Abstract
Elevated depressive symptoms are associated with cognitive deficits, while higher education protects against cognitive decline. This study was conducted to test if education level moderates the relationship between depressive symptoms and cognitive function. Seventy-three healthy, dementia-free adults aged 18–81 completed neuropsychological tests, as well as depression and anxiety questionnaires. Controlling for age, sex, and state anxiety, we found a significant interaction of depressive symptoms and education for immediate and delayed verbal memory, such that those with a higher education level performed well regardless of depressive symptomatology, whereas those with lower education and high depressive symptoms had worse performance. No effects were found for executive functioning or processing speed. Results suggest that education protects against verbal memory deficits in individuals with elevated depressive symptoms. Further research on cognitive reserve in depression-related cognitive deficits and decline is needed to understand the mechanisms behind this phenomenon.
Keywords: Depressive symptoms, Cognition, Memory, Cognitive reserve, Educational achievement
Introduction
Approximately two thirds of depressed patients experience cognitive deficits (Afridi, Hina, Qureshi, & Hussain, 2011; Rock, Roiser, Riedel, & Blackwell, 2014). These deficits occur in multiple domains including executive function, episodic memory, visuospatial memory, attention, and processing speed (McDermott & Ebmeier, 2009; Rock et al., 2014). Additionally, structural and functional brain abnormalities in frontotemporal regions that subserve these cognitive functions have been noted in individuals who suffer from depression (Dotson et al., 2014; Naismith, Norrie, Mowszowski, & Hickie, 2012). Cognitive deficits are present not only in major depressive disorder, but also in individuals who exhibit “minor” or subclinical levels of depression (Dotson, Resnick, & Zonderman, 2008; Dotson et al., 2014; Elderkin-Thompson et al., 2003). Despite multiple findings of cognitive deficits, there is variability in the pattern and severity of depression-related cognitive difficulties across individuals (Baune et al., 2010; McDermott & Ebmeier, 2009). This variability may be due in part to differences in the severity of depressive symptoms across studies (McDermott & Ebmeier, 2009). Variability may also result from the heterogeneous nature of depression, as it has been suggested that specific symptom dimensions of depression may be associated with unique cognitive impairments (Baune, Suslow, Arolt, & Berger, 2007; Elderkin-Thompson et al., 2003; McDermott & Ebmeier, 2009). Additionally, variability in the relationship between depressive symptoms and cognitive functioning may be affected by individual differences in cognitive reserve.
Cognitive reserve is a term used to describe the brain's ability to maintain adequate function despite neuropathology (Booth et al., 2013; Stern, 2002). Various proxies for cognitive reserve are used in the literature, most commonly vocabulary ability, leisure time activity, occupational attainment, and education (Baune et al., 2007). There is some debate over the extent to which education level accurately captures an individual's cognitive reserve (Reed et al., 2010; Zahodne et al., 2013), but studies have demonstrated a high correlation between education and alternative measures of reserve such as vocabulary knowledge (Mitchell, Shaughnessy, Shirk, Yang, & Atri, 2012; Siedlecki et al., 2009). Additionally, higher education levels have been shown to be a protective factor in nonpathological aging, as well as in multiple disease states, including dementia and Parkinson's disease (Cabello, Navarro, Latorre, & Fernández-Berrocal, 2014; Cohen et al., 2007; Valenzuela, & Sachdev, 2006).
Research examining the role of cognitive reserve in the relationship between depressive symptoms and cognitive dysfunction is limited. One study found that lower education and higher depressive symptoms were associated with poorer performance on a brief mental status questionnaire in older, community-dwelling men (Wight, Aneshensel, & Seeman, 2002). Another study found a similar interaction between education and depressive symptoms on task switching, verbal memory, and processing speed tasks in a group of depressed older adults (Avila et al., 2009). In contrast, a third study found no significant interactions between education level and depressive symptoms in predicting cognitive performance on tasks of processing speed, visuospatial functioning, executive functioning, language, or memory in older adults (Bhalla et al., 2005). Finally, a recent study found the reverse pattern of what would be expected, as older adults with higher levels of education showed more cognitive deficits at higher depressive symptoms than those with lower education (O'Shea et al., 2015).
Differing findings between studies may be partially explained by varying levels of education and depression severity; however, with such limited research on the subject, the effects of education on cognitive deficits in depression are still unclear. Additionally, previous studies have exclusively examined this relationship in older adults. It is, therefore, unknown whether the potential protective effects of education on cognitive deficits in depression are seen in younger adults or if it is unique to older individuals, who may be at greater risk for cognitive decline due to brain changes associated with aging (Fjell, & Walhovd, 2010). This study was, therefore, conducted to further explore the impact of education level and depressive symptoms on executive functioning, processing speed, and verbal memory in adults who ranged in age from 18 to 81 years. These domains were selected based on previous research indicating deficits in these areas in depressed individuals (McDermott, & Ebmeier, 2009; Rock et al., 2014). It was hypothesized that higher depressive symptoms and lower education levels would be associated with worse cognitive performance. Moreover, we predicted that higher education levels would be associated with less impact of depressive symptoms on cognitive functioning.
Methods
Participants
The sample included 73 community-dwelling adults between the ages of 18 and 81 years who were recruited from the University of Florida and the surrounding community via the Claude D. Pepper Registry for Research, local flyer and newspaper advertisements, and public service announcements. Inclusion criteria included right-handedness, native English speaking, normal or corrected-to-normal vision, an education of at least 9 years, and a score of >30 on the Telephone Interview for Cognitive Status (TICS; Brandt, Spencer, & Folstein, 1988). The TICS has a score range of 0–41, with higher scores reflecting better cognitive functioning. The cutoff of 30 has been shown to have a sensitivity of 94% as well as 100% specificity for differentiating between cognitively intact and demented individuals (Brandt et al., 1988). Participants were given the mood disorders, psychotic screener, substance use disorders, and anxiety disorder modules of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV; First, Spitzer, Gibbon, & Williams, 2002) to assess the presence of major psychopathology, other than unipolar depression or anxiety disorders, that would be an exclusionary criteria. Individuals with an anxiety disorder were not excluded from the study due to the high comorbidity of anxiety and depressive symptoms (Fava et al., 2000; Kvaal, McDougall, Brayne, Matthews, & Dewey, 2008). Instead, anxiety symptoms were controlled for in all analyses. Two participants met criteria for major depression, six for an anxiety disorder, and four for comorbid major depression and anxiety disorder based on SCID-IV administration. Other exclusionary criteria included a history of major medical or neurological illness, head trauma, learning disorders, current antiepileptic or antipsychotic use, and language comprehension difficulties. All participants gave written and verbal consent to participate in the study. All procedures were in compliance with the Declaration of Helsinki, and the protocol was approved by the University of Florida Health Science Center Institutional Review Board. Sample demographic data are presented in Table 1.
Table 1.
Demographic and cognitive characteristics of the sample
| Mean | SD | Range | |
|---|---|---|---|
| Demographics | |||
| Age | 38.49 | 22.40 | 18 to 81 |
| Sex (% women) | 72.60 | — | — |
| Race (% white) | 79.45 | — | — |
| Education (years) | 14.88 | 2.14 | 10 to 20 |
| CES-D | 10.03 | 10.70 | 0 to 45 |
| STAI-S | 33.92 | 12.31 | 20 to 70 |
| Cognitive performance (raw scores) | |||
| Trail Making A | 25.51 | 8.79 | 13 to 51 |
| Trail Making B | 57.17 | 23.48 | 27 to 137 |
| Stroop interference | 1.81 | 8.47 | −19.04 to 22.58 |
| HVLT-R total recall | 27.22 | 4.29 | 16 to 35 |
| HVLT-R delayed recall | 9.52 | 1.95 | 4 to 12 |
Notes: CES-D = Center for Epidemiologic Studies Depression Scale; STAI-S = State-Trait Anxiety Inventory, State scale; HVLT-R = Hopkins Verbal Learning Test-Revised.
Measures
Symptoms of depression were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977), a 20-item self-report questionnaire measuring the frequency and severity of depressive symptoms experienced in the past week that has been validated for use in both young and older community-dwelling adults (Haringsma, Engels, Beekman, & Spinhoven, 2004; Radloff, 1991). Each item on the CES-D is scored from 0 to 3, resulting in a potential total score range of 0–60, with higher scores signifying greater severity of depressive symptoms. State anxiety was assessed using the state form of the State-Trait Anxiety Inventory (STAI-S; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983).
Participants were administered a brief neuropsychological battery that focused on measures of executive functioning, processing speed, and verbal memory. The current study used the following measures: total immediate recall and delayed recall of the Hopkins Verbal Learning Test-Revised (HVLT-R; Brandt, & Benedict, 2001), completion time on the Trail Making Test parts A (TMT-A) and B (TMT-B; Reitan, 1992), and the Stroop Color–Word interference score (Golden, 1978). The Stroop inference score was calculated based on the traditional CW − CW′ formula, where CW corresponds to the color–word score and CW′ is the predicted color-word score, calculated based on the word (W) and color (C) subtest scores [(W × C)/(W + C)].
Statistical Analysis
Of the 73 subjects in the study, 1 had missing data for both TMT-A and TMT-B and 2 were missing data for the Stroop interference task. Additionally, three outliers (>3 SDs above the mean) were removed from the TMT-B analysis, resulting in sample sizes ranging from 69 to 73 for each test. Data were analyzed using linear regression models in SPSS version 21.0 (IBM Corp., Armonk, NY). Separate models were conducted for each of the aforementioned cognitive measures, with years of education and CES-D scores as independent variables and with age, sex, and STAI-S scores as covariates (see Table 1). All variables besides sex were continuous measures in the models. TMT-A and TMT-B scores were transformed via natural log and inverse transformations, respectively, to account for significant positive skew. Raw scores were used for Stroop interference, HVLT-R total recall, and HVLT-R delayed recall. Statistical significance was set at α ≤ 0.05.
Results
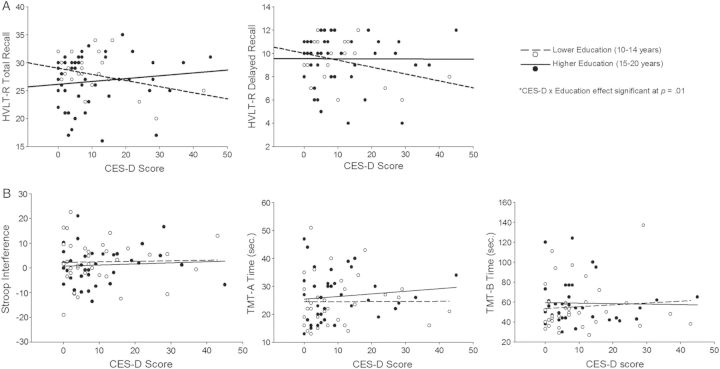
Results of the regression analyses are summarized in Table 2 and shown graphically in Fig. 1. Significant CES-D × education effects were observed for HVLT-R total recall [β = 0.27, t(66) = 2.59, p = .01] and HVLT-R delayed recall [β = 0.28, t(66) = 2.53, p = .01]. For both tests, higher depressive symptom severity was associated with worse performance in individuals with lower levels of education, but performance was preserved at higher depressive symptom severity in individuals with higher levels of education. This trend was seen in both younger and older adults when results were graphed separately. Additionally, a similar pattern was observed when individuals with CES-D scores >2 SDs above the mean were removed, suggesting that the results, while influenced by more severe depression scores, are not solely explained by them. There were no main effects of CES-D or education on any measure and no significant CES-D × education interactions for Stroop interference, TMT-A, or TMT-B.
Table 2.
Regression coefficients (B), standard error (SE), and degrees of freedom (df) for regression analysis
| TMT-A |
TMT-B |
Stroop |
HVLT-R total |
HVLT-R delayed |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE (df) | B | SE (df) | B | SE (df) | B | SE (df) | B | SE (df) | |
| CES-D | −.02 | 0.01 (66) | −.07 | 0.00 (63) | −.11 | 0.13 (65) | −.24 | 0.07 (67) | −.33 | 0.03 (67) |
| Education | −.01 | 0.02 (66) | .04 | 0.00 (63) | .08 | 0.46 (65) | −.01 | 0.24 (67) | −.00 | 0.11 (67) |
| CES-D × Education | .03 | 0.00 (65) | .02 | 0.00 (62) | .07 | 0.06 (64) | .27* | 0.03 (66) | .28* | 0.01 (66) |
Notes: TMT-A = Trail Making Test A; TMT-B = Trail Making Test B; Stroop = Stroop interference; HVLT-R = Hopkins Verbal Learning Test-Revised; CES-D = Center for Epidemiologic Studies Depression Scale.
*p < .05.
Fig. 1.
Results showing (A) significant CES-D × education effects on HVLT-R total recall and delayed recall and (B) nonsignificant CES-D × education effects on Stroop interference, TMT-A, and TMT-B. The open circles and dotted line indicate those with lower education (10–14 years) and the filled circles and solid line represent those with a higher level of education (15–20 years). Education groups are for graphical purposes only; the education level was used as a continuous variable in all statistical analyses. CES-D = Center for Epidemiologic Studies Depression Scale; HVLT-R = Hopkins Verbal Learning Test-Revised; TMT = Trail Making Test.
Discussion
Results from this study suggest that education level interacts with depressive symptoms to affect verbal memory, such that individuals with higher education levels may not show deficits despite elevated depressive symptoms, whereas those with lower education levels perform more poorly at increasingly elevated levels of depressive symptoms. This finding is consistent with previous work which found that education level serves as a protective factor against deficits in verbal memory in late-life depression (Avila et al., 2009) and against deficits in general cognitive status in older adults with elevated subclinical depressive symptoms (Wight et al., 2002). The current study extends previous findings to a sample that ranged from young adults to older adults, demonstrating that the effect is not limited to late life. Results are also similar to previous research on education's impact on cognition in multiple disease states, including Hepatitis C, Alzheimer's disease, and Parkinson's disease (Bieliauskas et al., 2007; Cohen et al., 2007; Stern, 2012).
Our results are not consistent with a recently published study which found that higher depressive symptoms were associated with memory, executive functioning, and language deficits in individuals with higher education, but not in those with lower education (O'Shea et al., 2015). These former findings suggest that those with higher education may lose the protective nature of education at increased depressive symptoms, in contrast to our finding that those with higher education are able to maintain performance at elevated depressive symptoms. This discrepancy may be due in part to differences between studies in the range of depressive symptoms. While both studies examined subclinical depressive symptoms, the previous study used the CES-D short form and scores ranged from 0 to 9, while we used the full 20-item CES-D and scores ranged from 0 to 45. Perhaps the greater variability in scores in our sample allowed us to detect relationships across a wider range of severity.
The verbal memory results are consistent with the cognitive reserve hypothesis, which postulates that those with higher levels of cognitive reserve can maintain cognitive performance despite neuropathology because they are able to utilize alternative cognitive strategies and neurological pathways to compensate for the effects of the disease (Stern, 2012). It is unclear whether higher education resulted in participants in our study using more effective strategies to remember words or if they were simply better able to manage the demands of the test compared with those with lower education. The effective implementation of strategies may also explain why we found a protective effect of education on memory but not on executive functions or processing speed, because mnemonic strategies are frequently taught in educational settings. Results of previous research examining a potentially protective effect of education on depression-related dysfunction in executive and speed abilities have been mixed, with one study finding significant protective effects (Avila et al., 2009), another noting no significant interactions (Bhalla et al., 2005), and a third study finding higher education associated with more decline (O'Shea et al., 2015). Although reasons for the discrepant findings are unclear, differences may be partially explained by differences in educational attainment between samples. Education moderated the relationships of depression with executive functioning and processing speed in a study that compared individuals with 1–4 years of education with those with 5 or more years (Avila et al., 2009) and in a study where the average level of education was relatively low (9.7 years; O'Shea et al., 2015), but not in the two studies, including the present study, that included samples with overall higher education levels (Bhalla et al., 2005). In the current study, education level ranged from 10 to 20 years, and in the study by Bhalla and colleagues (2005), the mean age of even their “low education” group was 11.3 years. Perhaps the protective effect of education on executive functioning and processing speed is apparent after just a few years of schooling, but plateaus or shows smaller gains as more years of education are attained. In contrast, educational attainment may continue to benefit verbal memory after more years of schooling. Alternatively, deficits in processing speed may be difficult to compensate for compared with verbal memory tasks, and therefore, increased education may not significantly assist in the retention of this ability. The specific tasks used to measure executive functioning and processing speed may have also affected the results, because alternative measures may have been more sensitive to the effects of depressive symptoms, education, or both.
Results from the current study suggest that individuals with elevated depressive symptoms and low education may be more susceptible to verbal memory deficits. Depression-related memory deficits are associated with functional disability, particularly in older adults. For example, impairments in everyday problem-solving ability in depressed older adults were mediated by memory and reasoning deficits in the Advanced Cognitive Training for Independent and Vital Elderly study (Yen, Rebok, Gallo, Jones, & Tennsteldt, 2012). In another study (Gallo et al., 2003), memory and problem-solving ability mediated the relationship between depression symptoms and everyday problem-solving and observed tasks of daily living. In conjunction with these findings, our results indirectly suggest that individuals with low education and elevated depressive symptoms may be at increased risk for functional decline. Future research should directly test this possible relationship.
This study sample was well educated overall and had mostly subclinical levels of depression. Research examining more severely depressed individuals, those with significantly lower education, and additional aspects of executive functioning may provide more insight into the protective nature of education on cognitive deficits in depression. The potential impact of age on the interactive effect of education and depressive symptoms on cognitive performance could not be assessed given the limited sample size, which precluded analyzing a three-way interaction between age, education, and CES-D scores. Our significant results across a wide age range suggest that the protective effect of education may not be unique to older adults, and therefore, future research would benefit from examining this potential three-way interaction. Additionally, although education is commonly used as a proxy for cognitive reserve, it may not fully capture all of the variance associated with cognitive reserve (Reed et al., 2010). Reed and colleagues (2010) suggested that cognitive reserve should be directly measured by examining the difference between expected and actual performance given brain pathology. In other words, the variance in cognitive performance that cannot be attributed to measured brain pathology could be identified as the variance due to cognitive reserve. Further research examining cognitive reserve's influence on cognitive deficits in depression by using either additional proxy measure for reserve, such as vocabulary knowledge, leisure activities, or occupational attainment or attempting to implement Reed's operational definition for cognitive reserve, would be beneficial to further understand the extent to which reserve protects against cognitive deficits in depression (Reed et al., 2010; Zahodne et al., 2013).
Overall, results suggest that memory deficits related to depressive symptoms may be attenuated by higher levels of education. This phenomenon may occur across the lifespan, not only in older adults who are more vulnerable to cognitive deficits. These results emphasize the importance of accounting for lower education levels during clinical evaluation, as less-educated individuals may be particularly vulnerable to memory deficits, even at subclinical depression levels. Gaining a better understanding of the neuropsychological deficits seen in subclinical depression and how education level affects these deficits will help to further clarify the role cognitive reserve plays in the neuropsychological functioning of individuals suffering from depressive disorders.
Funding
This work was supported by an Age-Related Memory Loss award from the McKnight Brain Research Foundation (VMD). VMD is partially supported by the University of Florida Claude D. Pepper Center, which is funded by the National Institute on Aging (P30 AG028740-01). SMS is supported by a grant from the National Institute of Aging (T32AG020499-11).
Conflict of Interest
None declared.
Acknowledgement
We thank Dr. Christopher Sozda for assistance with data collection.
References
- Afridi M. I., Hina M., Qureshi I. S., Hussain M. (2011). Cognitive disturbance comparison among drug-naive depressed cases and healthy controls. Journal of the College of Physicians and Surgeons – Pakistan 21 351–355. [PubMed] [Google Scholar]
- Avila R., Moscoso M. A. A., Ribeiz S., Arrais J., Jaluul O., Bottino C. M. C. (2009). Influence of education and depressive symptoms on cognitive function in the elderly. International Psychogeriatrics 21 560–567. [DOI] [PubMed] [Google Scholar]
- Baune B. T., Miller R., McAfoose J., Johnson M., Quirk F., Mitchell D. (2010). The role of cognitive impairment in general functioning in major depression. Psychiatry Research 176 183–189. [DOI] [PubMed] [Google Scholar]
- Baune B. T., Suslow T., Arolt V., Berger K. (2007). The relationship between psychological dimensions of depressive symptoms and cognitive functioning in the elderly—The MEMO-Study. Journal of Psychiatric Research 41 247–254. [DOI] [PubMed] [Google Scholar]
- Bhalla R. K., Butters M. A., Zmuda M. D., Seligman K., Mulsant B. H., Pollock B. G., et al. (2005). Does education moderate neuropsychological impairment in late-life depression? International Journal of Geriatric Psychiatry 20 413–417. [DOI] [PubMed] [Google Scholar]
- Bieliauskas L. A., Back-Madruga C., Lindsay K. L., Wright E. C., Kronfol Z., Lok A. S. F., et al. (2007). Cognitive reserve and neuropsychological functioning in patients infected with hepatitis C. Journal of the International Neuropsychological Society 13 687–692. [DOI] [PubMed] [Google Scholar]
- Booth A. J., Rodgers J. D., Schwartz C. E., Quaranto B. R., Weinstock-Guttman B., Zivadinov R., et al. (2013). Active cognitive reserve influences the regional atrophy to cognition link in multiple sclerosis. Journal of the International Neuropsychological Society 19 1128–1133. [DOI] [PubMed] [Google Scholar]
- Brandt J., Benedict R. H. B. (2001). Hopkins Verbal Learning Test-Revised. Professional manual. Lutz: FL: Psychological Assessment Resources. [Google Scholar]
- Brandt J., Spencer M., Folstein M. (1988). The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology 1 111–117. [Google Scholar]
- Cabello R., Navarro B., Latorre J. M., Fernández-Berrocal P. (2014). Ability of university-level education to prevent age-related decline in emotional intelligence. Frontiers in Aging Neuroscience 6 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen O. S., Vakil E., Tanne D., Nitsan Z., Schwartz R., Hassin-Baer S. (2007). Educational level as a modulator of cognitive performance and neuropsychiatric features in Parkinson disease. Cognitive and Behavioral Neurology 20 68–72. [DOI] [PubMed] [Google Scholar]
- Dotson V. M., Resnick S. M., Zonderman A. B. (2008). Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. American Journal of Geriatric Psychiatry 16 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson V. M., Szymkowicz S. M., Kirton J. W., Mclaren M. E., Green M. L., Rohani J. Y. (2014). Unique and interactive effects of anxiety and depressive symptoms on cognitive and brain function in young and older adults. Journal of Depression and Anxiety Suppl 1 22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin-Thompson V., Kumar A., Bilker W. B., Dunkin J. J., Mintz J., Moberg P. J., et al. (2003). Neuropsychological deficits among patients with late-onset minor and major depression. Archives of Clinical Neuropsychology 18 529–549. [DOI] [PubMed] [Google Scholar]
- Fava M., Rankin M. A., Wright E. C., Alpert J. E., Nierenberg A. A., Pava J., et al. (2000). Anxiety disorders in major depression. Comprehensive Psychiatry 41 97–102. [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, research version. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fjell A., Walhovd K. (2010). Structural brain changes in aging: Courses, causes and cognitive consequences. Reviews in the Neurosciences 21 187. [DOI] [PubMed] [Google Scholar]
- Gallo J. J., Rebok G. W., Tennsted S., Wadley V. G., Horgas A. The Advanced Cognitive Training for Independent & Vital Elderly Study Investigators. (2003). Linking depressive symptoms and functional disability in late life. Aging & Mental Health 7 469–480. [DOI] [PubMed] [Google Scholar]
- Golden C. J. (1978). Stroop color and word test: A manual for clinical and experimental uses. Chicago: Skoelting. [Google Scholar]
- Haringsma R., Engels G. I., Beekman A. T., Spinhoven P. (2004). The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. International Journal of Geriatric Psychiatry 19 558–563. [DOI] [PubMed] [Google Scholar]
- Kvaal K., McDougall F. A., Brayne C., Matthews F. E., Dewey M. E. (2008). Co-occurrence of anxiety and depressive disorders in a community sample of older people: Results from the MRC CFAS (Medical Research Council Cognitive Function and Ageing Study). International Journal of Geriatric Psychiatry 23 229–237. [DOI] [PubMed] [Google Scholar]
- McDermott L. M., Ebmeier K. P. (2009). A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders 119 1–8. [DOI] [PubMed] [Google Scholar]
- Mitchell M. B., Shaughnessy L. W., Shirk S. D., Yang F. M., Atri A. (2012). Neuropsychological test performance and cognitive reserve in healthy aging and the Alzheimer's disease spectrum: A theoretically driven factor analysis. Journal of the International Neuropsychological Society 18 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith S. L., Norrie L. M., Mowszowski L., Hickie I. B. (2012). The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Progress in Neurobiology 98 99–143. [DOI] [PubMed] [Google Scholar]
- O'Shea D. M., Fieo R. A., Hamilton J. L., Zahodne L. B., Manly J. J., Stern Y. (2015). Examining the association between late-life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. International Journal of Geriatric Psychiatry 30 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1 385–401. [Google Scholar]
- Radloff L. S. (1991). The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of Youth and Adolescence 20 149–166. [DOI] [PubMed] [Google Scholar]
- Reed B. R., Mungas D., Farias S. T., Harvey D., Beckett L., Widaman K., et al. (2010). Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 133 2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. (1992). Trail Making Test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychological Laboratory. [Google Scholar]
- Rock P. L., Roiser J. P., Riedel W. J., Blackwell A. D. (2014). Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine 44 2029–2040. [DOI] [PubMed] [Google Scholar]
- Siedlecki K. L., Stern Y., Reuben A., Sacco R. L., Elkind M. S., Wright C. B. (2009). Construct validity of cognitive reserve in a multiethnic cohort: The Northern Manhattan Study. Journal of the International Neuropsychological Society 15 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. D., Gorsuch R. L., Lushene R., Vagg P. R., Jacobs G. A. (1983). Manual of the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. International Neuropsychological Society. Journal 8 (3) 448–460. [PubMed] [Google Scholar]
- Stern Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. The Lancet Neurology 11 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M. J., Sachdev P. (2006). Brain reserve and cognitive decline: A non-parametric systematic review. Psychological Medicine 36 1065–1073. [DOI] [PubMed] [Google Scholar]
- Wight R. G., Aneshensel C. S., Seeman T. E. (2002). Educational attainment, continued learning experience, and cognitive function among older men. Journal of Aging and Health 14 211–236. [DOI] [PubMed] [Google Scholar]
- Yen Y., Rebok G., Gallo J., Jones R., Tennsteldt S. (2012). Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. Journal of Geriatric Psychiatry 19 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne L. B., Manly J. J., Brickman A. M., Siedlecki K. L., DeCarli C., Stern Y. (2013). Quantifying cognitive reserve in older adults by decomposing episodic memory variance: Replication and extension. Journal of the International Neuropsychological Society 19 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]