Abstract
In breast tumorigenesis, the metastatic stage of the disease poses the greatest threat to the affected individual. Normal breast cells with altered genotypes now possess the ability to invade and survive in other tissues. In this protocol, mouse mammary tumors are removed and primary cells are prepared from tumors. The cells isolated from this procedure are then available for gene profiling experiments. For successful metastasis, these cells must be able to intravasate, survive in circulation, extravasate to distant organs, and survive in that new organ system. The lungs are the typical target of breast cancer metastasis. A set of genes have been discovered that mediates the selectivity of metastasis to the lung. Here we describe a method of studying lung metastasis from a genetically engineered mouse model.. Furthermore, another protocol for analyzing mouse embryonic fibroblasts (MEFs) from the mouse embryo is included. MEF cells from the same animal type provide a clue of non-cancer cell gene expression. Together, these techniques are useful in studying mouse mammary tumorigenesis, its associated signaling mechanisms and pathways of the abnormalities in embryos.
Keywords: Medicine, Issue 99, Tumor, breast, lung, primary, MEF, embryo, fibroblasts, cancer, cell, mouse
Introduction
Metastasis is the final step in the progression of breast cancer 1. It involves a series of steps in which malignant cells are released from the primary tumor and disseminated to other organs 2. The cells must be able to intravasate, survive in circulation, extravasate to distant organs, and survive in that new organ system 3. Each step in tumorigenesis is tightly controlled by cells through various genetic and epigenetic changes 4. To study the mechanisms of breast cancer tumorigenesis, mouse model systems have been utilized due to their short lifespan, small size and fast breeding time. Some major advantages of using mouse models in cancer research are the molecular and physiological similarities they share with humans 5.
Established mouse and human cell lines have been used extensively in breast cancer research. Although we can obtain some information about the steps in metastasis using these cells, genetically manipulated animal models with tumor suppressors/oncogenes will give far more information about tumor development and metastasis starting from the embryonic stage. Thus, with these mouse models, we can obtain genetic and developmental regulation of tumor growth. Furthermore, the tumor microenvironment also plays an important role in tumor cell gene expression and it can be difficult to mimic the same scenario with studies using established cell lines. Primary human mammary tumors can also be used to study mouse mammary tumorigenesis. The main disadvantage of using isolated breast tumors is that this newly implanted tumor does not have the mouse’s vasculature and lymphatic tissue. Whereas the genetic mouse model systems for studying mammary tumorigenesis will allow us to monitor the steps of metastasis, analyze the tumor microenvironment, and access to the entire body 5.
The overall goal of this protocol is to study breast tumorigenesis in mice. It is known that breast cancers typically metastasize to the brain, lung and bone. This protocol allows for the isolation of primary mammary tumor cells, as well as, studying breast cancer metastasis to the lung. Furthermore, another protocol for analyzing mouse embryonic fibroblasts (MEFs) from the mouse embryo is included. Together, these techniques are useful in studying mouse mammary tumorigenesis.
Protocol
All mouse experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committees (IACUC) of LSU School of Medicine. All instruments must be autoclaved prior to dissection.
1. Preparation of Primary Cells from Mouse Breast Tumors
Use a mouse model that was genetically engineered with the Mouse Mammary Tumor Virus Polyoma Middle T-Antigen (MMTV-PyMT-transgenic mouse), which spontaneously develop tumors as previously described 6.
Sacrifice mice with Isoflurane (99.9%) and spray 75% ethanol to ensure a sterile environment (Figure 1A).
Open the skin from the area of the urethral orifice to the neck with scissors. Pin the skin down to allow easy access to organs (Figure 1B).
Isolate the mammary breast tumor and wash with cold sterile phosphate buffered saline (PBS) (pH 7.4) (Figure 1C).
Transfer the tumor to a sterile 10cm tissue culture plate and chop it with a sterile razor blade and scissors to approximately 1 mm3 pieces. Subsequent steps should be performed in a sterile laminar flow hood.
Incubate the chopped tumor tissue with Collagenase dissolved in serum free DMEM media at a final concentration of 1mg/ml on a rocker for 2 hr at 37 °C. Centrifuge the tumor tissue suspension for 3 min at 200 x g. NOTE: The Collagenase incubation is essential for enzymatic dissociation of the tissue.
Discard the supernatant and wash the precipitate 3 times with PBS (pH 7.4) by centrifuging for 3 min at 200 x g. After the final wash, decant the supernatant.
Resuspend the tissue with 2 ml of trypsin EDTA for 5 min at 37 °C while nutating. Centrifuge the suspension for 3 min at 200 x g and repeat the washes.
Resuspend the tissue pellet in normal growth medium (DMEM, 10% FBS, 2X pen/strep) onto a 10cm plate. Change the media every day for the next 3 days. Culture cells until desired cell number is obtained. NOTE: Approximately 1x107 cells will be isolated from this procedure. Other mouse mammary tumorigenesis models, such as MMTV-Neu, give lower yield of cells when compared to MMTV-PyMT. When using models that produce a lower yield, it is beneficial to begin with a larger tumor and culture cells longer.
2. Mouse Lung Extraction to Visualize Lung Metastasis
Prior to lung extraction, remove the liver and the diaphragm. Dislodge the ribs without disturbing the lungs.
With a fine pair of scissors, open the upper part of the trachea which is closer to the jugular vein.
Using a catheter, inject an agent such as 1 ml of Z-fix or India ink through the trachea to fill the lungs (Figure 2A). Embed the Z-fix Lungs in paraffin.
De-stain the lungs injected with India ink in Fekete’s solution (100 ml 70% alcohol, 10 ml of 10% buffered formalin, and 5 ml of glacial acetic acid) for 5 min. Define tumor nodules at secondary sites with a ≥ 0.5 mm in diameter, determine the nodules as metastasis. NOTE: Due to bronchi and bronchiole blockage by the tumor, tumor nodules cannot absorb 15% India ink, and thus normal lung tissue will be in black, and tumor nodules will be in white color 7. This will allow visualization of individual tumor nodules (Figure 2B).
3. Preparing Mouse Embryonic Fibroblasts (MEFs) from Mouse Embryos
Sacrifice pregnant mice with Isoflurane (99.9%) and spray 75% ethanol to ensure a sterile environment.
Open the skin from the area of the urethral orifice to the neck with scissors and isolate the embryos from the uterus.
Be careful not to disturb the embryos when opening the skin.
Remove the uterine horns and rinse them in cold sterile PBS (pH 7.4).
Separate the embryo from its placenta very carefully and place embryos in a 10cm plate containing ice cold PBS (pH 7.4).
Remove the head, liver and gut from the embryo and place the remaining portion in a 12 well tissue culture plate containing PBS (pH 7.4).
Transfer the embryo body to another 12 well plate containing trypsin EDTA and cut into small pieces (~ 1 mm3) using a sterile razor blade and scissors. Incubate the chopped material with 5 ml of trypsin EDTA for 5 min at 37 °C.
Inactivate trypsin by adding 1 ml Fetal Bovine Serum (FBS) to the chopped embryos. Centrifuge the suspension at 200 x g speed for 10 min, carefully remove the supernatant, resuspend the pellet with 1 ml of warm DMEM medium and transfer all the contents to a 10cm dish containing 10 ml of DMEM (with 20% FBS and 2X Penicillin/Streptomycin).
Change the medium every day for two days. Culture the cells until desired cell number is attained.
Representative Results
The tumor progression stage at which to sacrifice the animal depends on the particular study and IACUC guidelines. In Figure 1A, a 100 day old C57BL/6 mouse with the Mouse Mammary Tumor Virus Polyoma Middle T-Antigen (MMTV-PyMT) was sacrificed at three months. The skin was opened from the area of the urethral orifice to the neck with the scissors and the skin was pinned down to allow easy access to organs as shown in Figure 1B. This allowed easy access to the tumor that was obtained in Figure 1C. The primary cells obtained from the tumors are visualized in Figure 1D. Since many genes mediate breast cancer metastasis to the lung 1, we extracted the lungs (Figure 2A) to study the metastatic process. The lung was inflated with Z-fix and stained with India ink (Figure 2B) to visualize the tumor nodules. Figure 3 shows mouse embryonic fibroblasts isolated from FVB strain mouse embryos.
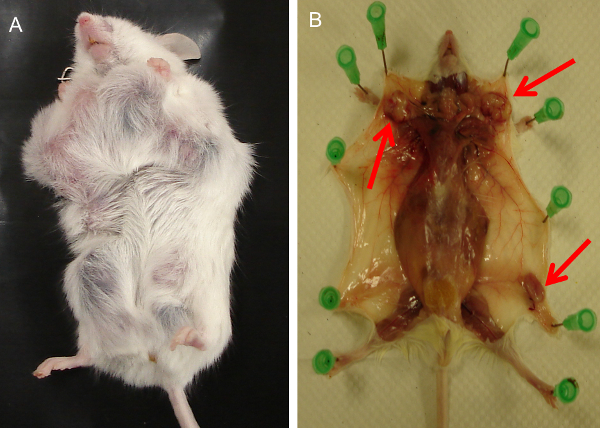 Figure 1. Preparation of Primary Cells from Breast Tumors. (A) A 100 day old female mouse with the Polyoma Middle T-Antigen (PyMT) was sacrificed. (B) The skin was pinned down to allow easy access to the mammary tumor. (C) The breast tumor was cut from the animal. (D) The isolated primary cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (scale bar: 100 μM). The images display the cells at day 3. Please click here to view a larger version of this figure.
Figure 1. Preparation of Primary Cells from Breast Tumors. (A) A 100 day old female mouse with the Polyoma Middle T-Antigen (PyMT) was sacrificed. (B) The skin was pinned down to allow easy access to the mammary tumor. (C) The breast tumor was cut from the animal. (D) The isolated primary cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (scale bar: 100 μM). The images display the cells at day 3. Please click here to view a larger version of this figure.
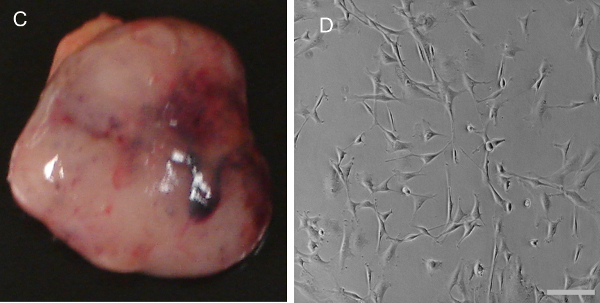 Figure 2. Mouse lung extraction to visualize lung metastasis. (A) The lung was inflated with Z-fix and (B) stained with India ink to visualize tumor nodules (scale bar: 500 μM). Please click here to view a larger version of this figure.
Figure 2. Mouse lung extraction to visualize lung metastasis. (A) The lung was inflated with Z-fix and (B) stained with India ink to visualize tumor nodules (scale bar: 500 μM). Please click here to view a larger version of this figure.
 Figure 3. Preparing Mouse Embryonic Fibroblasts (MEFs) from Mouse Embryos. MEF cells were extracted from a FVB mouse embryo (scale bar: 100 μM). Please click here to view a larger version of this figure.
Figure 3. Preparing Mouse Embryonic Fibroblasts (MEFs) from Mouse Embryos. MEF cells were extracted from a FVB mouse embryo (scale bar: 100 μM). Please click here to view a larger version of this figure.
Discussion
This protocol is for isolating primary cells from a fresh mouse tumor. Lysates prepared from this method are useful for protein, DNA and RNA analysis. Cancerous cells from the primary breast tumor often metastasize to the brain, lung and bone 1. Included is a technique to extract the lung for detection of metastasis. Staining the lung with an ink stain will allow for the visualization of tumor nodules as seen in Figure 2B. Fixing the lungs in Z-fix solution will allow us to make sections of lungs, and stain with antibodies that are appropriate to study metastatic mechanisms. Also included is a protocol for the isolation of mouse embryonic fibroblasts. This is useful for comparing the gene expression of the primary tumor cells with the embryonic fibroblast in the same animal type.
In the preparation of primary cells and MEFs, the most critical step is the mincing of the material. Without proper breakdown, low cell number will be achieved. If a low cell count is achieved, protocol can still be followed, but the cells will need to be cultured for a longer period of time.. In the lung extraction, proper staining and destaining is essential to allow for tumor nodule visualization. To extract RNA/protein from lungs, it will be good to use lungs that are unstained and unfixed. However, RNA can be extracted from fixed tissues as well 8.
Since the metastatic stage of breast cancer poses the greatest threat to the affected individual 2, it is important to visualize all components of breast tumorigenesis. A major challenge in understanding mechanisms of breast cancer progression is the availability of models that recapitulate the progression of the disease 9. Elucidation of the molecular mechanisms of breast tumorigenesis and metastasis are achieved by using animal models, such as mice 9, 10. The most common ways to induce mouse mammary tumors are genetically engineer the mice with pre-disposing mutations of oncogenes or tumor suppressors, introducing carcinogens, and injecting highly invasive cancer cells 10-12. Obtained tumors can then be further analyzed for gene expression. For breast cancer research, gene expression profiling is important since it predicts the clinical outcome of the patient 13.
Some limitations exist with the mouse model of studying mammary tumorigenesis. Although mice share molecular and physiological similarities with humans, they do not always recapitulate all aspects of human diseases5. This should be taken into consideration when using certain genetically engineered mice. Overall, mouse models provide a more realistic way to analyze mouse mammary tumorigenesis over other present models such as tissue culture and isolated human tumors. Taken together, this protocol allows for the further investigation of different aspects of breast tumorigenesis.
Disclosures
The authors have no disclosures.
Acknowledgments
We would like to thank NIH, LACaTS and Ochsner clinic foundation for the financial support.
References
- Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Natur. 2005;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Natur. 1980;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Wan L. Tumor Metastasis Moving New Biological Insights into the Clinic. Nat Med. 2013;19(11):1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7(9):654–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H. Accurate Identification of Experimental Pulmonary Metastases. J Natl Cancer Inst. 1966;36(4):641–645. doi: 10.1093/jnci/36.4.641. [DOI] [PubMed] [Google Scholar]
- Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60(4):1028–1034. [PubMed] [Google Scholar]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7(9):659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- Watts AM, Kennedy RC. Quantitation of Tumor Foci in an Experimental Murine Tumor Model Using Computer-Assisted Video Imaging. Anal Bioche. 1998;256(2):217–219. doi: 10.1006/abio.1997.2497. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, et al. Regression of Established Pulmonary Metastases and Subcutaneous Tumor Mediated by the Systemic Administration of High-Dose Recombinant Interleukin 2. J Exp Me. 1985;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Re. 2006;8(4):212. doi: 10.1186/bcr1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer L, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]


