Abstract
Trade-offs between competitive and parental strategies often are mediated by sex steroids. The mechanisms underlying steroid signaling and metabolism may therefore serve as targets of disruptive selection that leads to alternative behavioral phenotypes. White-throated sparrows exhibit two color morphs that differ in both competitive and parental behavior; white-striped (WS) birds engage in more territorial singing, whereas tan-striped (TS) birds provision nestlings more often. Although WS birds have higher levels of plasma testosterone (T) and estradiol than do TS birds, experimental equalization of these hormones does not abolish morph differences in singing. Neural sensitivity to sex steroids may differ between the morphs because the gene for estrogen receptor alpha (ERα) has been captured by a chromosomal rearrangement found only in the WS birds. We recently showed that expression of this gene differs between the morphs and may drive the behavioral polymorphism. First, the ERα promoter region contains fixed polymorphisms that affect transcription efficiency in vitro. Second, in a free-living population, local expression of ERα depends strongly on morph and predicts both territorial singing and parental provisioning. Differential ERα expression is particularly striking in the medial amygdala; WS birds have three times more ERα mRNA than do TS birds. This difference persists during the non-breeding season and is unaffected by exogenous T treatment. Finally, preliminary data generated by RNA-seq confirm that ERα expression in MeA is both differentially expressed and correlated with territorial singing. Together, these results suggest that ERα may be a target of disruptive selection that leads to alternative behavioral strategies. Our future directions include a more detailed analysis of the ERα promoter regions to determine the molecular basis of differential expression as well as gene network analyses to identify genes connected to ERα.
The white-throated sparrow as a model of life-history trade-offs
Disruptive selection that drives incompatible traits into alternative phenotypes is most likely to act on genes with multiple functions. Such genes include those that encode the action or regulation of hormones (Ketterson and Nolan 1992; Finch and Rose 1995; Rhen and Crews 2002; Sinervo and Svensson 2002; Nijhout 2003; Hau 2007; McGlothlin and Ketterson 2007; Miles et al. 2007). For example, trade-offs between territorial aggression and parenting (Trivers 1972) are thought to be mediated by sex steroids such as testosterone (T). In many species of fish, birds, rodents, and primates, high levels of circulating androgens are associated with increased intrasexual competition manifested as aggression or mating effort, whereas low levels are associated with increased parenting effort (e.g., Ketterson and Nolan 1994; McGlothlin et al. 2007). In humans, paternal care and fatherhood often have been associated with low plasma levels of T (Storey et al. 2000; Wynne-Edwards 2001; Fleming et al. 2002; Gray et al. 2002; Gray 2003), and high T levels associated with male–male aggression and competition (Booth et al. 1989; Bernhardt et al. 1998; Book et al. 2001).
In this review, we describe our ongoing research with a model organism particularly well-suited for understanding the mechanisms underlying the evolution of life-history trade-offs: the white-throated sparrow (Zonotrichia albicollis). This seasonally breeding songbird is ordinary in most respects; it is abundant throughout its range in eastern North America, defends territories during the breeding season, and is socially monogamous. Over the past several decades, however, research on this species has unearthed distinctive features. Within any population, about half of the birds have white and black stripes on the crown and a clear white throat. The rest have brown and tan stripes and a streaked throat. Before about 1970, field guides often labeled the two color types as male and female or adult and immature (e.g., Peterson 1961). Both of those categorizations, however, were incorrect. The variation in plumage corresponds to morphs, or alternative phenotypes. Once they molt into adult plumage (Fig. 1a), individuals are either “tan-striped” (TS) or “white-striped” (WS). Morph is fixed for life; individuals of this species do not switch between phenotypes. Breeding pairs typically consist of one TS and one WS bird (reviewed by Falls and Kopachena 2010). Together with the plumage morphs, this disassortative mating system makes this species unique among songbirds.
Fig. 1.
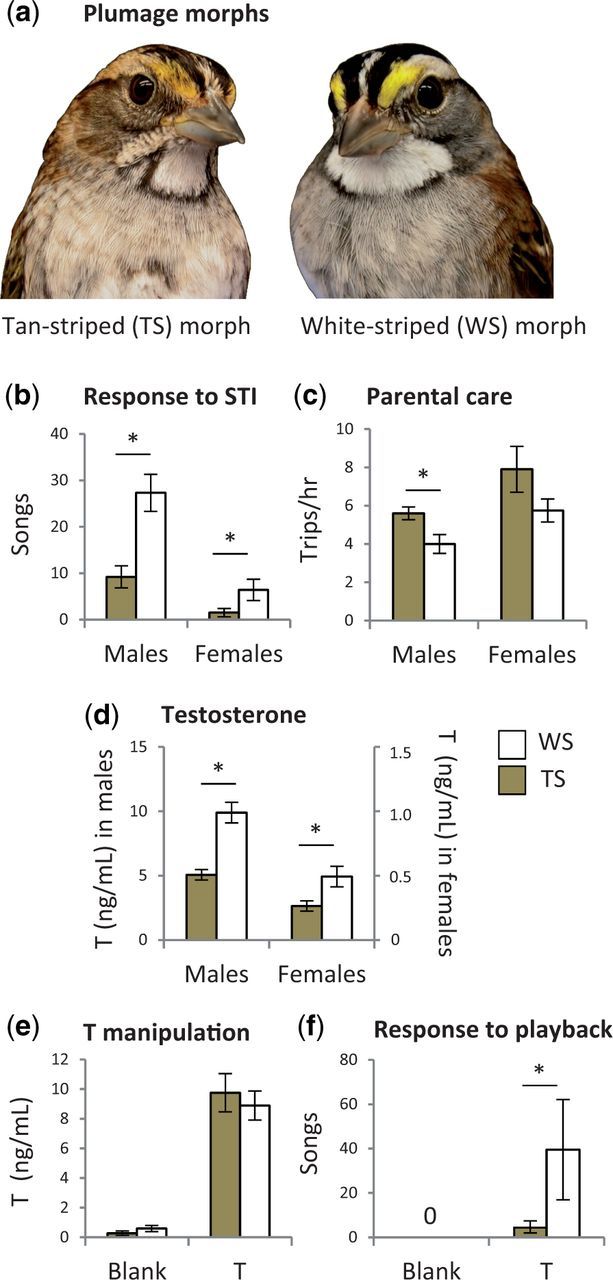
Morph differences in plumage, behavior, and hormones in white-throated sparrows. (a) Tan-striped (TS) and white-striped (WS) birds occur at equal rates. (b) WS birds of both sexes respond to STI with more singing than do TS birds. (c) TS males provision young in the nest at greater rates than do WS males (data from first brood). (d) Early in the breeding season, WS birds of both sexes have higher plasma testosterone (T) than do TS birds. (e) When T was manipulated in captive, non-breeding males to equalize T between the morphs, WS males nonetheless sang more in response to song playback than did TS males (f). (b), (c), and (d) redrawn from Horton et al. (2014a) and (e) and (f) from Maney et al. (2009) with permission.
The plumage morphs are interesting to behavioral biologists because they correspond to behavioral phenotypes that differ with respect to song rate and parental care. In both sexes, the WS birds sing at higher rates than do the TS birds (Fig. 1b). Song is used in this species primarily to defend territory, and most findings of morph differences in singing were obtained in the context of simulated territorial intrusion (STI; Lowther 1962; Jones 1987; Kopachena and Falls 1993a; Horton et al. 2014a). WS females sing at high rates compared with females of related species, whereas TS females rarely sing. In WS male × TS female pairs, therefore, most of the singing is done by the male whereas in pairs of the other morph type, the male and female share the singing about equally.
During the parental phase of the breeding season, males and females both bring food to the nestlings. The rate at which they do so, however, depends on morph—particularly for males. TS males provision their young more often than do WS birds (Horton et al. 2014a; Fig. 1c). Other authors have reported the same effect in females as well (Knapton and Falls 1983; Kopachena and Falls 1993b; cf. Horton and Holberton 2010). Interestingly, we have observed a morph difference in parenting only during first broods of the season. TS and WS males invest equally in the provisioning of replacement broods (Horton et al. 2014a). This finding is consistent with shifts in parental investment as the season progresses in other species (Biermann and Robertson 1981; Robinson et al. 2010). We hypothesize that late in the season, because fewer females are fertile, WS males gain little by investing in extra-pair copulations over nestling provisioning.
Endocrine correlates of behavioral polymorphism
The two morphs lie at either end of a continuum, with investment in the defense of resources and in mating success at one end and investment in current offspring at the other (Trivers 1972). In other words, they exemplify the classic trade-off between investing in territoriality and mating effort versus parental care, which in many birds is likely mediated by T (Ketterson and Nolan 1992; Hau 2007). In white-throated sparrows and related species, exogenous T treatment increases territorial singing and decreases parental behavior (Silverin 1980; Wingfield 1984; Hegner and Wingfield 1987; Schoech et al. 1998). These are precisely the behaviors that differ according to morph, so T is an excellent candidate for mediating morph differences in behavior—along with other hormones and neuropeptides that are modulated by T.
Plasma levels of T do not differ between the morphs during the non-breeding season (Schlinger 1987), when behavior is not polymorphic (Harrington 1973; Watt et al. 1984; Schlinger 1987; Schwabl et al. 1988; Piper and Wiley 1989; Dearborn and Wiley 1993; Wiley et al. 1999). During the breeding season, however, when morph-dependent behaviors emerge, T is higher in WS than in TS birds of both sexes (Spinney et al. 2006; Swett and Breuner 2009; Horton et al. 2014a). This difference is most pronounced early in the breeding season, when the birds are competing for territories (Fig. 1d). Plasma levels of the hormone estradiol (E2), which is synthesized from testosterone, are also higher in WS birds than in TS birds (Horton et al. 2014a). Because both singing and parental provisioning depend on plasma T (Silverin 1980; Wingfield 1984; Hegner and Wingfield 1987; Schoech et al. 1998; Lynn et al. 2009), it is tempting to speculate that morph differences in plasma levels of these hormones completely explain morph differences in both singing and parenting. Correlation, however, does not imply causation. In males, plasma T increases in response to STI (Wingfield and Hahn 1994) or to the presence of receptive females (Moore 1982; Dufty and Wingfield 1986; Wingfield and Monk 1994). A one-way causal effect of T on social behaviors, therefore, may not completely explain either polymorphic behavior or the inverse relationship between singing and parenting effort in this species.
To test whether morph differences in plasma steroids can explain the differences in behavior, we experimentally equalized plasma T in males (Maney et al. 2009). We treated non-breeding birds, in which plasma levels of sex steroids were low and the gonads were regressed, with silastic implants that elevated plasma T to levels typical of a WS male early in the breeding season. After treatment, plasma T did not differ between the morphs (Fig. 1e). We hypothesized that if morph differences in behavior can be explained by differences in T, we should not see any morph differences in behavior in this sample. We then performed song playbacks in the laboratory and recorded the birds’ vocal responses. Untreated, non-breeding birds did not sing in response to the playback (Fig. 1f). T-treated birds, however, did sing, and the WS birds sang at higher rates than did the TS birds (Fig. 1f). E2-treatment of non-breeding females produced the same result: WS females sang more than did their TS counterparts. This study showed clear evidence that morph differences in singing behavior cannot be completely explained by the differences in plasma levels of sex steroids in either sex. The results suggest an alternative hypothesis: that the brains of TS and WS birds differ in their sensitivity to sex steroids.
Estrogen receptor alpha polymorphism
In order to ask how the brains of the two morphs differ, we turn to their genetics. The plumage polymorphism has a genetic basis first described decades ago by Thorneycroft (1966, 1975), who compared the karyotypes of TS and WS birds. He noted that whereas all TS birds had two copies of a submetacentric chromosome 2, all WS birds had at least one copy of a metacentric homolog. He hypothesized that the metacentric arrangement, later denoted by Thomas et al. (2008) as ZAL2m, came about via a pericentric inversion. Using mapping and population genetics techniques, Thomas et al. (2008) showed that in fact ZAL2m contains at least two pericentric inversions (Fig. 2a), and that recombination is profoundly suppressed within the rearranged region. The resulting limited gene flow has caused the ZAL2m to diverge from the ZAL2, and the two haplotypes are now 1% different from each other (Huynh et al. 2011). Thus, odds are relatively high that alleles on each haplotype are expressed at different levels or even encode different proteins.
Fig. 2.
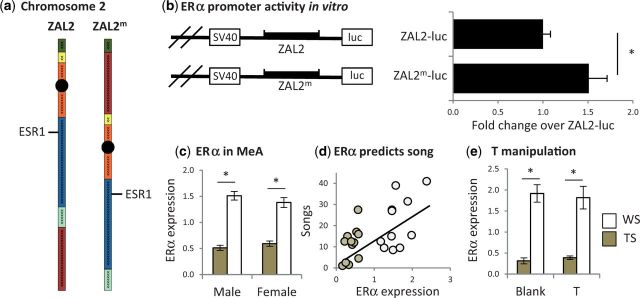
The expression of estrogen receptor alpha (ERα) mRNA has been affected by a chromosomal rearrangement. (a) The ESR1 gene has been captured by a rearrangement that consists of at least two pericentric inversions (see online version for color; Thomas et al. 2008). (b) In HeLa cells, a ZAL2m promoter construct inserted upstream of luciferase (luc) resulted in significantly more expression compared with the ZAL2 construct. (c) ERα mRNA expression in the medial amygdala (MeA) is significantly higher in WS than in TS birds of both sexes. (d) ERα expression in MeA significantly predicts STI-induced singing in free-living males. (e) When testosterone (T) was equalized between the morphs in captive males, the morph difference in MeA ERα expression persisted. (b), (c), and (d) redrawn from Horton et al. (2014b) with permission.
The differentiating region of ZAL2m provides an excellent target for investigating the genetic basis of the behavioral polymorphism in this species. Which genes inside the rearrangement are already known to affect aggression and parenting in songbirds? Steroid-sensitive behaviors often are correlated with the expression of steroid receptors (Cushing et al. 2004; Trainor et al. 2006; Ball and Balthazart 2008; Ketterson et al. 2009; Rosvall et al. 2012). In dark-eyed juncos (Junco hyemalis), a close relative of white-throated sparrows, aggressive responses to STI were positively correlated with the expression of estrogen receptor alpha (ERα in the ventral telencephalon [Rosvall et al. 2012]). Thus, variation in the expression of an important steroid receptor predicted variation in a steroid-dependent behavior—an intuitive result, given that the receptor confers sensitivity to E2, a major metabolite of T. In white-throated sparrows, the gene for ERα (ESR1) has been captured by the rearrangement on ZAL2m (Fig. 2a; Thomas et al. 2008) making it a prime candidate for driving variation in territorial singing and parenting behavior.
To determine whether the functioning of the receptor itself may have been affected by the rearrangement, we sequenced the coding region of the ESR1 gene on both ZAL2 and ZAL2m. We found two differences that affect the coding sequence of the receptor protein. The first was in the activation function (AF-1) domain, where several known coactivators bind, and the other in the ligand binding domain. These changes in the protein sequence of ERα are unlikely to significantly impact its function, however, because the substitutions found in the ZAL2m isoform are the same residues found in functional estrogen receptors in other species (Horton et al. 2014b). We therefore turned our attention to the upstream regions likely to contain promoters.
In contrast to the coding sequence, the promoter sequences upstream of the start site differ substantially between the haplotypes. Several of these changes occurred at putative binding sites for transcription factors. The ZAL2m allele, for example, has gained a binding site for Pbx-1, a transcription factor that affects the expression of estrogen receptors in humans (Cheung et al. 2009). This and many other changes in the ESR1 promoter sequence may drive differences in ERα expression. We tested this hypothesis in vitro by cloning the putative promoter regions into constructs containing a luciferase reporter gene. In HELA cells, the level of transcription was higher from the ZAL2m promoter than from the ZAL2 promoter (Horton et al. 2014b; Fig. 2b). Because mRNA transcription depends on many factors that may not have been available in our in vitro preparation, we could not conclude from this study that the ZAL2m promoter is more effective. We could, however, conclude that the promoter sequence we analyzed contains enough variation to affect transcription, which set the stage for in vivo comparisons.
Expression of ERα in free-living birds
To test for differential regulation in vivo, we quantified ERα mRNA expression in the brains of behaviorally characterized, breeding white-throated sparrows near Argyle, ME. We performed in situ hybridization using a radiolabeled riboprobe that did not span any SNPs—in other words, a riboprobe exactly complementary to both the ZAL2 and ZAL2m mRNAs. Using this technique, we found significant differences in ERα mRNA expression in eight regions of the brain known to be involved in social behavior. We will focus here on the most striking difference: ERα mRNA levels were nearly three times higher in WS than in TS birds in the medial amygdala (MeA; Fig. 2c). In both sexes, levels of MeA mRNA were nonoverlapping between the morphs; in other words, males and females could be accurately assigned to a morph simply by looking at ERα expression in MeA (Horton et al. 2014b).
The MeA is part of an interconnected social behavior network (Newman 1999; Goodson 2005) consisting of steroid-sensitive regions that contribute to a variety of social behaviors, including aggression and parenting. In animals that rely heavily on pheromonal communication, it receives massive projections from the accessory olfactory system (Scalia and Winans 1975). In birds, perhaps because they rely on vocal communication, MeA receives input from the auditory thalamus. This input reaches higher auditory association areas via the primary auditory cortex (Field L) and via MeA. MeA also receives input from the song control system, and thus connects vocal areas, the auditory system, and the social behavior network (Cheng et al. 1999). Lesions of MeA disrupt behavioral responses to social signals in ring doves and Japanese quail (Cheng et al. 1999; Thompson et al. 1998), and inhibit male-directed song in zebra finches (Ikebuchi et al. 2009). We therefore asked whether the profound morph difference in ERα expression in MeA was related to singing behavior. In males, we found a strong correlation between the expression and the vocal response to STI (Fig. 2d). In females, we found a nonsignificant trend in the same direction; our inability to demonstrate this relationship was likely caused by our lower sample size for females. Follow-up analysis in males demonstrated that ERα expression in MeA predicted singing even better than did morph. In other words, when morph was controlled, the correlation between ERα expression and singing persisted; in contrast, when the variation in ERα was controlled, the effect of morph on singing disappeared. Importantly, ERα expression predicted singing even when plasma levels of T and E2 were controlled in the analysis, further supporting our hypothesis that morph differences in singing behavior in this species cannot be explained simply by plasma levels of either sex steroid.
To confirm that morph differences in ERα mRNA do not depend on plasma T, we performed a second T-manipulation study. Non-breeding males of both morphs received subcutaneous silastic capsules containing either nothing or enough T to mimic plasma levels typical of WS males early in the breeding season. We quantified ERα mRNA expression in MeA after 2 weeks, during which time T reliably stimulates singing in captive males (Maney et al. 2009; Grozhik et al. 2014). Our results showed clearly that T-treatment had no effect on ERα expression in that region in either morph (Fig. 2e). This result suggests that morph differences in ERα expression in MeA in our free-living population (Fig. 2c) cannot be attributed solely to the differences in plasma T (Fig. 1d). Rather, changes in the promoter sequence may cause differential rates of ERα transcription on the ZAL2 and ZAL2m alleles (Fig. 2b). In other words, the rearrangement itself, or more precisely the resulting differentiation of ESR1, may cause differential expression of ERα mRNA by altering transcription efficiency.
Figure 2d shows a second interesting finding: a large effect of morph not only in the T-treated group but also in the control group. This result is curious because to date, no morph differences in behavior have been reported in non-breeding populations. In winter flocks and in laboratory-housed birds held on short days, morph does not predict dominance rank or aggression (Harrington 1973; Watt et al. 1984; Schlinger 1987; Schwabl et al. 1988; Piper and Wiley 1989; Dearborn and Wiley 1993; Wiley et al. 1999). In contrast, when birds are held on long days and undergo gonadal recrudescence, WS birds engage in significantly more aggression than do their TS cage-mates and outrank them on average (Watt et al. 1984; Maney and Goodson 2011). Morph differences in dominance and aggression therefore seem to depend on season (Maney and Goodson 2011). The large morph difference in ERα in MeA in non-breeding birds (Fig. 2c) suggests that although the morphs may not differ in aggressive behavior during the fall, a neurological substrate certainly exists to support such differences. Perhaps differences are not manifested in the fall because plasma T is negligible and aromatase activity is suppressed (Soma et al. 2003; Meitzen et al. 2007), meaning that local levels of E2 in MeA may be quite low. We are currently testing whether exogenous administration of E2 can rapidly affect behavior in non-breeding birds (see Heimovics et al. 2014) and whether those effects could depend on morph, as ERα expression in MeA would suggest.
Life-history trade-offs and the medial amygdala
Could life-history trade-offs be mediated entirely in the MeA? Cushing et al. (2004, 2008) provided some evidence for this hypothesis. They worked with two populations of prairie voles (Microtus ochrogaster), one from a monogamous, biparental population in Illinois and other from a population in Kansas with less male parental care and more promiscuity. Compared with the Illinois males, the Kansas males had higher levels of ERα mRNA expression in MeA (Cushing et al. 2004). Importantly, experimental overexpression of ERα in MeA profoundly inhibited parental behavior and increased interest in novel females (Cushing et al. 2008). Together with these and other studies in rodents (e.g., Murakami et al. 2011), our work on white-throated sparrows suggests that ERα in MeA may underlie the evolution of a suite of complex correlated traits that constitute a “personality” (Wolf 2007) or “behavioral syndrome” (Bell 2007) that maximizes territoriality and mate-seeking while minimizing prosocial behaviors such as monogamy and parenting. In white-throated sparrows, ERα in MeA was negatively correlated with parental provisioning, but that effect disappeared when plasma T and E2 were controlled (Horton et al. 2014b). Manipulation of plasma T did not affect ERα expression in MeA (Fig. 2d), but T could regulate expression in another region to affect behavior. For example, ERα expression in the medial preoptic area predicts parental provisioning (Horton et al. 2014b). If ERα expression in this region depends on plasma T, as it does in other species (e.g., Lisciotto and Morrell 1993), morph differences in that expression may be explained by differing levels of plasma steroids. We are currently testing this hypothesis.
Disruptive selection and chromosomal structure
The sequestration of life-history strategies into alternative phenotypes in white-throated sparrows resembles the evolution of behaviors that are sexually differentiated. The behaviors associated with the ZAL2m chromosome, namely higher territorial aggression and lower parental provisioning, are the same behaviors that differ by sex in this and many other species. It is interesting, therefore, to compare the ZAL2m chromosome to sex chromosomes. First, because of the disassortative mating system, each breeding pair consists of one bird with and one without ZAL2m. Second, because WS–WS pairs are rare, the ZAL2m is in a near-constant state of heterozygosity—not unlike the mammalian Y chromosome. This situation has suppressed recombination and created one of the largest blocks of linkage disequilibrium ever described in a vertebrate (Thomas et al. 2008). A major difference between ZAL2m and the mammalian Y, however, is that ZAL2m seems to be a healthy chromosome—although it is differentiating from its counterpart, it is not degenerating. We have not detected disrupted genes, repetitive elements, or other signatures of chromosome degeneration (Davis et al. 2011). That the chromosome appears healthy suggests that ZAL2m homozygotes occur in numbers sufficient to support recombination and gene flow.
We do know that the ZAL2m/2m genotype is not lethal because homozygotes, although rare, appear healthy. Out of approximately 700 birds genotyped in our laboratory since 2005, we have collected one “superwhite” bird with two copies of ZAL2m (Horton et al. 2013). Because she was a hatch-year female, she should have had rather dull plumage for a WS bird (Piper and Wiley 1989). She was as bright as an adult male, however (she is pictured in Fig. 1a, on the right). She was extremely vocal and aggressive, dominating opponents in behavioral tests. Her phenotype was thus an exaggerated version of a typical ZAL2/2m heterozygote, supporting the hypothesis that alleles inside the ZAL2m rearrangement confer high aggression. Two other superwhite birds have been described in the literature (Thorneycroft 1975; Falls and Kopachena 2010), but their behavior was not characterized systematically. Considering all genotyping done in our laboratory and by others (e.g., Romanov et al. 2009), three superwhite birds have been found among 1700 birds genotyped—a frequency of less than 1/500. Homozygotes are thus indeed rare, but apparently common enough to prevent degeneration of the rearranged chromosome (Davis et al. 2011). Mechanisms that prevent greater rates of homozygosity are not well-understood, but are likely to involve strong biases in mate choice and high aggression between males and females of the WS morph.
Future directions
Given that the ZAL2m rearrangement contains approximately 1000 genes (Thomas et al. 2008) and because a primary adaptive advantage of inversions is to bind together two or more co-adapted alleles (Dobzhansky 1970), we are under no illusion that behavioral polymorphism in the white-throated sparrow is caused by a single gene. In addition to ESR1, genes for gonadotropin receptors and a steroidogenic enzyme have been captured by the rearrangement and we are currently evaluating those. Moreover, we are now taking a discovery-based approach to identify all of the genes that are differentially expressed in the brain and that predict behavior. We recently completed an RNA-seq study of all transcripts in MeA in free-living, behaviorally characterized males (W. M. Zinzow-Kramer, unpublished data). So far, using this independent sample we have confirmed that ERα mRNA is expressed in MeA at higher levels in WS than in TS males (Fig. 3) and that its expression is correlated with the vocal response to STI. We are working now to identify networks of genes, connected to ERα and otherwise, that may function together to affect behavior.
Fig. 3.
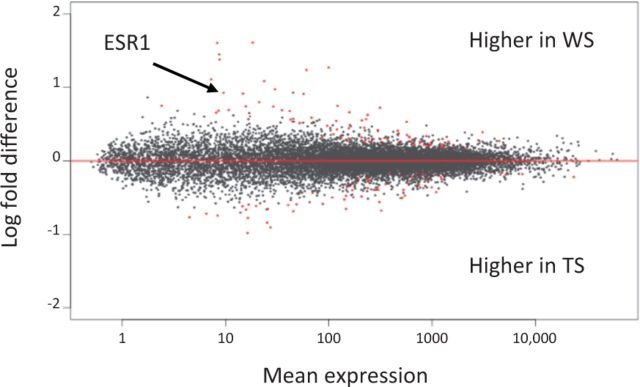
Analysis of RNA-seq data for all transcripts in the medial amygdala of 10 WS and 9 TS free-living males. Genes shown in red (see online version), which include ESR1, are differentially expressed.
Differentiation of the ZAL2m rearrangement has undoubtedly led not only to altered promoter efficiency but also to nonsynonymous changes in protein-coding regions. The complete genome sequences of a TS (ZAL2/2) bird and our superwhite (ZAL2m/2m) are now available, so we are working with our collaborators toward a complete SNP catalog. We will soon be able to identify every potential alteration in protein function, and will work toward identifying coadapted alleles. We believe that this species will prove to be an important model for understanding not only how chromosomal rearrangements affect the genes they capture, but also how and why they may confer selective advantages and persist in populations (Dobzhansky 1970).
Summary
Alternative life-history strategies in white-throated sparrows are determined, in part, by a rearrangement on the second chromosome. Because of the disassortative mating system in this species, the rearrangement is in a near-constant state of heterozygosity that limits gene flow. As a result, the rearranged chromosome 2 is differentiating from its counterpart, and genes inside the rearrangement are accumulating SNPs that affect gene transcription. The gene encoding ERα may contribute to the behavioral phenotype, as it is expressed differently in the two morphs and predicts both territorial and parental behaviors. We hypothesize that differentiation of this gene has played a causal role in the evolution of life-history strategies in this species.
Funding
This work was supported by the National Institutes of Health [1R01MH082833 to D.L.M.] and the National Science Foundation [SMA-1306132 to W.M.Z.-K. and IOS-0723805 to D.L.M.]. SICB provided financial assistance for attendance at the symposium.
Acknowledgments
The authors wish to thank all of the people who contributed to the work discussed here, including Jamie Davis, Will Hudson, Ignacio Moore, Cliff McKee, Justin Michaud, Eric Ortlund, Sandra Shirk, Greg Tharpe, Jim Thomas, and Elaina Tuttle.
References
- Ball GF, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Phil Trans R Soc B. 2008;363:1699–710. doi: 10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AM. Future directions in behavioural syndromes research. Proc R Soc B Biol Sci. 2007;274:755–61. doi: 10.1098/rspb.2006.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt PC, Dabbs JM, Fielden JA, Lutter CD. Testosterone changes during vicarious experiences of winning and losing among fans at sporting events. Physiol Behav. 1998;65:59–62. doi: 10.1016/s0031-9384(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Biermann GC, Robertson RJ. An increase in parental investment during the breeding season. Anim Behav. 1981;29:487–9. [Google Scholar]
- Book AS, Starzyk KB, Quinsey VL. The relationship between testosterone and aggression: a meta-analysis. Aggr Viol Behav. 2001;6:579–99. [Google Scholar]
- Booth A, Shelley G, Mazur A, Tharp G, Kittok R. Testosterone, and winning and losing in human competition. Horm Behav. 1989;23:556–71. doi: 10.1016/0018-506x(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Cheng M, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav Evol. 1999;53:243–70. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- Cheung CL, Chan BY, Chan V, Ikegawa S, Kou I, Ngai H, Smith D, Luk KD, Huang QY, Mori S, et al. Pre-B-cell leukemia homeobox 1 (PBX1) shows functional and possible genetic association with bone mineral density variation. Hum Mol Genet. 2009;18:679–87. doi: 10.1093/hmg/ddn397. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le WW, Hoffman GE. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 2004;1016:247–54. doi: 10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. J Neurosci. 2008;28:10399–403. doi: 10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JK, Mittel LB, Lowman JJ, Thomas PJ, Maney DL, Martin CL, Comparative Sequencing Program NISC, Thomas JW. Haplotype-based genomic sequencing of a chromosomal polymorphism in the white-throated sparrow (Zonotrichia albicollis) J Heredity. 2011;102:380–90. doi: 10.1093/jhered/esr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn DC, Wiley RH. Prior residence has a gradual influence on the dominance in captive white-throated sparrows. Anim Behav. 1993;46:39–46. [Google Scholar]
- Dobzhansky T. New York: Columbia University Press; 1970. Genetics of the evolutionary process. [Google Scholar]
- Dufty AM, Wingfield JC. The influence of social cues on the reproductive endocrinology of male brown-headed cowbirds: field and laboratory studies. Horm Behav. 1986;20:222–34. doi: 10.1016/0018-506x(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Falls JB, Kopachena JG. Ithaca: Cornell Lab of Ornithology; 2010. White-throated sparrow (Zonotrichia albicollis), The Birds of North America Online. (Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/128.) [Google Scholar]
- Finch CE, Rose MR. Hormones and the physiological architecture of life-history evolution. Quart Rev Biol. 1995;70:1–52. doi: 10.1086/418864. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PB, Kahlenberg SM, Barret ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with low testosterone in males. Evol Hum Behav. 2002;23:193–201. [Google Scholar]
- Gray PB. Marriage, parenting, and testosterone variation among Kenyan Swahili men. Am J Phys Anthropol. 2003;122:279–86. doi: 10.1002/ajpa.10293. [DOI] [PubMed] [Google Scholar]
- Grozhik AV, Horozsko CP, Horton BM, Voisin DA, Maney DL. Hormonal regulation of vasotocin receptor mRNA in a seasonally breeding songbird. Horm Behav. 2014;65:254–63. doi: 10.1016/j.yhbeh.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington BA. Aggression in winter resident and spring migrant white-throated sparrows in Massachusetts. Bird Banding. 1973;44:314–5. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays. 2007;292:133–44. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Hegner RE, Wingfield JC. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. Auk. 1987;104:462–9. [Google Scholar]
- Heimovics SA, Ferris JK, Soma KK. Non-invasive administration of 17β-estradiol rapidly increases aggressive behavior in non-breeding, but not breeding, male song sparrows. Horm Behav. 2014;69C:31–8. doi: 10.1016/j.yhbeh.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Horton BM, Holberton RL. Morph-specific variation in baseline corticosterone and the adrenocortical response in breeding white-throated sparrows (Zonotrichia albicollis) Auk. 2010;127:540–8. [Google Scholar]
- Horton BM, Moore IT, Maney DL. New insights into the hormonal and behavioural correlates of polymorphism in white-throated sparrows. Anim Behav. 2014a;93:207–19. doi: 10.1016/j.anbehav.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Hudson WH, Ortlund EA, Shirk S, Thomas JW, Young ER, Zinzow-Kramer WM, Maney DL. Estrogen receptor α polymorphism in a species with alternative behavioral phenotypes. Proc Natl Acad Sci USA. 2014b;111:1443–8. doi: 10.1073/pnas.1317165111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Hu Y, Martin CL, Bunke BP, Matthews BS, Moore IT, Thomas JW, Maney DL. Behavioral characterization of a white-throated sparrow homozygous for the ZAL2m chromosomal arrangement. Behav Genet. 2013;43:60–70. doi: 10.1007/s10519-012-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh LY, Maney DL, Thomas JW. Chromosome-wide linkage disequilibrium caused by an inversion polymorphism in the white-throated sparrow. Heredity. 2011;106:537–46. doi: 10.1038/hdy.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi M, Hasegawa T, Bischof HJ. Amygdala and socio-sexual behavior in male zebra finches. Brain Behav Evol. 2009;74:250–7. doi: 10.1159/000264660. [DOI] [PubMed] [Google Scholar]
- Jones JG. 1987. Use of space by male white-throated sparrows (Zonotrichia albicollis) (Unpublished doctoral thesis). Toronto, ON: University of Toronto. [Google Scholar]
- Ketterson ED, Nolan V. Hormones and life histories—an integrative approach. Am Nat. 1992;140:S33–62. doi: 10.1086/285396. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V. Male parental behavior in birds. Ann Rev Ecol Syst. 1994;25:601–28. [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr Comp Biol. 2009;49:365–79. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapton RW, Falls JB. Differences in parental contribution among pair types in the polymorphic white-throated sparrow. Can J Zool. 1983;61:1288–92. [Google Scholar]
- Kopachena JG, Falls JB. Re-evaluation of morph-specific variations in parental behavior of the white-throated sparrow. Wilson Bull. 1993a;105:48–59. [Google Scholar]
- Kopachena JG, Falls JB. Aggressive performance as a behavioral correlate of plumage polymorphism in the white-throated sparrow (Zonotrichia albicollis) Behaviour. 1993b;124:249–66. [Google Scholar]
- Lisciotto CA, Morrell JI. Circulating gonadal steroid hormones regulate estrogen receptor mRNA in the male rat forebrain. Brain Res Mol Brain Res. 1993;20:79–90. doi: 10.1016/0169-328x(93)90112-3. [DOI] [PubMed] [Google Scholar]
- Lowther JK. 1962. Colour and behavioral polymorphism in the white-throated sparrow, Zonotrichia albicollis [Unpublished doctoral thesis]. Toronto, ON: University of Toronto. [Google Scholar]
- Lynn SE, Prince LE, Schook DM, Moore IT. Supplementary testosterone inhibits paternal care in a tropically breeding sparrow, Zonotrichia capensis. Physiol Biochem Zool. 2009;82:699–708. doi: 10.1086/605915. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goodson JL. Neurogenomic mechanisms of aggression in songbirds. Adv Genet. 2011;75:83–119. doi: 10.1016/B978-0-12-380858-5.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Lange HS, Raees MQ, Sanford SE. Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Horm Behav. 2009;55:113–20. doi: 10.1016/j.yhbeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Ketterson ED. Hormone-mediated suites as adaptations and evolutionary constraints. Phil Trans R Soc Lond B Biol Sci. 2007;363:1611–20. doi: 10.1098/rstb.2007.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am Nat. 2007;170:864–75. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–57. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DB, Sinervo B, Hazard LC, Svensson EI, Costa D. Relating endocrinology, physiology and behaviour using species with alternative mating strategies. Funct Ecol. 2007;21:653–65. [Google Scholar]
- Moore MC. Hormonal responses of free-living male white-crowned sparrows to experimental manipulation of female sexual behavior. Horm Behav. 1982;16:323–9. doi: 10.1016/0018-506x(82)90030-7. [DOI] [PubMed] [Google Scholar]
- Murakami G, Hunter RG, Fontaine C, Ribeiro A, Pfaff D. Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur J Neurosci. 2011;34:469–77. doi: 10.1111/j.1460-9568.2011.07761.x. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–57. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nijhout FH. Development and evolution of adaptive polyphenisms. Evol Dev. 2003;5:9–18. doi: 10.1046/j.1525-142x.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- Peterson RT. Boston: Houghton Mifflin; 1961. A field guide to Western birds. [Google Scholar]
- Piper WH, Wiley RH. Distinguishing morphs of the white-throated sparrow in basic plumage. J Field Ornithol. 1989;60:73–83. [Google Scholar]
- Rhen T, Crews D. Variation in reproductive behaviour within a sex: neural systems and endocrine activation. J Neuroendocrinol. 2002;14:517–31. doi: 10.1046/j.1365-2826.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Robinson TJ, Siefferman L, Risch TS. Seasonal trade-offs in reproductive investment in a multi-brooded passerine. Condor. 2010;112:390–8. [Google Scholar]
- Romanov MN, Tuttle EM, Houck ML. The value of avian genomics to the conservation of wildlife. BMC Genomics. 2009;10(Suppl. 2):S10. doi: 10.1186/1471-2164-10-S2-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub R, Ketterson ED. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc Biol Sci. 2012;279:3547–55. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schlinger BA. Plasma androgens and aggressiveness in captive winter white-throated sparrows (Zonotrichia albicollis) Horm Behav. 1987;21:203–10. doi: 10.1016/0018-506x(87)90045-6. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Ketterson ED, Nolan V, Jr, Sharp PJ, Buntin JD. The effect of exogenous testosterone on parental behavior, plasma prolactin, and prolactin binding sites in dark-eyed juncos. Horm Behav. 1998;34:1–10. doi: 10.1006/hbeh.1998.1455. [DOI] [PubMed] [Google Scholar]
- Schwabl H, Ramenofsky M, Schwabl-Benzinger I, Farner DS, Wingfield JC. Social status, circulating levels of hormones, and competition for food in winter flocks of the white-throated sparrow. Behaviour. 1988;107:107–21. [Google Scholar]
- Silverin B. Effects of long-acting testosterone treatment on free living pied flycatchers, Ficedula hypoleuca, during the breeding season. Anim Behav. 1980;28:906–12. [Google Scholar]
- Sinervo B, Svensson E. Correlational selection and the evolution of genomic architecture. Heredity. 2002;89:329–38. doi: 10.1038/sj.hdy.6800148. [DOI] [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5-alpha reductase, and 5-beta reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J Neurobiol. 2003;56:209–21. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Spinney LH, Bentley GE, Hau M. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis) Horm Behav. 2006;50:762–71. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walch CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Swett MB, Breuner CW. Plasma testosterone correlates with morph type across breeding substages in male white-throated sparrows. Physiol Biochem Zool. 2009;82:572–9. doi: 10.1086/605392. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Caceres M, Lowman JJ, Morehouse CB, Short ME, Baldwin EL, Maney DL, Martin CL. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement that suppresses recombination. Genetics. 2008;179:1455–68. doi: 10.1534/genetics.108.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): a comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–29. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- Thorneycroft HB. Chromosomal polymorphism in the White-throated Sparrow, Zonotrichia albicollis (Gmelin) Science. 1966;154:1571–2. doi: 10.1126/science.154.3756.1571. [DOI] [PubMed] [Google Scholar]
- Thorneycroft HB. A cytogenetic study of the white-throated sparrow, Zonotrichia albicollis. Evolution. 1975;29:611–21. doi: 10.1111/j.1558-5646.1975.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor alpha in select brain nuclei are associated with individual differences in aggression. Horm Behav. 2006;50:338–45. doi: 10.1016/j.yhbeh.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. “Sexual Selection and the Descent of Man”. Aldine: Chicago; 1972. pp. 139–79. [Google Scholar]
- Watt DJ, Ralph CJ, Atkinson CT. The role of plumage polymorphism in dominance relationships of the white-throated sparrow. Auk. 1984;101:110–20. [Google Scholar]
- Wiley RH, Steadman L, Chadwick L, Wollerman L. Social inertia in the white-throated sparrows results from recognition of opponents. Anim Behav. 1999;57:453–63. doi: 10.1006/anbe.1998.0991. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Androgens and mating systems: testosterone-induced polygyny in normally monogamous birds. Auk. 1984;101:665–71. [Google Scholar]
- Wingfield JC, Hahn TP. Testosterone and territorial behavior in sedentary and migratory sparrows. Anim Behav. 1994;47:77–89. [Google Scholar]
- Wingfield JC, Monk D. Behavioral and hormonal responses of male song sparrows to estradiol-treated females during the non-breeding season. Horm Behav. 1994;28:146–54. doi: 10.1006/hbeh.1994.1012. [DOI] [PubMed] [Google Scholar]
- Wolf M, van Doorn GS, Leimar O, Weissing FJ. Life-history trade-offs favour the evolution of animal personalities. Nature. 2007;447:581–4. doi: 10.1038/nature05835. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm Behav. 2001;40:139–45. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]


