and crop tools were used to pass through the dura, retract the inferior temporal gyrus and expose the dome of the aneurysm. The patient's posterior cerebral artery was found to be running parallel to the exposed field, obscuring the neck of the aneurysm.
The transylvian approach was modelled after performing a pterional craniotomy. The sylvian fissure was dissected to expose branches of the middle cerebral artery (MCA) that would eventually need to be retracted. The MRI volume was then cropped away in the sagittal view to identify the M1 branch and the vascular complex surrounding the basilar tip aneurysm. Analysis from this vantage point also suggested obstruction of the aneurysm neck via the P1 branch of the posterior cerebral artery and the posterior communicating artery.
The modified pterional approach was conducted via an orbitozygomatic craniotomy allowing for approach through the oculomotor–carotid canal or the optico-carotid canal. The posterior clinoid was found to obstruct the line-of-sight. Following further drilling, it was found that the aneurysm neck was difficult to see through the optico–carotid triangle, even after several angle readjustments. The oculomotor-carotid canal also showed neck obstruction from clinoid. These views suggested that fenestrated clips would have to be used.
After evaluating the various surgical approaches utilising the training system, it was deemed that an endovascular approach should be considered first. Having discussed this at length with the patient's family, the family decided to forego any treatment. The child sustained a haemorrhage 138 days later from which she did not recover.
Discussion
Multiple trials afforded the resident the opportunity to adjust approaches and maximise the line-of-sight in surgical planning. The environment provides the trainee the opportunity to both plan and re-evaluate the patient-specific obstructions and difficulties seen with each approach. Residents and students with varying levels of training were able to use the models and identify different points of obstruction, and readjust the line-of-sight to get a sense of what would need to be done intra-operatively to successfully clip the aneurysm. Senior residents and attending physicians came to similar conclusions, but commented on the inability to simulate adequate retraction and resultant vessel deformation, which would allow for a more accurate surgical simulation. Also noted was the inability to account for small perforating vessels around the basilar artery and posterior cerebral artery (PCA)/MCA branches, a limitation of the CTA imaging modality within the VR environment.
The virtual environment with full rotational capabilities permits the user to simulate the proper surgical set-up, patient positioning and unlimited opportunities to ‘drill’ the ideal craniotomy. The use of volume segmentation to isolate and colourise the object vascular pathology as well as the fusion of CTA and MRI images provide the trainee with the opportunity to discover and appropriately address patient-specific anatomical variances and structural impediments to aneurysm clipping presented by various surgical approaches. Furthermore, it allows the neurosurgery resident to attempt the surgical planning and virtually simulate the visual portion of the operative experience of cases.
Given the risks associated with aneurysm surgery, as was noted in the case reported here, an endovascular approach was eventually deemed to be the optimal intervention. The rise in successful endovascular therapies has led to a relative decrease in the number of microsurgical operations performed yearly, leaving a lesser number of operations to be distributed across trainees. Inherent in the technology's appeal is the ability for the student or resident to perform multiple trials on the patient-specific models, without having to be in the OR. This particular interface was limited by a lack of appropriate haptics and ability to appropriately simulate retraction, but it qualitatively offers an appropriate visual experience with respect to vascular and parenchymal anatomy. Future studies will quantitatively assess the educational benefits of this modality and develop appropriate metrics.5
Learning points.
The three-dimensional environment is gaining acceptance as both a training environment and a tool for operative planning in neurosurgery.
Extending the spectrum of use to include medical student education would allow the experience in the operating room to be more familiar and thus more educational.
Refinements such as haptic interfaces and tissue deformation algorithms will be necessary before virtual environments can realistically be used to preselect students with exceptional dexterity; currently available systems provide an appropriate avenue for familiarising medical students and novice residents with the surgical anatomy they will encounter in the operating room, while affording more advanced residents an opportunity to explore various surgical approaches and the benefits and complications afforded by each.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Spicer MA, Apuzzo ML. Virtual reality surgery: neurosurgery and the contemporary landscape. Neurosurgery 2003;52:489–97; discussion 96-7. [DOI] [PubMed] [Google Scholar]
- 2.Wright DL, Rolland JP, Kancherla AR. Using virtual reality to teach radiographic positioning. Radiol Technol 1995;66:233–8; quiz 9-40. [PubMed] [Google Scholar]
- 3.Baur C, Guzzoni D, Georg O. VIRGY: a virtual reality and force feedback based endoscopic surgery simulator. Stud Health Technol Inform 1998;50:110–16. [PubMed] [Google Scholar]
- 4.Henn JS, Lemole GM, Jr, Ferreira MA, et al. Interactive stereoscopic virtual reality: a new tool for neurosurgical education. Technical note. J Neurosurg 2002;96:144–9. [DOI] [PubMed] [Google Scholar]
- 5.Bajka M, Tuchschmid S, Fink D, et al. Establishing construct validity of a virtual-reality training simulator for hysteroscopy via a multimetric scoring system. Surg Endosc 2010;24:79–88. [DOI] [PubMed] [Google Scholar]