Abstract
STUDY QUESTION
Are urinary BPA concentrations associated with in vitro fertilization (IVF) outcomes among women attending an academic fertility center?
SUMMARY ANSWER
Urinary BPA concentrations were not associated with adverse reproductive and pregnancy outcomes among women from a fertility clinic.
WHAT IS KNOWN ALREADY
Bisphenol A (BPA), an endocrine disruptor, is detected in the urine of most Americans. Although animal studies have demonstrated that BPA reduces female fertility through effects on the ovarian follicle and uterus, data from human populations are scarce and equivocal.
STUDY DESIGN, SIZE AND DURATION
This prospective cohort study between 2004 and 2012 at the Massachusetts General Hospital Fertility Center included 256 women (n = 375 IVF cycles) who provided up to two urine samples prior to oocyte retrieval (total N = 673).
PARTICIPANTS/MATERIALS, SETTINGS, METHODS
Study participants were women enrolled in the Environment and Reproductive Health (EARTH) Study. Intermediate and clinical end-points of IVF treatments were abstracted from electronic medical records. We used generalized linear mixed models with random intercepts to evaluate the association between urinary BPA concentrations and IVF outcomes adjusted by age, race, body mass index, smoking status and infertility diagnosis.
MAIN RESULTS AND THE ROLE OF CHANCE
The specific gravity-adjusted geometric mean of BPA was 1.87 µg/l, which is comparable to that for female participants in the National Health and Nutrition Examination Survey, 2011–2012. Urinary BPA concentrations were not associated with endometrial wall thickness, peak estradiol levels, proportion of high quality embryos or fertilization rates. Furthermore, there were no associations between urinary BPA concentrations and implantation, clinical pregnancy or live birth rates per initiated cycle or per embryo transfer. Although we did not find any associations between urinary BPA concentrations and IVF outcomes, the relation between BPA and endometrial wall thickness was modified by age. Younger women (<37 years old) had thicker endometrial thickness across increasing quartiles of urinary BPA concentrations, while older women (≥37 years old) had thinner endometrial thickness across increasing quartiles of urinary BPA concentrations.
LIMITATIONS, REASONS FOR CAUTION
Limitations to this study include a possible misclassification of BPA exposure and difficulties in extrapolating the findings to the general population.
WIDER IMPLICATIONS OF THE FINDINGS
Data on the relation between urinary BPA concentrations and reproductive outcomes remain scarce and additional research is needed to clarify its role in human reproduction.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by NIH grants R01ES022955, R01ES009718 and R01ES000002 from the National Institute of Environmental Health Sciences (NIEHS) and grant T32DK00770316 from the National Institute of Child Health and Human Development (NICHD). None of the authors has any conflicts of interest to declare. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Keywords: bisphenol A, IVF outcomes, epidemiology, reproductive health, endocrine disruptor
Introduction
Bisphenol A (BPA) is a high production volume chemical that has received substantial scientific and regulatory attention over the past decade. BPA is widely used in the manufacture of a variety of consumer products such as polycarbonate plastics, epoxy resin liners of canned foods, some dental sealants and composites, and thermal receipts (Ehrlich et al., 2014). BPA was detected in over 90% of urine samples obtained from participants in the 2003–2004 and 2011–2012 National Health and Nutrition Examination Survey (NHANES) (Calafat et al., 2008; Centers for Disease Control and Prevention (CDC), 2015), showing that there is widespread general population exposure (Vandenberg et al., 2007). Aglycone (unconjugated) BPA has weak estrogenic activity through binding with estrogen receptors α and β (Gould et al., 1998, Kuiper et al., 1998). In addition, aglycone BPA has high affinity for two membrane-bound estrogen receptors, G protein-coupled estrogen receptor 30 (GPR30) (Dong et al., 2011) and membrane estrogen receptor alpha (mERα) (Wozniak et al., 2005), as well as for an orphan nuclear estrogen-related receptor gamma (ERRγ) (Matsushima et al., 2007, Okada et al., 2008). BPA has also been shown in experimental animal studies to bind to the androgen receptor, peroxisome proliferator-activated receptor γ, and thyroid hormone receptor (Richter et al., 2007).
These endocrine activities of BPA have been shown to lead to adverse reproductive outcomes in animal models. Animal data have shown that BPA primarily affects female fertility through its effects on the ovarian follicle and uterus. For example, BPA adversely affects oocyte meiosis (Hunt et al., 2003; Brieno-Enriquez et al., 2011), interferes with germ cell nest breakdown (Rivera et al., 2011), reduces the primordial follicle pool by stimulating their initial recruitment and subsequent follicle development until the antral stage (Rivera et al., 2011), alters ovarian steroidogenesis (Fernandez et al., 2010; Xi et al., 2011), modifies normal uterine morphology (Berger et al., 2010), and impairs uterine receptivity and ova-implantation (Berger et al., 2007, 2008; Crawford and Decatanzaro, 2012). There is limited evidence on the effect of BPA on pregnancy outcomes. Low-dose BPA exposure (<1600 µg/day) has reduced the number of live pups born in exposed CD1 mice (Cabaton et al., 2011) and Holtzman rats (Salian et al., 2009b). Moreover, it has decreased the number of total pups born to unexposed female Holtzman rats mated to neonatally and gestationally exposed males of the same strain (Salian et al., 2009a,b). However, other studies in mice and rats have shown that higher-dose BPA exposure (up to 50 mg/day) is not associated with the number of live pups or total number of delivered pups (Howdeshell et al., 2008; Tyl et al., 2008; Thuillier et al., 2009; Kobayashi et al., 2010, 2012; Ryan et al., 2010; Xi et al., 2011; Nanjappa et al., 2012).
Several studies have investigated the impact of BPA on female reproductive and pregnancy outcomes (Galloway et al., 2010; Mok-Lin et al., 2010; Bloom et al., 2011a,b; Fujimoto et al., 2011; Ehrlich et al., 2012a,b). We previously reported that in women undergoing in vitro fertilization (IVF), urinary BPA concentrations were inversely associated with peak serum estradiol levels, number of oocytes at retrieval (overall and mature), and number of normally fertilized oocytes (Mok-Lin et al., 2010; Ehrlich et al., 2012b). We also reported that higher urinary BPA concentrations were associated with a suggestive decrease in trend of implantation (Ehrlich et al., 2012a). The objective of the current analysis was to reevaluate, in a much larger number of women from the same cohort (Mok-Lin et al., 2010; Ehrlich et al., 2012a,b), the associations of urinary BPA concentrations with early IVF outcomes (i.e. peak estradiol, oocyte yield, fertilization rate). In addition, we expand on our previous research (Mok-Lin et al., 2010; Ehrlich et al., 2012a,b) by exploring the potential relationships of urinary BPA concentrations with clinical pregnancy and live birth outcomes.
Materials and Methods
Study population
Study participants were women enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established to evaluate environmental and dietary determinants of fertility (Hauser et al., 2006). Women between 18 and 45 years old were eligible to participate and ∼60% of those contacted by the research nurses enrolled. The current analysis includes 256 women who completed at least one IVF cycle between November 2004 and April 2012 (n = 375 cycles) at the Massachusetts General Hospital (MGH) Fertility Center, and had provided at least one urine sample for the measurement of BPA per IVF cycle. IVF cycles in which women used an oocyte donor (n = 18) or cryopreservation-thaw cycles (n = 34) were excluded from the present study. The study was approved by the Human Studies Institutional Review Boards of the MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC). Participants signed an informed consent after the study procedures were explained by a research nurse and all questions were answered.
Clinical management and assessment of outcomes
Clinical information was abstracted from the patient's electronic medical record by research staff. Follicle stimulating hormone (FSH) and estradiol concentrations were measured in blood serum, collected on the third day of the menstrual cycle, using an automated electrochemiluminescence immunoassay at the MGH Core Laboratory as previously described (Mok-Lin et al., 2010). The peak estradiol concentration was defined as the highest level of estradiol prior to oocyte retrieval, which was obtained on the day of trigger with hCG. Subsequent to an infertility evaluation, each patient was assigned an infertility diagnosis by a physician at the MGH Fertility Center according to the Society for Assisted Reproductive Technology (SART) definitions as previously described (Mok-Lin et al., 2010). The participant's date of birth was collected at entry, and weight and height were measured by the nurse. Body mass index (BMI) was calculated as weight (in kilograms) per height (in meters) squared.
Women underwent one of three controlled ovarian stimulation IVF treatment protocols on Day 3 of induced menses after completing a cycle of oral contraceptives: (i) luteal phase GnRH-agonist protocol, (ii) follicular phase GnRH-agonist/Flare protocol or (iii) GnRH-antagonist protocol. Lupron dose was reduced at, or shortly after, the start of ovarian stimulation with FSH/hMG in the luteal phase GnRH-agonist protocol. FSH/hMG and GnRH-agonist or GnRH-antagonist was continued to the day of trigger with human chorionic gonadotrophin (hCG). Throughout the monitoring phase of the subject's IVF treatment cycle, estradiol levels were obtained (Elecsys Estradiol II reagent kit, Roche Diagnostics). Oocyte retrieval was completed when follicle dimensions on transvaginal ultrasound reached 16–18 mm and the estradiol level reached at least 500 pg/ml. Patients were monitored during gonadotrophin stimulation for serum estradiol, follicle size measurements and counts, and endometrial thickness through to 2 days before oocyte retrieval. Human chorionic gonadotrophin (hCG) was administered ∼36 h before the scheduled oocyte retrieval procedure to induce ovulation. Details of the oocyte retrieval have been previously described (Mok-Lin et al., 2010).
Embryologists determined the total number of oocytes retrieved per cycle and classified them as germinal vesicle, metaphase I, metaphase II (MII) or degenerated. Oocytes underwent either conventional IVF or intracytoplasmic sperm injection (ICSI) as clinically indicated. Embryologists determined the fertilization rate 17–20 h after insemination as the number of oocytes with two pronuclei divided by the number of MII oocytes inseminated. We classified embryo quality based on morphology and number of blastomeres, ranging from 1 (best) to 5 (worst) on Day 2 and 3. For analysis, we classified embryos as best quality if they had 4 cells on Day 2, 8 cells on Day 3, and a morphologic quality score of 1 or 2 on Days 2 and 3 (Veeck and Zaninovic, 2003). An overall score of 1 or 2 was considered high quality, 3 was considered intermediate quality and 4 or 5 indicated poor quality embryos.
In women who underwent an embryo transfer, clinical outcomes were assessed. Implantation was defined as a serum β-hCG level >6 mIU/ml, typically measured 17 days (range 15–20 days) after oocyte retrieval. An elevation in β-hCG with the confirmation of an intrauterine pregnancy on an ultrasound at 6 weeks was considered a clinical pregnancy. A live birth was defined as the birth of a neonate on or after 24 weeks gestation.
Urine sample collection and BPA measurements
Women provided up to two spot urine samples per IVF cycle, with the first one (not necessarily a fasting sample) collected between Day 3 and Day 9 of the gonadotrophin phase, and the second one, always a fasting sample, collected on the day of oocyte retrieval, prior to the procedure or administration of intravenous fluids. Urine was collected in a sterile, clean polypropylene specimen cup at the MGH Fertility Center. Specific gravity (SG), which was used to correct BPA concentrations for urine dilution, was measured at room temperature using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement within an hour of the urine being produced. The urine was then divided into aliquots, frozen and stored at −80°C. Samples were shipped on dry ice overnight to the CDC where they were stored at or below −40°C until analysis. The urinary concentrations of the sum of free and conjugated BPA species (total BPA) were measured using online solid-phase extraction (SPE) coupled with isotope dilution-high-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS), as described before (Ye et al., 2005). First, 100 µl of urine was treated with β-glucuronidase/sulfatase (Helix pomatia, H1; Sigma Chemical Co, St. Louis, MO, USA) to hydrolyze the BPA-conjugated species. BPA was then retained and concentrated on a C18 reversed-phase size-exclusion SPE column (Merck KGaA, Germany), separated from other urine matrix components using a pair of monolithic HPLC columns (Merck KGaA), and detected by negative ion-atmospheric pressure chemical ionization-MS/MS. The limit of detection (LOD) was 0.4 µg/l. In addition to study samples, each analytical run included low-concentration and high-concentration quality control materials, prepared with spiked pooled human urine, and reagent blanks to assure the accuracy and reliability of the data (Ye et al., 2005). BPA concentrations were adjusted for dilution using the following formula: Pc = P[(1.015 − 1)/SG − 1], where Pc is the SG-corrected BPA concentration (µg/l), P is the measured BPA concentration (µg/l) of the urine sample, and 1.015 is the mean SG concentration in the study population (Smith et al., 2012). The geometric mean of the SG-adjusted BPA concentrations from two spot urine samples collected during each IVF cycle was used as a measure of cycle-specific urinary BPA concentration. For cycles with only one urine sample (∼20%), the BPA concentration for that single urine sample was used as the cycle-specific urinary BPA concentration. BPA concentrations below the LOD were assigned a value equal to the LOD divided by the square root of 2 prior to SG adjustment as described previously (Meeker et al., 2010).
Statistical analysis
Demographic and baseline reproductive characteristics of the women are presented using median ± interquartile ranges (IQRs) or percentages. Women's exposures to BPA were categorized into quartiles of urinary BPA concentrations (based on the woman's cycle-specific SG-adjusted geometric mean of BPA as described above) with the lowest quartile considered as the reference group. Associations between urinary BPA concentrations and demographics and baseline reproductive characteristics were evaluated using Kruskal–Wallis tests for continuous variables and chi-squared tests for categorical variables (data not shown). Multivariable generalized linear mixed models with random intercepts were used to evaluate the association between urinary BPA concentrations and IVF outcomes. A Poisson distribution and log link function were specified for oocyte counts, a normal distribution and identity link function were specified for endometrial wall thickness and E2 trigger levels, and a binomial distribution and logit link function were specified for embryo quality, fertilization rates, and clinical outcomes (implantation, clinical pregnancy and live birth). To explore whether associations between urinary BPA concentrations and IVF outcomes were modified by age and insemination method, product cross-product term of quartiles of urinary BPA concentrations and the modifier (both as ordinals variables) was entered into the model. Tests for linear trends (Rosner, 2000) were conducted using the median values of each quartile of urinary BPA concentration as a continuous variable. To allow for better interpretation of the results, population marginal means (Searle et al., 1980) are presented which accounts for all the covariates in the model.
Confounding was assessed using prior knowledge on biological relevance and descriptive statistics from our study population through the use of directed acyclic graphs (Weng et al., 2009). The variables considered as potential confounders included factors previously related to IVF outcomes in this and other studies, and factors associated with BPA exposure and IVF outcomes in this study, regardless of whether they had been previously described as predictors of IVF outcomes (Table I). Because collection of the samples included in this analysis spanned 8 years, and during this period urinary BPA concentrations and IVF success rates may have changed, a variable for calendar year was considered but not retained in the models because the estimates remained very similar with and without inclusion of calendar year. Final models were adjusted for age (continuous), BMI (continuous), race (white versus nonwhite), smoking status (never versus ever) and infertility diagnosis (male factor, female factor, unexplained). All tests were two-tailed and the level of statistical significance was set at 0.05. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
Table I.
Baseline characteristics of 256 women in the Environment and Reproductive Health Study (EARTH) by quartiles of specific gravity adjusted urinary BPA concentrations (μg/l).
| Total cohort | Q1 | Q4 | P, valuea | |
|---|---|---|---|---|
| Median (IQR) or N (%) | Median (IQR) or N (%) |
|||
| Baseline characteristics | ||||
| Age, years | 35.0 (32.5, 39.0) | 36.0 (33.0, 39.0) | 35.0 (32.0, 39.0) | 0.44 |
| Race/ethnic group, n (%) | ||||
| White/Caucasian | 211 (82.4) | 60 (74.1) | 35 (87.5) | 0.06 |
| Black | 6 (2.4) | 1 (1.2) | 3 (7.5) | |
| Asian | 21 (8.2) | 12 (14.8) | 1 (2.5) | |
| Other | 18 (7.0) | 8 (9.9) | 1 (2.5) | |
| Body mass index, kg/m2 | 23.0 (21.0, 26.0) | 23.0 (21.5, 25.9) | 22.5 (21.3, 24.4) | 0.62 |
| Ever smoker, n (%) | 71 (27.7) | 19 (23.5) | 13 (32.5) | 0.70 |
| Education, n (%) | 0.86 | |||
| < College graduate | 20 (7.8) | 5 (6.6) | 4 (10.5) | |
| College graduate | 77 (30.1) | 21 (28.0) | 13 (34.2) | |
| Graduate degree | 140 (59.1) | 49 (65.3) | 21 (55.3) | |
| Baseline reproductive characteristics | ||||
| Previous IUI, n (%) | 120 (46.7) | 33 (40.7) | 16 (40.0) | 0.27 |
| Previous IVF, n (%) | 61 (23.8) | 24 (29.6) | 9 (22.5) | 0.03 |
| Initial infertility diagnosis, n (%) | 0.77 | |||
| Male factor | 94 (36.7) | 32 (39.5) | 17 (42.5) | |
| Female factor | 78 (30.5) | 22 (27.2) | 14 (35.0) | |
| Diminished ovarian reserve | 18 (7.0) | 4 (4.9) | 3 (7.5) | |
| Endometriosis | 18 (7.0) | 6 (7.4) | 3 (7.5) | |
| Ovulation disorders | 22 (8.6) | 4 (4.9) | 5 (12.5) | |
| Tubal | 18 (7.1) | 6 (7.4) | 3 (7.5) | |
| Uterine | 2 (0.8) | 2 (2.4) | 0 (0) | |
| Unexplained | 84 (32.8) | 27 (33.3) | 9 (22.5) | |
| Initial treatment protocol, n (%) | ||||
| Antagonist | 29 (11.3) | 12 (14.8) | 7 (17.5) | 0.51 |
| Flareb | 41 (16.0) | 13 (16.1) | 7 (17.5) | |
| Luteal phase agonistc | 186 (72.3) | 56 (69.1) | 26 (65.0) | |
| Initial ICSI cycles, n (%) | 130 (53.3) | 44 (55.7) | 21 (53.8) | 0.83 |
| E2 trigger levels, pmol/l | 2017.0 (1453.0, 2639.0) | 2100.0 (1530.0, 2540.5) | 1773.0 (1325.0, 2630.0) | 0.39 |
| Day 3 FSH levels, IU/ l | 6.9 (5.8, 8.3) | 6.8 (5.6, 8.5) | 7.4 (6.3, 8.2) | 0.17 |
| Embryo transfer day, n (%) | ||||
| No embryos transferred | 27 (10.5) | 8 (9.9) | 1 (2.5) | |
| Day 2 | 13 (5.7) | 3 (4.1) | 1 (2.6) | 0.52 |
| Day 3 | 134 (55.5) | 48 (65.8) | 25 (64.1) | |
| Day 5 | 82 (35.8) | 22 (30.1) | 13 (33.3) | |
| Number of embryos transferred, n (%) | ||||
| No embryos transferred | 27 (10.5) | 8 (9.9) | 1 (2.5) | 0.09 |
| 1 embryo | 29 (11.3) | 9 (11.1) | 6 (15.0) | |
| 2 embryos | 150 (58.6) | 44 (54.3) | 27 (67.5) | |
| 3+ embryos | 47 (18.6) | 20 (24.7) | 6 (15.0) | |
BPA, bisphenol A; IQR, interquartile range; N, number; IUI, intrauterine insemination, IVF, in vitro fertilization, ICSI, intracytoplasmic sperm injection.
aFrom Kruskal–Wallis test for continuous variables and χ2 tests for categorical variables.
bFollicular phase GnRH-agonist/Flare protocol.
cLuteal phase GnRH-agonist protocol.
Results
The 256 women included in this analysis were predominantly Caucasian (82%) and the majority (71%) had never smoked (Table I). These women had a median age of 35 years (IQR: 33–39) and a median BMI of 23.0 kg/m2 (IQR: 21.0–26.0). The primary SART diagnosis at enrollment was male factor (37%) followed by unexplained infertility (33%) and female factor infertility (31%) (ovulation disorders were the most common followed by tubal disorders, diminished ovarian reserve, endometriosis, and uterine disorders). Luteal phase GnRH-agonist protocols were the most commonly used stimulation protocol in the first treatment cycle (72%). Women had a median day 3 FSH of 6.9 IU/l. Caucasian and African-American women had higher urinary BPA concentrations as compared with Asian women (P, trend = 0.06). No other baseline characteristics were significantly related to urinary BPA concentrations.
The geometric mean of the 673 SG-adjusted urine samples provided by the 256 women (contributing to 375 IVF cycles) was 1.87 µg/l (Table II), comparable to that for females in NHANES 2009–2010 and 2011–2012 (2.09 and 1.91 µg/l, respectively) (Centers for Disease Control and Prevention (CDC), 2015) and similar to our earlier analysis (2.56 µg/l) (Ehrlich et al., 2012a). Two urine samples were collected in 80% (299/375) of the IVF cycles. Detectable concentrations of BPA were measured in 88.7% (604/673) of urine samples (Table II).
Table II.
Distribution of cycle-specific geometric mean of urinary BPA concentrations (μg/l) among 256 women in the Environment and Reproductive Health Study (EARTH) undergoing 375 IVF cycles (673 urine samples).
| Percentile |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detection rate | GM (SD) | Min | 10th | 25th | 50th | 75th | 90th | 95th | Max | |
| BPA | 88.7% | 2.06 (2.20) | <LOD | 0.57 | 0.89 | 1.47 | 2.40 | 3.87 | 5.48 | 22.07 |
| SG-adj BPA | 1.87 (1.57) | <LOD | 0.74 | 0.97 | 1.38 | 2.24 | 3.50 | 4.87 | 16.55 | |
All values below LOD were assigned a value equal to the LOD divided by √2. Seven (1.9%) cycle-specific BPA concentrations were < LOD and are included in the percentiles. There were 69 (10.3%) individual urine samples which had BPA concentrations < LOD. There was an 88.7% detection rate for all individual urine samples [SG range (1.001–1.038)].
< LOD, below limit of detection (0.4 μg/l); Max, maximum; Min, minimum; SG-adj BPA, specific-gravity adjusted BPA concentrations.
The associations of urinary BPA concentrations with endometrial thickness and ovarian stimulation outcomes are shown in Table III. Although the overall trend was not statistically significant in adjusted models, women with urinary BPA concentrations in the highest quartile had slightly fewer total oocytes (n = 9.9) and MII oocytes (n = 8.3) compared with women in the lowest quartile (N = 10.9, P = 0.13; and N = 9.1, P = 0.21, respectively). Urinary BPA concentrations were not related to endometrial wall thickness or to peak estradiol levels. Similarly, urinary BPA concentrations were not associated with the proportion of high-quality embryos or the fertilization rates, either overall (Table III) or when IVF and ICSI cycles were examined separately (data not shown).
Table III.
Specific gravity adjusted urinary bisphenol A concentrations in relation to ovarian stimulation and endometrial thickness outcomes among 256 women in the Environment and Reproductive Health Study (EARTH) contributing to 375 fresh IVF cycles from an infertility clinic.
| Total oocyte yield, n |
MII oocyte yield, n |
|||
|---|---|---|---|---|
| Urinary BPA concentrations (μg/l) | Unadjusted | Adjusteda | Unadjusted | Adjusteda |
| Q1 [<LOD–0.96] | 11.0 (10.0, 12.1) | 10.9 (9.9, 12.0) | 9.2 (8.3, 10.1) | 9.1 (8.2, 10.0) |
| Q2 [0.97–1.37] | 11.0 (10.0, 12.1) | 10.8 (9.9, 11.9) | 9.1 (8.2, 10.0) | 9.0 (8.1, 9.9) |
| Q3 [1.38–2.20] | 11.1 (10.1, 12.3) | 10.9 (9.9, 12.0) | 9.8 (8.9, 10.8) | 9.6 (8.7, 10.6) |
| Q4 [2.24–16.55] | 9.9 (10.0, 11.0) | 9.9 (8.9, 11.0) | 8.3 (7.5, 9.3) | 8.4 (7.6, 9.3) |
| P, trendb | 0.09 | 0.13 | 0.13 | 0.21 |
|
Endometrial wall thickness, mm |
E2 trigger levels, pmol/l |
|||
| Urinary BPA concentrations (μg/l) | Unadjusted | Adjusteda | Unadjusted | Adjusteda |
| Q1 [<LOD–0.96] | 9.9 (9.5, 10.3) | 9.9 (9.5, 10.3) | 2147.5 (1971.7, 2323.4) | 2112.9 (1938.3, 2287.4) |
| Q2 [0.97–1.37] | 10.2 (9.8, 10.6) | 10.2 (9.8, 10.6) | 2149.9 (1973.0, 2326.8) | 2132.2 (1957.3, 2306.9) |
| Q3 [1.38–2.20] | 10.6 (10.2, 11.0) | 10.6 (10.2, 11.0) | 2099.9 (1923.9, 2275.8) | 2098.2 (1923.8, 2272.6) |
| Q4 [2.24–16.55] | 10.1 (9.7, 10.6) | 10.2 (9.7, 10.6) | 1999.5 (1818.6, 2180.1) | 2009.3 (1829.9, 2188.7) |
| P, trendb | 0.76 | 0.63 | 0.17 | 0.31 |
|
>1 Best embryo qualityc, proportion |
Fertilization, rate |
|||
| Urinary BPA concentrations (μg/l) | Unadjusted | Adjusteda | Unadjusted | Adjusteda |
| Q1 [<LOD–0.96] | 0.38 (0.28, 0.49) | 0.39 (0.28, 0.50) | 0.72 (0.68, 0.76) | 0.72 (0.68, 0.77) |
| Q2 [0.97–1.37] | 0.47 (0.36, 0.57) | 0.47 (0.36, 0.58) | 0.69 (0.64, 0.73) | 0.69 (0.64, 0.73) |
| Q3 [1.38–2.20] | 0.44 (0.33, 0.55) | 0.43 (0.33, 0.54) | 0.70 (0.65, 0.74) | 0.70 (0.65, 0.74) |
| Q4 [2.24–16.55] | 0.45 (0.35, 0.56) | 0.45 (0.34, 0.56) | 0.73 (0.68, 0.77) | 0.73 (0.68, 0.77) |
| P, trendb | 0.52 | 0.61 | 0.44 | 0.51 |
aData are presented as predicted marginal means (95% CI) adjusted for age (continuous), BMI (continuous), smoking status (never and ever), race (white and others) and infertility diagnosis (male, female and unexplained).
bTests for trend were performed using the median concentration of urinary bisphenol A in each group as a continuous variable in the model.
cWe classified embryos as best quality if they had 4 cells on Day 2, 8 cells on Day 3, and a morphologic quality score of 1 or 2 on Days 2 and 3.
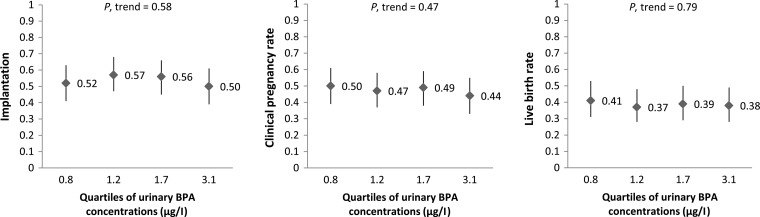
No significant dose–response associations were observed between urinary BPA concentrations and implantation, clinical pregnancy and live birth rates per initiated cycle in models adjusted for age, BMI, race/ethnicity, smoking status and primary infertility diagnosis (Fig. 1). The adjusted differences (95% confidence interval (CI)) in implantation, clinical pregnancy and live birth rates for women in the highest quartile of urinary BPA concentration compared with women in the lowest quartile were −0.02 (−0.13 to 0.09), −0.06 (−0.17 to 0.05) and −0.03 (−0.13 to 0.08), respectively. Because infertility diagnosis might be related to earlier BPA exposure and thus may be an intermediate on the causal pathway to IVF outcome, we conducted a sensitivity analysis removing infertility diagnosis and the results remained non-significant. Moreover, we performed analyses using urinary BPA concentrations as a continuous variable and there were no associations with any of the IVF outcomes (data not shown).
Figure 1.
Adjusted rates (95% CI) in clinical outcomes per initiated cycle according to specific gravity adjusted urinary BPA concentrations (μg/l). Models are adjusted for age (continuous), BMI (continuous), smoking status (never and ever), race (white and others) and infertility diagnosis (male, female and unexplained). Tests for trend were performed using the median concentration of urinary BPA in each quartile as a continuous variable in the model. Implantation was defined as a serum β-hCG level >6 mIU/ml typically measured 17 days (range 15–20 days) after oocyte retrieval, clinical pregnancy as the presence of an intrauterine pregnancy confirmed by ultrasound and live birth as the birth of a neonate on or after 24 weeks gestation. Medians of each quartile of urinary BPA concentrations (μg/l) are presented.
In order to facilitate comparisons of our results with our earlier publication based on a smaller subset of women from the same cohort, sensitivity analyses were carried out exploring the relation between urinary BPA concentrations and implantation per embryo transferred (Supplementary Table SI). We found no associations with these outcomes with the expanded population and extended follow-up. We also analyzed our data using a median of SG = 1.024 based on previous literature (Boeniger et al., 1993; Teass et al., 1993) and used in our earlier publication (rather than 1.015 used in the present analysis) and adjusted for the same set of covariates used in the previous publication (protocol type, Day 3 FSH result and number of embryo transferred) (Supplementary Table SI). We observed no association of BPA with infertility treatment outcomes in these supplementary analyses.
In evaluating whether effects of BPA depended on other factors, we observed significant effect modification by age in the association between BPA and endometrial wall thickness (P, interaction = 0.02). The adjusted differences (95% CI) in endometrial wall thickness for women with urinary BPA concentrations in the top quartile, compared with women in the bottom quartile, were +1.07 (+0.20, +1.30) among younger (P, trend = 0.06) and −0.60 (−1.30, +0.10) among older women (P, trend = 0.08). There was no evidence of significant heterogeneity of the relation between urinary BPA concentrations and other preclinical and clinical outcomes (P, interactions >0.1) by age.
Discussion
We evaluated the association of urinary concentrations of BPA with IVF outcomes among 256 women (n = 375 IVF cycles) attending a fertility clinic at the Massachusetts General Hospital and compared our results with our earlier publications on a smaller group of women from the same cohort (peak estradiol among 84 women and 112 cycles, implantation among 137 women and 180 cycles, and response to ovarian stimulation among 174 women and 237 cycles) (Mok-Lin et al., 2010; Ehrlich et al., 2012a,b). We also expanded upon our previous publications by exploring the relationships of urinary BPA concentrations with clinical pregnancy and live birth rates. In the present analysis, we found no associations of urinary BPA concentrations with measures of ovarian stimulation (oocyte yield), endometrial thickness, embryo quality, fertilization rates, implantation, clinical pregnancy and live birth rates.
Although the results for total and MII oocyte yield were not significant in our current analyses, they were in similar directions with our earlier publications on a smaller sample size in the EARTH cohort (Mok-Lin et al., 2010; Ehrlich et al., 2012b). First, Mok-Lin et al. (2010) reported that urinary BPA concentrations were inversely associated with the number of oocytes retrieved per cycle and peak serum estradiol levels in 84 women contributing to 112 IVF cycles (Mok-Lin et al., 2010). Later, Ehrlich et al. (2012b) reported that women with higher urinary BPA concentrations had significantly lower serum peak E2, oocyte yield, MII oocyte count, and number of normally fertilizing oocytes among 174 women contributing 237 cycles (Ehrlich et al., 2012b). Previously, we also published an analysis that found a suggestive relationship (P, trend = 0.12) between higher urinary BPA concentrations and increased implantation failure among 137 women undergoing 180 IVF cycles (Ehrlich et al., 2012a). However, in our present analyses, we did not find an association between higher urinary BPA concentrations and lower implantation. (Note that in the current analysis we explored associations with implantation rather than implantation failure.) To better compare our current results on a larger sample size to our earlier published results, we ran several sensitivity analyses. The inverse association of urinary BPA with oocyte yield and serum peak E2, and the borderline association between BPA and implantation found previously were not confirmed in the present larger and more powerful analysis. We determined, by comparing our previous and current analyses, that time trends in urinary BPA concentrations or IVF success rates could not account for differences in results. Furthermore, although our earlier analysis was per embryo transfer whereas our current was per initiated cycle, we ran sensitivity analyses and determined that this did not account for difference in results. Therefore, the most likely explanation is that our earlier preliminary analysis on a much smaller sample size might have yielded spurious findings that differed from the current analysis with larger sample size. There are few other studies on BPA and reproductive outcomes among women from a fertility clinic. A small study conducted at the University of California in San Francisco, found no associations of serum BPA concentrations with embryo cleavage rate or fragmentation in 27 women undergoing IVF and participating in the prospective cohort Study of Metals and Assisted Reproductive Technologies (Bloom et al., 2011b). Accordingly, the Longitudinal Investigation of Fertility and the Environmental Study showed that neither female nor male BPA urinary concentrations were associated with time to pregnancy among 501 couples recruited upon discontinuing contraception to become pregnant between 2005 and 2009 (Buck Louis et al., 2014).
We also found no association between urinary BPA concentrations with clinical pregnancy and live birth in our study population. Due to scarce human data, we considered it important to compare our result with experimental animal data. Consistent with our results, BPA exposure was not associated with the number of live pups or total number of delivered pups (Howdeshell et al., 2008; Tyl et al., 2008; Thuillier et al., 2009; Kobayashi et al., 2010, 2012; Ryan et al., 2010; Xi et al., 2011; Nanjappa et al., 2012) in mice and rats. However, other studies showed contradictory results in the same type of animals (Salian et al., 2009a,b; Cabaton et al., 2011). Despite these inconsistent results on pregnancy outcomes, there is experimental evidence for effects of BPA on folliculogenesis and oocyte meiosis. A recent review concluded that in animal models, BPA adversely affects oocyte meiosis, interferes with germ cell nest breakdown, reduces the primordial follicle pool by stimulating their initial recruitment and subsequent follicle development until the antral stage, alters ovarian steroidogenesis, modifies normal uterine morphology, and impairs uterine receptivity and ova-implantation (Peretz et al., 2014).
Although we did not find any association between urinary BPA concentrations and IVF outcomes in this study population, the relation between BPA and endometrial wall thickness was modified by age. Younger women (<37 years old) had thicker endometrial thickness across increasing quartiles of urinary BPA concentrations while older women (≥37 years old) had thinner endometrial thickness across increasing quartiles of urinary BPA concentrations. It is unclear why this association was modified by age and further studies are needed to corroborate this finding.
Our study has several limitations worth noting. One limitation is the uncertainty of extrapolating our results to women from the general population. Also, misclassification of BPA exposure based on spot urine sample is possible because BPA is a short-lived chemical and exposures are likely to be episodic in nature (Ye et al., 2011; Braun et al., 2012; Lassen et al., 2013). This would likely attenuate associations. Strengths of our study include its prospective design which minimizes the risk of reverse causation, the comprehensive adjustment of possible confounding variables, and the adequate power (80%) of the study which was able to detect clinically relevant difference of 29% in clinical pregnancy rates and 27% in live birth rates between women in the top and bottom quartiles of urinary BPA concentrations.
In conclusion, we found little evidence of adverse associations of urinary BPA concentrations with reproductive and pregnancy outcomes among women from a fertility clinic. We are currently using the same cohort to explore associations of reproductive and pregnancy outcomes with additional chemicals, such as phthalates and parabens. Furthermore, when there is an adequate sample size, we propose to explore potential effects of mixtures that include BPA on reproductive health.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
R.H. and J.E.C. were involved in study concept and design, and critical revision for important intellectual content of the manuscript. P.L.W. contributed to method modification and provided statistical expertise. L.M.-A. analyzed data, drafted the manuscript and had a primary responsibility for final content. L.M.-A., Y.-H.C., J.E.C., P.L.W. and R.H. interpreted the data. S.E. contributed to the statistical analyses. A.J.G. reviewed the statistical analysis. J.C.P., J.B.F. and A.M.C. were involved in acquisition of the data. All authors were involved in the critical revision of the manuscript and approved the final manuscript.
Funding
This work was supported by NIH grants R01ES022955, R01ES009718, and R01ES000002 from the National Institute of Environmental Health Sciences (NIEHS) and grant T32DK00770316 from the National Institute of Child Health and Human Development (NICHD).
Conflict of interest
None of the authors has any conflicts of interest to declare. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Xiaoyun Ye, Xiaoliu Zhou, Josh Kramer and Tao Jia of CDC for their technical assistance with BPA measurements. We also acknowledge all members of the EARTH study team, specifically the Harvard T.H. Chan School of Public Health research nurses, Jennifer B. Ford and Myra G. Keller, research staff, Ramace Dadd and Patricia Morey, and physicians and staff at Massachusetts General Hospital fertility center. A special thanks go to all of the study participants.
References
- Berger RG, Hancock T, deCatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol 2007;23:138–144. [DOI] [PubMed] [Google Scholar]
- Berger RG, Shaw J, deCatanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17β-estradiol. Reprod Toxicol 2008;26:94–99. [DOI] [PubMed] [Google Scholar]
- Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol 2010;30:393–400. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Kim D, vom Saal FS, Taylor JA, Cheng G, Lamb JD, Fujimoto VY. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril 2011a;96:672–677.e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, vom Saal FS, Kim D, Taylor JA, Lamb JD, Fujimoto VY. Serum unconjugated bisphenol A concentrations in men may influence embryo quality indicators during in vitro fertilization. Environ Toxicol Pharmacol 2011b;32:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 1993;54:615–627. [DOI] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect 2012;120:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieno-Enriquez MA, Robles P, Camats-Tarruella N, Garcia-Cruz R, Roig I, Cabero L, Martinez F, Caldes MG. Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum Reprod 2011;26:2807–2818. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril 2014;101:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect 2011;119:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables (February, 2015). Atlanta, GA, USA: Department of Health and Human Services, Centers for Disease Control and Prevention, 2015, http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf. [Google Scholar]
- Crawford BR, Decatanzaro D. Disruption of blastocyst implantation by triclosan in mice: impacts of repeated and acute doses and combination with bisphenol-A. Reprod Toxicol 2012;34:607–613. [DOI] [PubMed] [Google Scholar]
- Dong S, Terasaka S, Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut 2011;159:212–218. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect 2012a;120:978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod 2012b;27:3583–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA 2014;311:859–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect 2010;118:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril 2011;95:1816–1819. [DOI] [PubMed] [Google Scholar]
- Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Money C, McCormack P, Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI Adult Population Study. Environ Health Perspect 2010;118:1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol 1998;142:203–214. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 2006;17:682–691. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male Long Evans Hooded rat. Toxicol Sci 2008;102:371–382. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 2003;13:546–553. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ohtani K, Kubota H, Miyagawa M. Dietary exposure to low doses of bisphenol A: effects on reproduction and development in two generations of C57BL/6J mice. Congenit Anom 2010;50:159–170. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kubota H, Ohtani K, Hojo R, Miyagawa M. Lack of effects for dietary exposure of bisphenol A during in utero and lactational periods on reproductive development in rat offspring. J Toxicol Sci 2012;37:565–573. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998;139:4252–4263. [DOI] [PubMed] [Google Scholar]
- Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Main KM, Skakkebaek NE, Jorgensen N, Kranich SK, Andersson AM. Temporal variability in urinary excretion of bisphenol A and seven other phenols in spot, morning, and 24-h urine samples. Environ Res 2013;126:164–170. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J Biochem 2007;142:517–524. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, Ye X, Hauser R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol 2010;30:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 2010;33:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod 2012;86:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ Health Perspect 2008;116:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect 2014;122:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 2007;24:199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera OE, Varayoud J, Rodriguez HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol 2011;32:304–312. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics, 5th edn Pacific Grove, CA: Duxbury Press, 2000. [Google Scholar]
- Ryan BC, Hotchkiss AK, Crofton KM, Gray LE. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci 2010;114:133–148. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology 2009a;265:56–67. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci 2009b;85:742–752. [DOI] [PubMed] [Google Scholar]
- Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat 1980;34:216–221. [Google Scholar]
- Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, Ye X, Ford J, Keller M, Meeker JD et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect 2012;120:1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teass AW, DeBord DG, Brown KK, Cheever KL, Stettler LE, Savage RE, Weigel WW, Dankovic D, Ward E. Biological monitoring for occupational exposures to o-toluidine and aniline. Int Arch Occup Environ Health 1993;65:S115–S118. [DOI] [PubMed] [Google Scholar]
- Thuillier R, Manku G, Wang Y, Culty M. Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells. Microsc Res Tech 2009;72:773–786. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol Sci 2008;104:362–384. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–177. [DOI] [PubMed] [Google Scholar]
- Veeck L, Zaninovic N. An Atlas of Human Blastocysts. New York: Parthenon Publishing Group, 2003. [Google Scholar]
- Weng H-Y, Hsueh Y-H, Messam LLM, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol 2009;169:1182–1190. [DOI] [PubMed] [Google Scholar]
- Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect 2005;113:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Lee CKF, Yeung WSB, Giesy JP, Wong MH, Zhang X, Hecker M, Wong CKC. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD-1 mice. Reprod Toxicol 2011;31:409–417. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem 2005;77:5407–5413. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect 2011;119:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.