Abstract
Introduction:
Recommended dosage of oral nicotine replacement therapy (NRT) product is often not achieved in smoking cessation attempts. n-6-propylthiouracil (PROP) bitter taste phenotype may be a potential risk factor for non-adherence to oral NRT products due to their bitter taste. There is limited literature on this phenotype in the context of smoking and none in relation to oral NRT pharmacotherapy.
Methods:
The association of PROP taste phenotype with NRT usage and sensory response to products was examined. In a cross-over experimental design, 120 participants received a 1 week supply of nicotine inhalers and 1 week of nicotine lozenges with random assignment to order. Mixed effects linear model analyses were conducted.
Results:
PROP taste phenotype and taste receptor genotype were not associated with NRT usage or sensory response to NRT, after adjusting for other factors. However, PROP non-tasters used a higher number of lozenges per day (continuous exposure) than nicotine cartridges (intermittent exposure). Unexpectedly, half of baseline PROP non-tasters shifted to taster phenotype 2 weeks after smoking cessation or reduction. Menthol cigarette smokers identified higher NRT strength of sensation scores than nonmenthol smokers. Taste receptor genotype was related to PROP taste phenotype (Kendall τ = .591, p = .0001).
Conclusions:
A nonsignificant relationship of PROP phenotype and NRT usage may be associated with NRT under-dosing and limited variance in the outcome variable. PROP non-tasters’ greater use of lozenges is consistent with nicotine exposure being less aversive to non-tasters. Further research of this and other factors impacting NRT usage are warranted to effectively inform smoking cessation pharmacotherapy.
Introduction
Adult cigarette smoking prevalence is estimated at 18.1% or 42.1 million individuals with a slowing in the decline of adult smoking in recent years.1 Nicotine replacement therapy (NRT) significantly increases the rate of cessation compared to placebo. In a Cochrane meta-analysis, the pooled risk ratio for abstinence for any NRT form relative to control was 1.60 (95% CI = 1.53–1.68).2 However, there has been differential adherence with various NRT products.3
Nicotine is bitter tasting4 and can elicit pain and burning in the airways when tobacco smoke is inhaled.5,6 Aversion to the bitter taste of nicotine may reduce the risk of smoking as well as the severity of nicotine dependence in those who choose to smoke.7,8 On the other hand, bitter taste may be a potential risk factor for non-adherence to oral NRT in smokers who wish to quit.
Bitter taste sensitivity varies widely across individuals. The ability to taste phenylthiocarbamide (PTC) and n-6-propylthiouracil (PROP) is a common phenotypic marker for individual differences in bitterness perception that may be related to smoking behavior and nicotine use. For example, early work identified a relationship between smoking and threshold sensitivity to PROP with significantly fewer smokers among those with lower PROP thresholds (i.e., greater acuity).9,10 In the general population, about 30% are insensitive to the bitter taste of PROP, whereas ~45% and 25%, are moderately or extremely responsive to PROP bitterness. These groups are called PROP medium and super-tasters, respectively.11,12 Two studies reported a higher percentage (~50%) of PTC/PROP non-tasters among smokers than in the general population.7,8 Moreover, PTC non-tasters had higher nicotine dependence scores.8
Phenotypic variation in PROP/PTC taste sensitivity is controlled by sequence variation in the bitter receptor gene TAS2R38 that accounts for a major portion (55%–85%), but not all of the variance in sensitivity.13,14 Polymorphisms in TAS2R38 code for two common haplotypes, PAV (the sensitive form) and AVI (the insensitive one). Several rare forms (e.g., AAI, AAV) also exist and are typically associated with intermediate sensitivities. The rarity varies by race as reported in Mangold and others’15 study where 20.8% of Blacks had an AAI or AAV haplotype. Carriers of two insensitive alleles (AVI/AVI) are considered non-tasters, and those with at least one sensitive allele (PAV/AVI or PAV/PAV) are considered tasters.16,17 Genetic analyses support the association between phenotypic taste blindness to PROP and smoking behaviors. For example, a population-based study showed that AVI homozygotes consumed more cigarettes/day than other genotypes.18 In a study in smokers, the AVI haplotype was associated with greater nicotine dependence in Black females, but not in White males or females as measured by smoking quantity, heaviness of smoking and the Fagerström Test of Nicotine Dependence.15 Although Cannon et al.19 found no differences in smoking prevalence between individuals with PAV and AVI haplotypes, those with the AAV (intermediate) haplotype had a lower prevalence of smoking (49%) compared to those without this haplotype (70%). Interestingly, the PAV haplotype was associated with lower taste motivation for smoking than the AVI haplotype, consistent with the protective role of the PAV haplotype in bitter taste avoidance.19
The association between the bitter taste of PROP and NRT products has not been reported. The aims of this study were to: examine the association between PROP taste phenotype and use of NRT products (nicotine inhaler and lozenge for 1 week each) in cigarette smokers during smoking abstinence; characterize the relationship between PROP taste phenotype and sensory experiences of oral NRT products (nicotine inhaler and lozenge for 1 week each); and investigate the interaction of PROP taste phenotype and NRT product type on NRT usage. A secondary aim was to assess relationships between TAS2R38 genotypes and PROP taste phenotypes in the participants.
Methods
A cross-over experimental design with 120 participants was implemented. Each participant received a 1 week supply of nicotine inhalers and 1 week of nicotine lozenges with random assignment to order. Stratified recruitment of PROP phenotype was implemented to yield a similar number of tasters and non-tasters of PROP. At baseline, a history and physical examination were completed by an advanced practice nurse. NRT usage and sensory responses to NRT were assessed at baseline and the end of week 1 and 2 in The Ohio State University Clinical Research Center (OSUCRC). Participants received US$100 at the end of each completed week and complimentary parking. Data collection was completed over 22 months.
Sample
Inclusion criteria were 18 to 55 years of age, cigarette smoker >1 year of at least 10 cigarettes per day, willing to quit smoking during the 2-week study, not pregnant, lactating, taking prescription medication altering taste, or experiencing an acute or chronic physical or mental illness. Initial recruitment generated a higher proportion of tasters of PROP.
To obtain a balanced sample of tasters and non-tasters of PROP a two-step amended IRB approved protocol was implemented 12 months into data collection. Based on PROP taste phenotype results in the first step, the participant was informed that they would either end the study at that point and receive US$10, or go on to the 2-week treatment study. This screening process eventually yielded 48.3% of the sample as non-tasters of PROP. If a participant did not complete the full 2-week study, we enrolled another participant to achieve complete data on 120 individuals. Due to family and/or work commitments, seven participants did not complete the study.
Measures
PROP taste phenotype was determined at baseline enrollment and at the end of week 2. Participants rated the intensity of the taste of a 1.5cm diameter paper disk with 1.0mol/L NaCl solution first, followed by the PROP filter paper disk impregnated with a 50 mmol/L PROP solution.20 We used the Labeled Magnitude Scale21 to assess perceived intensity of NaCl and PROP ranging from “barely able to detect” (0mm) to “strongest imaginable oral sensation” (100mm). Three taster groups were classified as: non-tasters with intensity rating of 15mm or less, medium tasters from 16mm through 67mm, and super tasters above 67mm. When a participant gave a borderline rating on PROP, it was compared to the participant’s rating of NaCl to clarify group assignment. This screening procedure has been validated against standard methods using PROP solutions20 and used in numerous PROP-tasting studies.22–25 After careful examination of the data, tasters were combined into one group to simplify data analysis.
Daily use of NRT was defined as the number of nicotine lozenges and nicotine inhaler cartridges recorded daily by the participant on Teleform® logs specific to oral nicotine lozenge or inhaler. NRT product was packaged providing participants sufficient quantities for each week-long period. Nearly all of the sample received 4mg unflavored nicotine lozenges based on body weight, with a small number receiving 2-mg lozenges. Participants were provided seven daily sheets on which to record the number of lozenges or cartridges at intervals throughout the day, congruent with their random assignment to order of NRT. During OSUCRC visits at the end of each week, they brought in these sheets along with unused NRT product and used packaging to validate usage self-report.
Sensory responses to NRT were assessed with the Duke Sensory Scale instrument which has three subscales: strength of sensations during cigarette smoking in five locations of chest, windpipe, throat, tongue, and nose, liking of the product, and level of satisfaction with the product.26 Responses were from 0 (not at all) to 7 (extremely strong). Baseline sensory data on cigarette use was obtained as well as daily sensory experiences on the three subscales with each NRT product. An average for each condition (1 week) was computed for each of the three subscales with higher scores indicating increased strength of sensations, liking, and satisfaction. Scores of strength of sensation for each location were summed. The strength of sensation subscale was modified to include only throat, tongue, and nose locations yielding three items as the study focus was on oral NRT.
Scores on the 6-item Fagerström Test for Nicotine Dependence (FTND) instrument range from 0 to 10 with higher scores indicating greater dependence.27
To estimate level of nicotine replacement, salivary cotinine concentrations at the end of each treatment week were obtained and compared to admission baseline cotinine concentration. A ratio for each of the two time points was computed. Cotinine is the major proximate metabolite of nicotine with approximately 70% of nicotine converted to cotinine with a half-life of 16hr.28 Assays were conducted by J2Laboratories with gas chromatography mass spectrometry.
To validate short-term nonsmoking status during NRT treatment as well as relapse, carbon monoxide (CO) in exhaled air in ppm was measured at baseline and the end of week 1 and 2. The half-life of CO is approximately 4hr pending activity level and a ≥8 ppm in exhaled air was considered an active smoking level.28 The Bedfont Mini-Smokerlyzer (Innovative Marketing) was used to measure CO. Smoking abstinence was defined as those who self-reported nonsmoking with CO <8 ppm at weeks 1 and 2. Relapsers were defined by self-reported smoking status and/or CO >8 ppm at weeks 1 and 2. Smoking status for each week was included in mixed effects linear model analyses. The log of cigarettes per day for each week provided interval level data and replaced dichotomous relapse status in alternative model analyses.
To determine taste receptor genotype, the process included DNA purification from whole blood, polymerase chain reaction (PCR) amplification, and single nucleotide polymorphism (SNP) genotyping assay using Taqman® universal PCR Master Mix and the specific Taqman® SNP genotyping assay. Three SNP’s from Applied Biosystems (C9506827_10, C9506826_10, C8876467_10) yielded PAV, AAV, AAI and AVI haplotypes. Equipment included Fisher Scientific® PCR work station, Thermo Scientific® Nanodrop 2000 spectrophotometer, and BioRad® CFX96 real-time thermal cycler in our laboratory. Three levels of taste receptor genotype were used in model analyses: the sensitive, insensitive, and intermediate sensitivity forms.
Analysis Plan
For the first aim, the goal was to examine the association of PROP taste phenotype and NRT usage of the two therapies. Frequency of usage (average number of lozenges or cartridges per day) was the response. To analyze the data, a mixed effects linear model was constructed. The fixed effects were phenotype, NRT product type, addiction level, ratio of cotinine at the end of each week to baseline, and a variable indicating whether the subject relapsed during the week. The variable participant was included as a random effect to account for the repeated measures nature of the data. For this aim, the main parameter of interest was the coefficient representing the association of phenotype with frequency of use adjusting for other factors. For the second aim, the model was similar with the response variable being the individual’s average score on one of the three sensory subscales across the week. The coefficient for phenotype indicated whether sensory scores were different across PROP phenotypes after adjusting for other effects and accounting for individual differences. For the third aim, the model used to address the first aim was extended to include an interaction between phenotype and NRT product. This interaction was used to test whether the difference between inhaler usage and lozenge usage was different between tasters and non-tasters of PROP while accounting for possible covariate effects. In addition to fitting the original models, several alternative models were explored. First, each model was modified to include a set of additional covariates in the following configurations: (a) interaction of relapse indicator with FTND, (b) main effect of gender and several interactions (gender × product, gender × FTND, and week × product), and (c) indicator of menthol and interaction of menthol × product. After constructing all of these models, each of the original and augmented models was run again substituting log of cigarettes per day for the relapse indicator. All statistical analyses were performed using IBM SPSS version 19.0.
Power Analysis
An “a priori” power analysis indicated that 54 individuals per group would be required to detect a significant effect of phenotype (α = 0.05, one-sided) for aim 1 with 80% power if the within-subject correlation was 0.7. The observed within-subject correlation was slightly lower than anticipated (0.681) and enrollment (minus attrition) was greater than expected (58 and 62 for non-tasters and tasters, respectively). Consequently, the study achieved the desired power level almost exactly. Reported p values are two-sided and can be halved to produce corresponding one-sided values.
Results
Sociodemographic characteristics of the sample, smoking behavior variables, and description of model parameters are detailed in Table 1. Other findings of note were that while this was not a smoking cessation trial, there were 34.5% who were nonsmokers during the lozenge phase and 37.3% were nonsmokers during the nicotine inhaler phase. Those who relapsed decreased from an average 15 cigarettes per day at baseline to 4–5 cigarettes per week (median). Alcohol history and use was assessed in the sample. Twenty-one participants (17.5%) reported not using alcohol, while the average years of alcohol use among those who used alcohol was 10.83±8.39 years. Further, in the typical 4-week period during which the participant completed baseline data collection, 60.9% reported 1 or 2 drinks on 10 or fewer days. Of the 60 participants who reported 3–4 drinks per day in the period, the majority reported that this occurred on 4 or fewer days per month. Among non-tasters of PROP, 36.8% smoked menthol cigarettes at baseline, and among tasters, 43.5% smoked menthol cigarettes. There was no significant difference detected using a chi-squared test (p = .575). For FTND, a similar analysis was performed and there was no evidence of association between PROP taste phenotype and FTND (p = .239). Menthol usage was almost identical among the three taste receptor groups: 32.4% were classified as diplotypes associated with insensitive non-taster, 54.5% were diplotypes associated with intermediate sensitivity, and 41.9% were diplotypes associated with sensitive tasters. There was no significant difference detected by chi-squared test (p = .387). Similarly, no significant association was found between FTND and three taste receptor groups (p = .690).
Table 1.
Sociodemographic Characteristics and Model Parameter Descriptions (N = 120)
| Variable | M (SD) | % |
|---|---|---|
| Female | 47.5 | |
| Education ≤high school | 36.7 | |
| White | 65.8 | |
| Black | 27.5 | |
| Menthol cigarette smokers | 40.3 | |
| Age (years) | 32.1(10.3) | |
| PROP taste phenotype non-tasters | 48.3 | |
| Baseline cigarettes/day | 15.4 (5.7) | |
| Baseline cotinine (ng/ml) | 329 (180) | |
| Cotinine ratio week 1:baseline | 0.756 (.56) | |
| Cotinine ratio week 2:baseline | 0.840 (.78) | |
| Years of regular smoking | 14 (9.9) | |
| Time to first cigarette (minutes) | 22 (20.7) | |
| Average nicotine lozenges/day | 4.7 (2.4) | |
| Average nicotine inhalers/day | 4.1 (2.2) | |
| With lozenge—strength of sensation score | 10.68 (3.38) | |
| With lozenge—liking score | 2.54 (1.65) | |
| With lozenge—satisfaction score | 3.27 (1.69) | |
| With inhaler—strength of sensation score | 9.94 (3.63) | |
| With inhaler—liking score | 3.70 (1.27) | |
| With inhaler—satisfaction score | 3.83 (1.78) | |
| Baseline FTND | 5.34 (1.72) | |
| Relapse week 1 | 61.3 | |
| Relapse week 2 | 66.9 | |
| Cigarettes/day (log) week 1 | 1.17 (1.21) | |
| Cigarettes/day (log) week 2 | 1.45 (1.28) |
FTND = Fagerström Test for Nicotine Dependence; PROP = n-6-propylthiouracil.
Aim 1 examined the association of PROP taste phenotype with use of two oral NRT products. PROP taste phenotype was not found to be associated with the number of cartridges or lozenges used per day on average after adjusting for other factors (p = .647). NRT usage was significantly related to product type with nicotine lozenge usage being greater than that of nicotine inhaler (p = .044). In addition, participants used 0.486 more lozenges and cartridges per day during the first week than they did in the second week, after adjusting for other factors (p = .034). Higher cotinine ratios were significantly linked to greater number of lozenges and cartridges used per day after adjusting for other factors (p = .001). Doubling a subject’s cotinine ratio was associated with an additional 1.371 lozenges or cartridges per day on average. When adding an interaction of phenotype and NRT product to the model (to address Aim 3), we found that non-tasters used a higher number of lozenges per day than cartridges (0.654 more units per day, p = .045), while PROP tasters showed no significant difference in product usage (p = .387).
Aim 2 focused on the association of PROP taste phenotype and sensory experiences of the two NRT products in three separate analyses: strength of sensation, liking, and satisfaction scores. PROP taste phenotype was not associated with strength of sensation, liking or satisfaction. Week was a significant variable in the strength of sensation model, where sensory score was 0.796 higher in week 1 versus week 2 after adjusting for other factors in the model (p = .032). Adding gender to the model, NRT product was not associated with strength of sensation among females, while males had 1.19 higher scores for lozenges than for inhalers (p = .019). An alternative analysis examined the 5-item version of the strength of sensation sensory scale and considered the addition of an indicator of menthol cigarette usage (and its interaction with product) to the model. Subjects who used menthol cigarettes provided an average sensory score 2.25 points higher than those who did not (p = .011). A further modification of the model to use log of cigarettes per day instead of the relapse indicator found a similar result, with menthol users scoring 2.47 points higher on average (p = .013).
PROP taste phenotype and nicotine dependence level were not significant in the average liking score after adjusting for other factors. Participants provided a liking score 0.882 lower for lozenges than for cartridges after adjusting for other factors (p < .001). Subjects who relapsed provided a liking score 1.005 lower, on average, than those who did not relapse (p < .001).
PROP taste phenotype, week, and cotinine ratio were not associated with the average NRT satisfaction score after adjusting for other factors. Higher addiction levels were significantly linked to higher satisfaction scores (p = .027). Participants who relapsed provided a satisfaction score 0.963 lower on average than those who did not relapse after adjusting for other factors (p < .001). Modifying of the model to use log of average cigarettes per day instead of the relapse indicator and adding terms for gender, product × week, gender × product, and gender × FTND, we found a significant interaction between product and week (p = .043). When using an inhaler, average satisfaction was 0.857 greater in the second week than in the first week. When using lozenges, average satisfaction was 0.169 lower in the second week than in the first week.
A secondary aim was to determine each participant’s taste receptor gene polymorphisms. Analyses for the first three study aims were conducted with bitter taste genotype replacing the PROP taste phenotype variable and no meaningful differences in results were found.
Secondarily, we assessed the relationship of taste receptor gene polymorphisms and PROP taste phenotype. At baseline the relationship was significant with Kendall τ = 0.591 (p = .0001), while the correlation at the end of the 2-week study was 0.432 (p = .0001). Frequency of PROP taster and non-taster phenotype at baseline and week 2 by taste receptor diplotype is presented in Table 2.
Table 2.
Frequency of PROP Taster and Non-Taster Phenotype at Baseline and Week 2 by Taste Receptor Diplotype
| Taster status phenotype (PROP) | ||||
|---|---|---|---|---|
| Baseline | Week 2 | |||
| Diplotypes | Taster | Non-taster | Taster | Non-taster |
| */PAV | 57 | 17 | 70 | 4 |
| AVI/AVI | 2 | 30 | 18 | 14 |
| AVI/AAV | 2 | 5 | 4 | 3 |
| AVI/AAI | 1 | 5 | 3 | 3 |
| AAI/AAI | 0 | 1 | 1 | 0 |
| Totals | 62 | 58 | 95 | 25 |
PROP = n-6-propylthiouracil.
Patterns of PROP taste phenotype over the 2 week study include: 18.3% were non-tasters at both time points, 50% were tasters at both time points, while 30% shifted from non-taster at baseline to some form of taster at 2 weeks and 1.7% (n = 2) moved from taster to non-taster over 2 weeks. Two weeks of smoking abstinence (or reduction) was associated with increased responsiveness to bitter (PROP) and salty (NaCl) taste, and more than half of the non-tasters were classified as tasters at the end of the study. Mean intensity ratings for both stimuli rose from baseline to week 2 (for NaCl, 30.2±2.1 vs. 42.3±2.1 and for PROP, 35.9±3.3 vs. 53.2±2.9; p < .01 for both). Figure 1 depicts the regression of intensity ratings of NaCl and PROP at baseline and at week 2 controlling for age and gender. The shaded box indicates participants classified as non-tasters of PROP at both time points. Data suggest that current smoking blunts general taste sensation and may interfere with PROP phenotyping.
Figure 1.
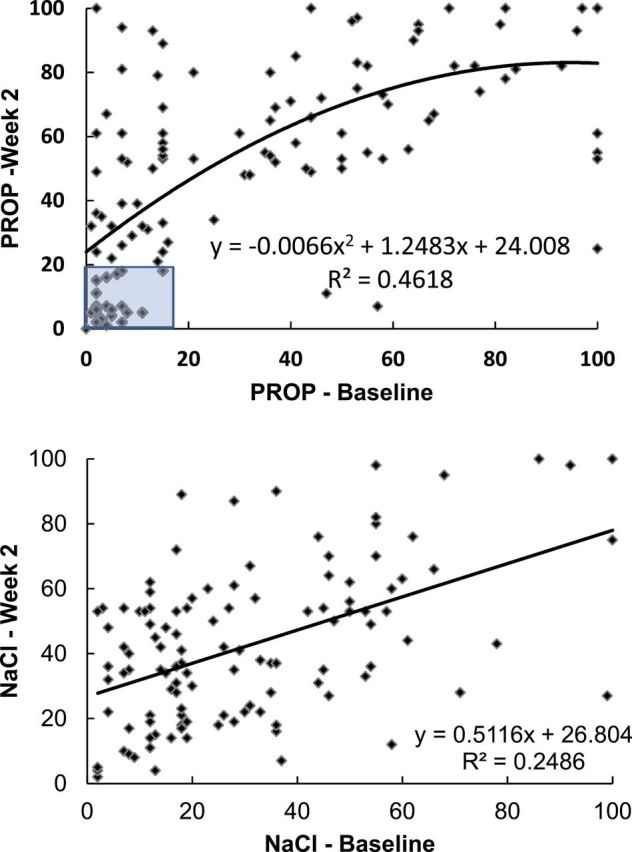
Regression of intensity ratings of n-6-propylthiouracil (PROP) and NaCl at baseline and week 2.
Discussion
PROP taste phenotype was not significantly related to NRT usage in this sample. The only PROP-related finding in this study was that non-tasters used more lozenges than cartridges per day, which is congruent with oral nicotine exposure being less aversive among non-tasters who perceive less bitterness. However, there was under-dosing of NRT with averages of 4.7±2.4 lozenges per day and 4.1±2.2 nicotine inhaler cartridges per day. This is considerably less than the recommended dosage of 9 lozenges per day or 6–16 nicotine inhaler cartridges per day.29 A wider range of NRT usage would have facilitated a more adequate assessment of the mechanisms of bitter taste phenotype and NRT usage. This is a limitation of the study since this response variable had minimal variation.
Lozenge intake in the current study was lower than that reported by Shiffman30 where high lozenge users consumed 10.2±2.5 lozenges per day, while low lozenge users reported 5.1±1.9 lozenges per day. Also, the 2002 Shiffman and colleagues’ study reported 9.1±3.8 lozenges (4mg) per day in high nicotine dependence participants and 7.4±4.0 lozenges (2mg) per day in low nicotine dependence participants.31 More in line with lozenge use in the current study were the reported 4.8±3.3 lozenges per day in a trial comparing transdermal nicotine and lozenges.32
The range of nicotine inhaler daily use ranged from 2.6 to 6.6 per day in studies where these data were available. Schneider and colleagues reported average use of nicotine inhalers at 6.0–6.6 per day over 6 weeks.33 Similarly, nicotine inhaler use was 6.4±2.9 per day in a randomized double-blind study.34 In a double-blind trial with nicotine inhalers, participants in active treatment used an average 3.8 per day (range 0–14).35 A study of nicotine inhaler purchase by participants compared over-the-counter to a health-care-provider condition to assess effectiveness.36 Daily use was 2.64±1.9 cartridges in an environment where NRT was not provided as part of research. Consuming less than the recommended NRT dosage presents concerns as well as the need for strategies to enhance adherence.
In Aim 2, while PROP phenotype was not significantly related to sensory experiences after adjusting for other factors in the model, scores on liking and satisfaction were associated with product type, relapse status, and nicotine dependence. Several significant associations were logical such as relapsers experiencing slightly lower liking scores and less satisfaction of NRT than non-relapsers. A positive association between nicotine dependence and satisfaction with NRT is consistent with expectations. The finding of significantly higher average strength of sensation scores among those who smoked menthol cigarettes at baseline is of interest in that menthol cigarette smokers may find oral NRT products more acceptable since these individuals experienced stronger sensations with the product. Some established menthol smokers may actively seek stronger sensory attributes as noted by Kreslake Wayne, and Connolly.37 Further exploration is warranted to determine if higher strength of sensation responses to NRT among menthol smokers, as in the current study, are perceived positively or not.
The relationship of taste receptor genotype and PROP taste phenotype at baseline of 0.591 suggests a strong correlation, but not a perfect congruence between measures. These findings are consistent with previously reported phenotype-genotype relationships ranging from 55% to 85%.13,38 Other factors including the number of fungiform papillae14,39 and TAS2R38 receptor mRNA expression in taste cells40 may play a role in defining PROP phenotypes.
Smoking is associated with greater loss of smell function than taste,41 and recovery of smell function is slow following smoking cessation.42 Nevertheless, higher taste detection thresholds have been observed in smokers43 and may be related to lower fungiform papillae density and functionality44,45 and to reduced gene expression in several sub-types of TAS2R receptors (including TAS2R38).46 The present study revealed an increase in taste intensity for both NaCl and PROP measured at week 2 of a smoking abstinence trial, consistent with a short-term recovery in taste function. Only Mullings et al.47 studied changes in taste following brief smoking abstinence. Results showed that after 12hr of abstinence, taste thresholds were lower following a denicotinized cigarette than a nicotinized one.47 The present findings raise interesting questions about the potential for rapid recovery of taste function with short-term smoking abstinence or reduction. In rodents, chronic nicotine exposure leads to morphological changes in taste buds.48 Nicotine also stimulates gustatory neurons in the nucleus of the solitary tract leading to a reduction in taste-evoked responses.49 Thus, one could speculate that reduced consumption of nicotine during smoking abstinence could lead to the opposite effect, that is, improvement in short-term taste function in chronic smokers.
Finally, classification of participants by their PROP ratings at week 2 did not improve associations between PROP phenotype and NRT use. It is possible that a longer and more complete abstinence period is needed to stabilize taste responses, especially for PROP phenotyping.
Conclusions
While PROP phenotype was not significantly related to NRT usage, this may be associated with NRT under-dosing and limited variance in the outcome variable. Further research of this and other factors impacting NRT usage are warranted to effectively inform smoking cessation pharmacotherapy. A short 2-week trial of an oral NRT product in this study provided incentive and self-efficacy for 35% of the sample to achieve short-term smoking cessation. Participants’ increased ability to taste PROP after 2 weeks of smoking cessation or reduction provides novel information.
Funding
This work was supported by the National Institutes of Health (grant number DA024765 R21); the National Center for Research Resources, Ohio State University Clinical Research Center (grant number UL1RR025755); and the Ohio State University Comprehensive Cancer Center Cancer Control Program.
Declaration of Interests
None declared.
References
- 1. Agaku IT, King BA, Dube SR. Current cigarette smoking among adults—United States, 2005–2012. Morb Mortal Wkly Rep. 2014;63:29–34. http://www.cdc.gov.proxy.lib.ohio-state.edu/mmwr/PDF/wk/mm6302.pdf Accessed August 4, 2014. [PMC free article] [PubMed] [Google Scholar]
- 2. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012. , Issue 11. Art. No: CD000146. 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 3. West R, Hajek P, Nilsson F, Foulds J, May S, Meadows A. Individual differences in preferences for and responses to four nicotine replacement products. Psychopharmacology. 2001;153:225–230. [DOI] [PubMed] [Google Scholar]
- 4. Scott TR, Giza BK, Yan J. Electrophysiological responses to bitter stimuli in the primate cortex. Ann N Y Acad Sci. 1998;855:498–501. [DOI] [PubMed] [Google Scholar]
- 5. Hummel T, Hummel C, Pauli E, Kobal G. Olfactory discrimination of nicotine-enantiomers by smokers and non-smokers. Chem Senses. 1992;17:3–21. [Google Scholar]
- 6. Thürauf N, Kaegler M, Dietz R, Barocka A, Kobal G. Dose-dependent stereoselective activation of the trigeminal sensory system by nicotine in man. Psychopharmacology (Berl). 1999;142:236–243. [DOI] [PubMed] [Google Scholar]
- 7. Enoch MA, Harris CR, Goldman D. Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted? Addict Behav. 2001;26:399–404. [DOI] [PubMed] [Google Scholar]
- 8. Snedecor SM, Pomerleau CS, Mehringer AM, Ninowski R, Pomerleau OF. Differences in smoking-related variables based on phenylthiocarbamide “taster” status. Addict Behav. 2006;31:2309–2312. [DOI] [PubMed] [Google Scholar]
- 9. Fischer R, Griffin F, Kapan AR. Taste thresholds, cigarette smoking, and food dislikes. Med Exp. 1963;9:151–167. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan AR, Glanville EV, Fischer R. Taste thresholds for bitterness and cigarette smoking. Nature. 1964;202:1366. [DOI] [PubMed] [Google Scholar]
- 11. Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP Tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56:1165–1171. [DOI] [PubMed] [Google Scholar]
- 12. Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr. 2008;28:367–388. [DOI] [PubMed] [Google Scholar]
- 13. Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. [DOI] [PubMed] [Google Scholar]
- 14. Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 2008;33:255–265. [DOI] [PubMed] [Google Scholar]
- 15. Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD. Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet. 2008;45:578–582. [DOI] [PubMed] [Google Scholar]
- 16. Bufe B, Breslin PAS, Kuhn C, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duffy VB, Davidson AC, Kidd JR, et al. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 2004;28:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller M, Liu X, Wohland T, et al. TAS2R38 and its influence on smoking behavior and glucose homeostasis in the German Sorbs. PLoS One. 2013;8:e80512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cannon DS, Baker TB, Piper ME, et al. Associations between phenylthiocarbamide gene polymorphisms and cigarette smoking. Nicotine Tob Res. 2005;7:853–858. [DOI] [PubMed] [Google Scholar]
- 20. Zhao L, Kirkmeyer SV, Tepper BJ. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 2003;78:625–633. [DOI] [PubMed] [Google Scholar]
- 21. Green BG, Dalton P, Cowart BJ, Shaffer GS, Rankin KM, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. [DOI] [PubMed] [Google Scholar]
- 22. Cabras T, Melis M, Castagnola M, et al. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS One. 2012;7:e30962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirkmeyer SV, Tepper BJ. Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem Senses. 2003;28:527–536. [DOI] [PubMed] [Google Scholar]
- 24. Shafaie Y, Koelliker Y, Hoffman DJ, Tepper BJ. Energy intake and diet selection during buffet consumption in women classified by the 6-n-propylthiouracil bitter taste phenotype. Am J Clin Nutr. 2013;98:1583–1591. [DOI] [PubMed] [Google Scholar]
- 25. Tepper BJ, Koelliker Y, Zhao L, et al. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity (Silver Spring). 2008;16:2289–2295. [DOI] [PubMed] [Google Scholar]
- 26. Westman EC, Behm FM, Rose JE. Airway sensory replacement combined with nicotine replacement for smoking cessation: a randomized, placebo controlled trial using a citric acid inhaler. Chest. 1995;107:1358–1364. [DOI] [PubMed] [Google Scholar]
- 27. Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and assessment. Ear Nose Throat J. 1992;11:763–767. [PubMed] [Google Scholar]
- 28. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. [DOI] [PubMed] [Google Scholar]
- 29. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 30. Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction. 2007;102:809–814. [DOI] [PubMed] [Google Scholar]
- 31. Shiffman S, Dresler CM, Hajek P, Gilburt SJA, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Arch Intern Med. 2002;162:1267–1276. [DOI] [PubMed] [Google Scholar]
- 32. Schnoll RA, Martinez E, Tatum KL, et al. Nicotine patch vs. nicotine lozenge for smoking cessation: an effectiveness trial coordinated by the Community Clinical Oncology Program. Drug Alcohol Depend. 2010;107:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schneider NG, Olmstead R, Nilsson F, Mody FV, Franzon M, Doan K. Efficacy of a nicotine inhaler in smoking cessation: a double-blind, placebo-controlled trial. Addiction. 1996;91:1293–1306. [PubMed] [Google Scholar]
- 34. Hjalmarson A, Nilsson F, Sjöström L, Wiklund O. The nicotine inhaler in smoking cessation. Arch Intern Med. 1997;157:1721–1728. [PubMed] [Google Scholar]
- 35. Tønnesen P, Nørregard J, Mikkelsen K, Jørgensen S, Nilsson F. A double-blind trial of a nicotine inhaler for smoking cessation. JAMA. 1993;269:1268–1271. [PubMed] [Google Scholar]
- 36. Leischow SJ, Ranger-Moore J, Muramoto ML, Matthews E. Effectiveness of the nicotine inhaler for smoking cessation in an OTC setting. Am J Health Behav. 2004;28:291–301. [DOI] [PubMed] [Google Scholar]
- 37. Kreslake JM, Wayne GF, Connolly GN. The menthol smoker: tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob Res. 2008;10:705–715. [DOI] [PubMed] [Google Scholar]
- 38. Prodi DA, Drayna D, Forabosco P, et al. Bitter taste study in a sardinian genetic isolate supports the association of phenylthiocarbamide sensitivity to the TAS2R38 bitter receptor gene. Chem Senses. 2004;29:697–702. [DOI] [PubMed] [Google Scholar]
- 39. Melis M, Alzori E, Cabras S, et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PLoS One. 2013;8e74151. 10.1371/journal.pone.0074151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lipchock SV, Mennella JA, Spielman AI, Reed DR. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am J Clin Nutr. 2013;98:1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255:1121–1126. [DOI] [PubMed] [Google Scholar]
- 42. Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–1236. [PubMed] [Google Scholar]
- 43. Sato K, Endo S, Tomita H. Sensitivity of three loci on the tongue and soft palate to four basic tastes in smokers and non-smokers. Acta Otolaryngol. 2002;546:74–82. [DOI] [PubMed] [Google Scholar]
- 44. Fischer ME, Cruickshanks KJ, Schubert CR, et al. Factors related to fungiform papillae density: the beaver dam offspring study. Chem Senses. 2013;38:669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pavlidis P, Gouveris C, Kekes G, Maurer J. Changes in electrogustometry thresholds, tongue tip vascularization, density and form of the fungiform papillae in smokers. Eur Arch Otorhinolaryngol. 2014;27:2325–2331. [DOI] [PubMed] [Google Scholar]
- 46. Aoki M, Takao T, Takao K, Koike F, Suganuma N. Lower expressions of the human bitter taste receptor TAS2R in smokers: reverse transcriptase-polymerase chain reaction analysis. Tob Induc Dis. 2014;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mullings EL, Donaldson LF, Melichar JK, Munafò MR. Effects of acute abstinence and nicotine administration on taste perception in cigarette smokers. J Psychopharmacol. 2010;24:1709–1715. [DOI] [PubMed] [Google Scholar]
- 48. Tomassini S, Cuoghi V, Catgalani E, Casini G, Bigiani A. Long-term effects of nicotine on rat fungiform taste buds. Neuroscience. 2007;13:803–810. [DOI] [PubMed] [Google Scholar]
- 49. Simons CT, Boucher Y, Carstens MI, Carstens E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J Neurophysiol. 2006;96:1877–1886. [DOI] [PubMed] [Google Scholar]


