Abstract
The complex microstructure of salivary gland pleomorphic adenoma is examined in relation to function. Events related to secretion of macromolecules and absorption, responses to the altered microenvironment and controversies concerning epithelial–mesenchymal transition versus modified myoepithelial differentiation are explored. Their effects on tumor cell phenotypes and arrangements are emphasized. Heterotopic differentiation and attempts at organogenesis are also considered. The approach allows interpreting microstructure independently of histogenetic perceptions, envisaging the tumor cells as a continuum, endorsing luminal structures as the principal components, and defining pleomorphic adenoma as a benign epithelial tumour characterized by variable epithelial–mesenchymal transition, secretion/differentiation and metaplasia.
Keywords: Pleomorphic adenoma, Benign mixed tumor, Differentiation, Epithelial–mesenchymal transition, Myoepithelial cells, Metaplasia, Secretion, Function
Introduction
While the interpretation of histochemical and ultrastructural features of mammalian salivary glands in intimate relation to function has been gratifying [1–3], the histological assessment of epithelial salivary tumors is conventionally undertaken in isolation from function. Similar to histogenetic considerations [4], it may be argued that with regard to diagnosis or management, the functional histology of these tumors is “interesting but unimportant”. It is, however, felt that such an approach would increase understanding and hone diagnostic skills in turn. In this article the functional histology of the morphologically complex salivary gland pleomorphic adenoma (PA) is examined in the hope that alternate interpretations and/or explanations of established observations are reached. The article is not intended as a conventional review of the histology, ultrastructure, histochemistry and immunohistochemistry of PA and the references have been selected in view of the various arguments explored here.
Morphological Perception of PA: Historical Considerations, Catalogue of Components and Definitions
Early investigators noted the infinite variations in structure of PA [5]. Although Masson [5] adequately described the epithelial and mesenchymal-like tissues of the tumor and their relations, it was Willis [6] who attempted cataloguing the various components (Table 1). Willis’ scheme did not gain popularity and we propose the alternative summarized in Table 2.
Table 1.
Structural components of PA according to Willis [6]
| 1. Nearly normal-looking salivary tissue |
| 2. Atypical glandular tissue |
| 3. Atypical glandular tissue with marginal epithelial sprouting |
| 4. Solid epithelial formations |
| 5. Epithelial formations with cystic spaces |
| 6. Cornifying stratified epithelial structures |
| 7. Epithelial filaments and networks in a mucinous matrix |
| 8. The so-called “cartilage-like tissue” |
Table 2.
Histological components of PA
| 1. Silhouette (nodular, multinodular); solid or cystic |
| 2. Epithelial cellular (parenchymal): stromal ratio |
| 3. Capsule (thickness, completeness, involved or penetrated) |
| 4. Epithelial cellular components |
| Arrangements (solid, bilayered or multilayered luminal, rounded or angular, interlacing fascicular, schwannomatous; ratios) |
| Cell types |
| Criterion (1)Topography, (2) Morphological phenotype, (3) Interpretation |
| Types (1) luminal, non-luminal; (2) cuboidal, columnar (various), basal, spindled (non-descript, leiomyomatous), oval, stellate, angular, clear, plasmacytoid, oncocytic, squamous (epidermoid), mucous, seromucous, serous, apocrine, adipocytic, sebaceous; ratios; (3) intercalated ductal-like, neoplastic myoepithelial/modified myoepithelial |
| 5. Stromal components |
| Types (myxoid, chondroid, fibrous/hyalinised, osseous, adipocytic) |
| Contents (mucosubstances, collagens, elastin) |
| 6. Boundary between cellular and stromal components (demarcated, indistinct/fraying off) |
| 7. Crystalloids, microliths, pigmentation |
| 8. Vascular structures, nerves |
| 9. Non-epithelial cells (dendritic, macrophages, lymphoid cells, mast cells, myofibroblasts) |
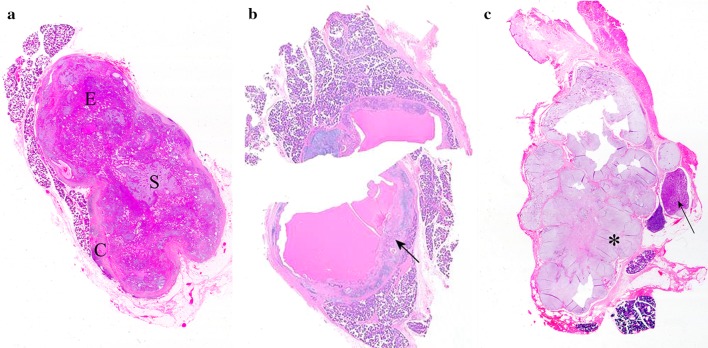
Examination at scanning magnification of routinely-prepared sections from PA resections allows appreciation of the contour, ratio of cellular and stromal components and the usually solid or, rarely, cystic nature of the primary tumor, as well as the multiple nodules of recurrent lesions (Fig. 1).
Fig. 1.
Scanned histological sections of parotid PAs. a Bosselated, circumscribed, solid tumor with an equal epithelial (E): stromal (S) ratio, which is incompletely surrounded by capsule (C). b Largely cystic tumor divided in two; the arrow indicates a typical area. c Multinodular, recurrent tumor; while most of the nodules are stroma-rich and myxoid (asterisk), other are cell-rich and of a monomorphic appearance (arrow) (Unless otherwise specified, the pictures are from sections stained with hematoxylin and eosin. Zooming on the electronic format of the picture would allow appreciation of detail difficult to be seen in print)
Foote and Frazell [4] suggested that PA can be conveniently classified according to its cellular: stromal components ratio, which has been further developed by Seifert et al. [7] to include the appearance of the principal stromal component (Fig. 2). The classification is practical and may lead to interesting information. For instance, PAs of minor salivary glands are often more cellular than their counterparts in the parotid [8]. Nevertheless, such a classification lacks clinical significance [4] and is currently unused.
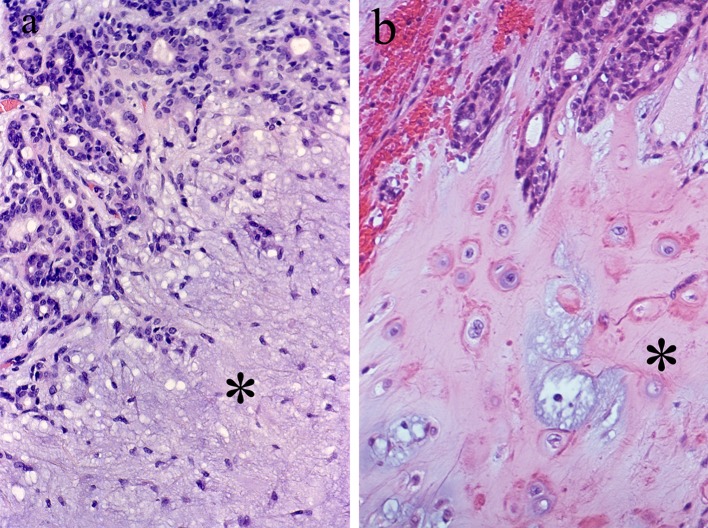
Fig. 2.
Typical histology of PA. Luminal structures are present at the upper part of the pictures. a Myxoid stroma (asterisk). b Chondroid stroma (asterisk)
The significance of the variously complete and thick tumor capsule in relation to surgical management has been recently reviewed and amply illustrated [9] and is not further discussed here.
The application of the topographically descriptive terms luminal and non-luminal to describe the variously dyscohesive or non-cohesive tumor cells is simple and precise. Further description of morphologically distinct phenotypes (Table 2) is acceptable as well. Although widely popular, characterising luminal and non-luminal tumor cells as intercalated ductal-like or neoplastic myoepithelial/modified myoepithelial, respectively [10–12], seems less satisfactory. Such characterisation together with histogenetic notions allocating the origin of PA to the ducto-acinar junction where intercalated ductal and myoepithelial cells prevail [10, 11, 13], are in part speculative and so open to debate [14]. This is further explored below.
Of the various cellular arrangements, the luminal structures having an inner layer of cuboidal eosinophilic cells and outer layer(s) of clear cells (Fig. 3a) deserve special reference (For a review of clear cells in salivary tissues, see [14]). Such a “biphasic” arrangement is often seen in the glandular parenchyma of the mature human female breast (Fig. 3b). This similarity possibly influenced pioneers in salivary pathology who also had a special interest in breast lesions [15, 16], encouraging them to interpret the non-luminal cells of PA as neoplastic myoepithelia [14]. Bundled arrangements of non-luminal, spindled cells simulating leiomyomatous or schwannomatous appearances are also known to exist [17].
Fig. 3.
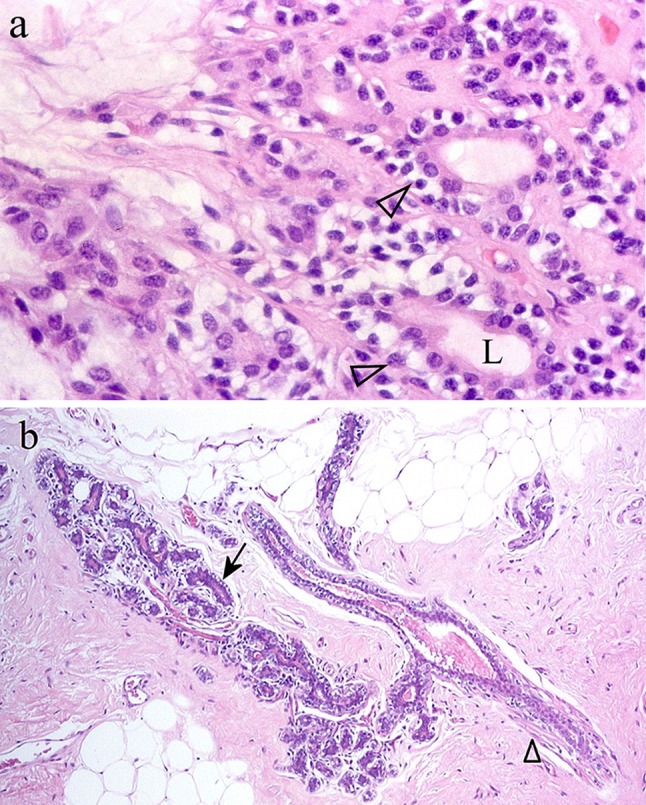
a Bilayered, luminal structures of PA (arrowheads) showing an outer layer of clear cells. L Lumen. b Ductule and related cluster of acini from a case of simple fibrocystic disease of the breasts. While clear, rounded myoepithelial cells are on the outer aspect of acini (arrow), elongated myoepithelial cells with eosinophilic cytoplasm/differentiated myofibrils surround the ductule (arrowhead). Comparison of the figures explains why clear cells in PA had been interpreted as myoepithelial
The extracellular mucosubstances of PA have been histochemically characterized (Fig. 4). Mucosubstances in lumina usually consist of neutral or carboxylated glycoproteins and are produced by luminal cells, whereas those in stroma are glycosaminoglycans (e.g. hyaluronan, chondroitin sulfate) largely, although not exclusively, produced by the non-luminal cells [18, 19]. Immunohistochemistry assisted in localizing the core proteins of glycosaminoglycans (e.g. aggrecan, lumican, perlecan) [19–21]. Excessive, newly formed, extracellular elastin is often present in the stroma (Fig. 5) (see [14]).
Fig. 4.
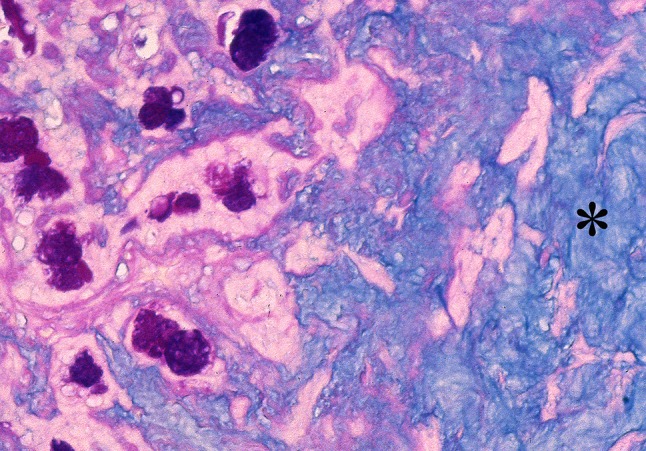
PA stained by Alcian Blue at pH 2.5 followed by periodic acid-Schiff (AB pH 2.5-PAS). Luminal structures containing a mixture of neutral and acid glycoproteins staining royal blue are seen at the left part of the picture. The asterisk indicates stromal glycosaminoglycan staining sky blue
Fig. 5.
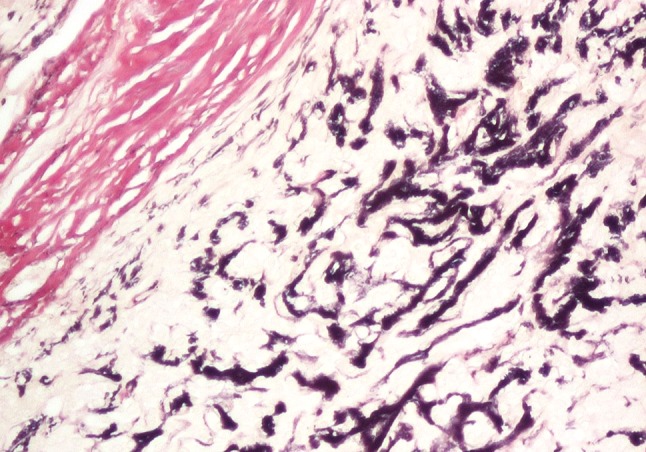
PA stained by elastic—van Gieson. Copious, newly formed elastin staining black is seen. Part of the collagenous capsule that stains red is at the upper left of the picture
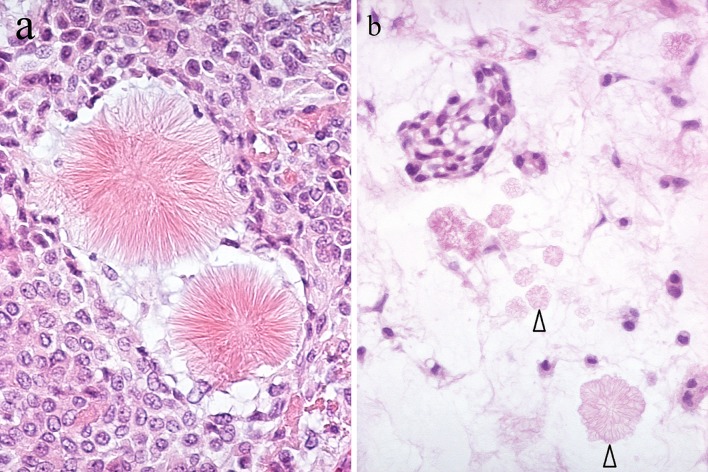
Stellate fibrillar collagen and crystalloids (Fig. 6) have been recently reviewed [14] and are not further discussed at this time.
Fig. 6.
a Collagenous, stellate fibrillar structures. b Multiple tyrosine crystalloids of floral appearance (arrowheads) in myxoid stroma. An aggregate of dyscohesive, non-luminal, tumor cells is at the upper left part of the picture
Thick-walled vessels regarded as abnormal veins [22], can be seen in PA (Fig. 7). They are probably pre-existing structures trapped within the growing tumor, unrelated to the modest expression of vascular endothelial growth factor in tumor cells [23, 24] and of little diagnostic significance. Small nerve fascicles may be detected (Fig. 8), but neuro-effector relationships are unlikely to be in play here. In 1965, Garrett [25], used acetylcholinesterase enzyme histochemistry and recorded little or no coupling of nerves with tumor cells, and recent attempts at localizing neuropeptide immunoreactivities seem uninformative [26]. The absence of neuro-effector relationships in PA is further discussed below.
Fig. 7.
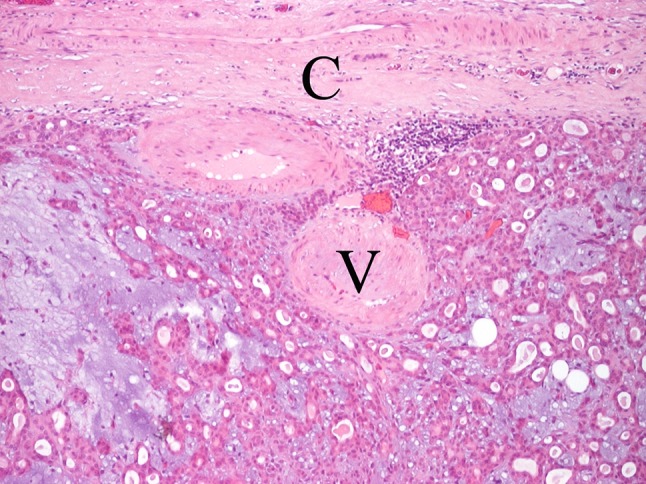
Subcapsular venous structures (V). C capsule
Fig. 8.
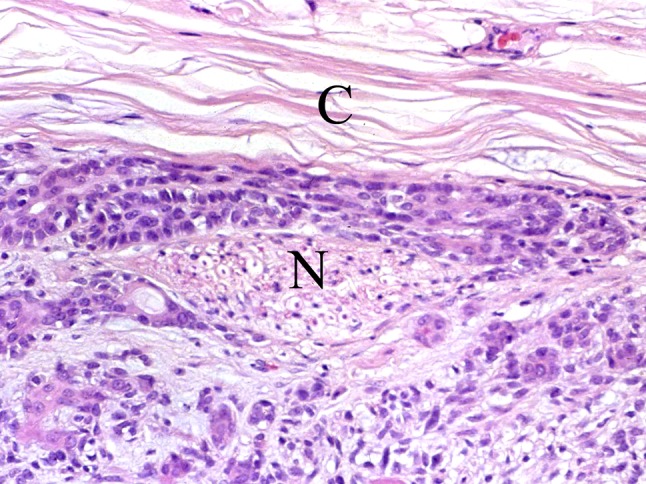
A small subcapsular nerve fascicle (N). C capsule
Changes in the morphological perception of PA are reflected in the diagrams provided by Masson, Hamperl and Dardick (their Figs. 3.18/Plate IV, 29j and 1, respectively) [5, 13, 15], and the various definitions (Table 3) [4, 16, 27–29]. Masson [5] did not consider a myoepithelial component, emphasizing progressive dys/non-cohesion of tumor cells via intercellular secretion and mesenchymatous transformation instead. Hamperl [15] championed the cause of neoplastic myoepithelial cells and Dardick et al. provided electron-microscopical refinement [11, 13, 30]. Only the definitions endorsed by the World Health Organization refer to a myoepithelial component (Table 3) [28, 29]. The picturesque description of dyscohesive or non-cohesive cellular aggregates as “a swarm of bees” (Table 3) [29], proved unpopular. Many definitions allude to a “mucoid” stromal component (Table 3) [16, 29, 30], which is confusing. “Mucoid” and “myxoid” should denote glycoproteins secreted by epithelial cells, and glycosaminoglycans usually produced by mesenchymal or mesenchymal-like cells, respectively.
Table 3.
Definitions of PA
| Foote and Frazell [4] | The term “mixed tumor” designates the commonest group of major salivary gland tumors that characteristically show combined traits of epithelial and connective tissue-like growth |
| Thackray and Lucas [16] | Pleomorphic adenoma is a circumscribed tumor characterized microscopically by its pleomorphic or mixed appearance, and its clearly recognizable epithelial tissue intermingled with areas of mucoid, myxoid, or chondroid appearance |
| Seifert [29] | A tumor of variable capsulation characterized microscopically by architectural rather than cellular pleomorphism. Epithelial and modified myoepithelial elements intermingle with tissue of mucoid, myxoid or chondroid appearance. The epithelial and myoepithelial components form ducts, strands, sheets or structures resembling a swarm of bees. Squamous metaplasia is found in about 25 % of pleomorphic adenomas |
| Ellis and Auclair [27] | Mixed tumor is a benign, epithelial-derived tumor composed of cells that demonstrate both epithelial and mesenchymal differentiation |
| Eveson et al. [30] | Pleomorphic adenoma is a tumor of variable capsulation characterized microscopically by architectural rather than cellular pleomorphism. Epithelial and modified myoepithelial elements intermingle most commonly with tissue of mucoid, myxoid or chondroid appearance |
Non-epithelial cells in PA are outside the scope of this article, but see “Organogenesis in PA” below.
Functional Morphological Perception of PA: The Principles
The fundamental function of salivary glands is to produce and release macromolecules in conjunction with transport of water and electrolytes, which is effected via differentiation of the glandular parenchyma towards luminal acinar and various ductal cells [3]. A different line of differentiation ensures support of the secretory cells and motor activities and results in non-luminal/basal epithelial cells, the distal representatives of which assume myoid features to become myoepithelial cells [31, 32]. Another salivary glandular function is absorption and processing of luminal material, which are effected by ductal cells [2, 3]. It is likely that these functions are altered in a neoplastic setting and the morphological manifestations of those alterations shall be examined in turn. Heterotopic/non-innate lines of differentiation and attempts at organogenesis shall also be considered.
Apart from intrinsic cellular alterations, tumorigenesis affects the microenvironment. Tumor cells would respond to this by adapting and expressing phenotypic changes [33, 34]. These shall be examined and areas overlapping with disordered differentiation will be identified.
Secretory Events/Differentiation in PA
The discussion shall be limited to secretion of organic products, in view of the fact that little or no work has been so far directed to an examination of fluid secretion in salivary tumors. Tissue cultures of PA [35, 36] and enzymatic dispersion of salivary tissue are, however, possible. Cellular preparations thus obtainable could be examined by means of electrophysiological techniques and microfluorimetry, to assess whether responses to neurohormonal stimuli and signalling processes are affected in salivary neoplasia. The methodologies have provided relevant information on experimentally effected pathological atrophy and Sjögren syndrome [37, 38].
The morphological manifestations of secretory events in PA can be classified according to the techniques used for their demonstration (Table 4).
Table 4.
Secretory events and differentiation in PA
| 1. Demonstrable by histochemistry/electron microscopy |
| 2. Non-specific, demonstrable by routine histology |
| 3. Specific, demonstrable by routine histology |
| Mucous cells |
| Seromucous cells |
| Serous cells |
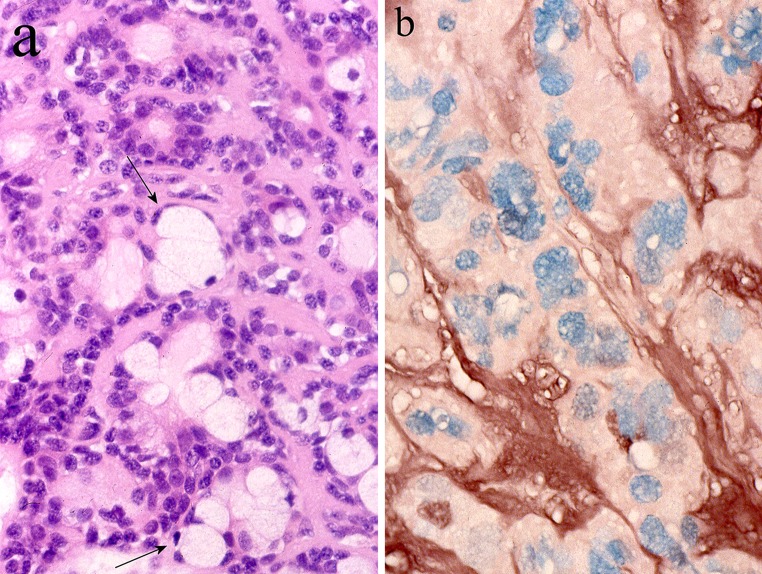
Mucosubstance histochemistry localizes neutral or carboxylated glycoproteins in apical rims of luminal cells (Fig. 9) [18], where scanty, small, variably dense secretory granules, usually unipartite, can be detected by electron microscopy [39]. Histochemically demonstrable calcium in PA is also periluminally concentrated (Fig. 10) and possibly relates to packing of glycoproteins into granules [40]. The glycoproteins and secretory granules of PA resemble those of intercalated ducts in normal salivary glands [18, 39], which supports its alleged origin from the ducto-acinar junction [10, 11, 13]. An alternative explanation would be that tumor cells in PA, although polarized, are only able to synthesize limited, immature, secretory glycoproteins. The absence of neuro-effector relationships in PA may influence the synthesis. A sequence of post-translational modifications in which sulfation is a late event, effects maturity of secretory glycoproteins and that the tumor cells lack sulfated mucosubstances indicates that such modifications are not advanced [18, 33, 41]. The alternative explanation considered above, independent of site-specific histogenetic perceptions, accords with tumorigenic models emphasizing similarities between tumor cells and immature cells; evidence indicating that proliferative—hence, tumorigenic capacity lies with all types of salivary glandular cells [14], and the variable amylase, proline-rich protein, lysozyme, lactoferrin and MUC2 immunoreactivities in PA [33, 42–44].
Fig. 9.
PA stained by high-iron diamine followed by AB at pH 2.5 (HID-AB). Carboxylated, non-sulfated glycoproteins (sky blue) are seen in the apical cytoplasm of luminal cells around a lumen (L). Sulfated glycosaminoglycan (brown) is present in the stroma (S)
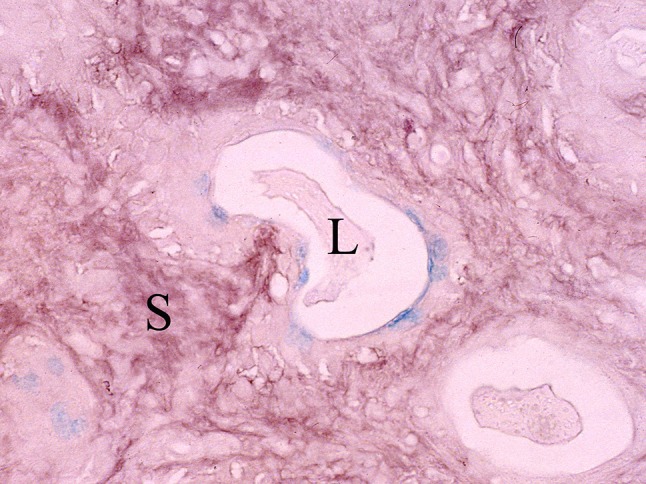
Fig. 10.
Cryostat section of PA stained with glyoxal bis(2-hydroxyanil) for ionized and ionizable calcium. Weak stain (arrow) partly outlines a luminal structure (L)
The presence of mucosubstances in lumina of PA (Fig. 4) suggests that synthesized secretory glycoproteins are appropriately sorted and targeted for exocytosis. Synthesized plasmalemma-anchored glycoproteins, like MUC1 (epithelial membrane antigen), seem variously sorted; while some reach the cell surface, others accumulate intracellularly [33, 45]. It has been suggested that increased synthesis and/or retention of segregated, variably mature, secretory glycoproteins in PA effect enlargement and cytoplasmic granulation of non-descript tumor cells and, in turn, acquisition of acinar phenotypes [33]. This would explain the occasional, routine histological finding of short columnar tumor cells that show fine apical cytoplasmic granulation or amorphous mucoid material (Figs. 11, 12), as well as mucous, seromucous, and serous cells (Figs. 13, 14). Histochemical features of mucous and seromucous cells in PA have been previously described [33]; the presence of –SH groups and absence of sulfated mucosubstances therein (Fig. 13b), indicative of early post-translational modifications [2, 41], are consistent with the alternative explanation discussed above. The processes are envisaged as a continuum and plasticity is a likely feature of salivary parenchyma. In experimental hypertrophy and hyperplasia of the salivary glands, intercalated ductal cells accumulate secretory granules and assume an acinar appearance [46].
Fig. 11.
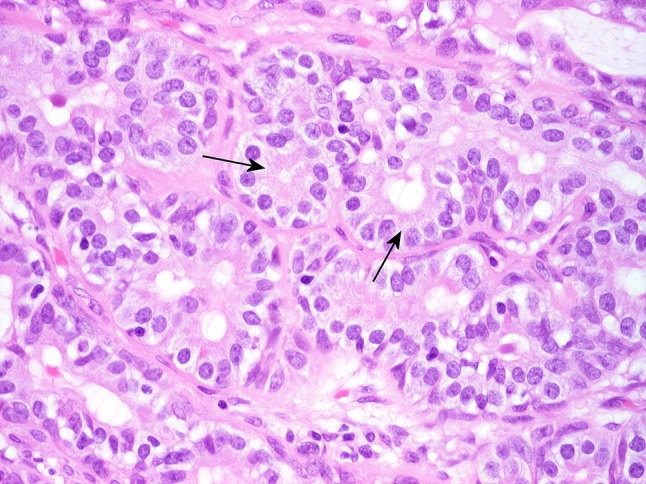
Cohesive luminal structures lined by short columnar tumor cells that contain adluminal small amphophilic secretory granules (arrows). The increased height and cytoplasmic granulation distinguish those cells from normal intercalated ductal cells
Fig. 12.
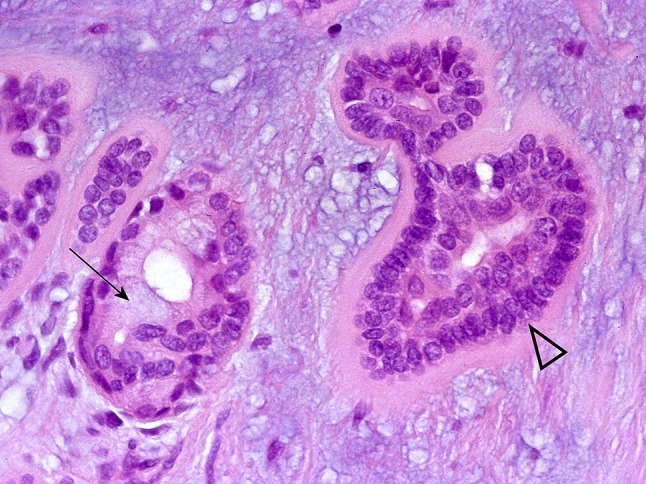
Adluminal mucoid material (arrow). The arrowhead indicates basal cell arrangements rimmed by hyalinised material; they resemble those of salivary basal cell adenomas and cutaneous cylindroma and illustrate similarities between salivary and dermal adnexal tumors
Fig. 13.
a Tumor mucous cells in acinar arrangements (arrows). b Staining by HID-AB shows carboxylated, non-sulfated glycoproteins (sky blue) in the mucous cells, which contrast with the stromal, sulfated glycosaminoglycan (brown)
Fig. 14.
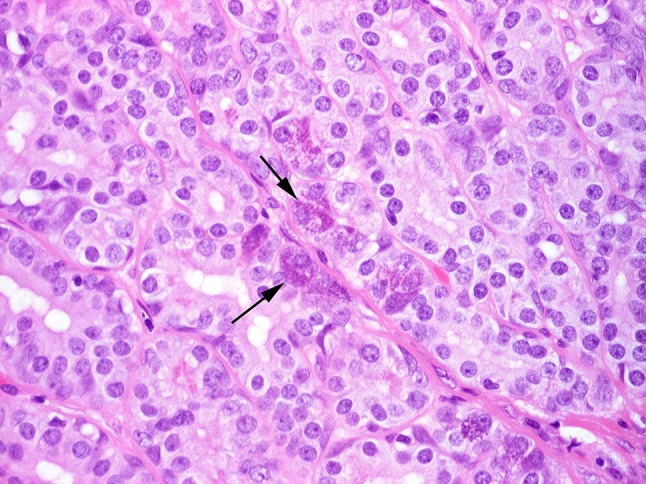
Cohesive luminal structures interspersed with serous-like tumor cells (arrows)
Except for acini and intercalated ducts, secretory granules are also found in the periluminal rims of salivary striated ducts [47], where kallikrein (KLK) activity has been localized with the use of immunohistochemistry and enzyme histochemistry [48, 49]. Luminal structures lined by tall columnar cells with eosinophilic cytoplasm and central nuclei, which resemble striated ducts, are an occasional histological feature in PA (Fig. 15). Tissue KLKs, a family of 15 serine proteases, include KLK3 (prostate specific antigen) [50]. Prostate specific antigen immunoreactivity has been detected in periluminal rims of human striated ducts (Fig. 15, inset), and focally in some PAs [51, 52]. In the context of salivary pathology, such immunoreactivity has been regarded as an immunohistochemical or diagnostic pitfall [52, 53] rather than linked up with KLK3; immunohistochemistry for KLKs had not been applied to the characterisation of the so-called “striated duct adenoma” [54]. Of the other members of the family, KLKs 8, 10, 13, and 14 have been immunohistochemically localized in PA [55–58].
Fig. 15.
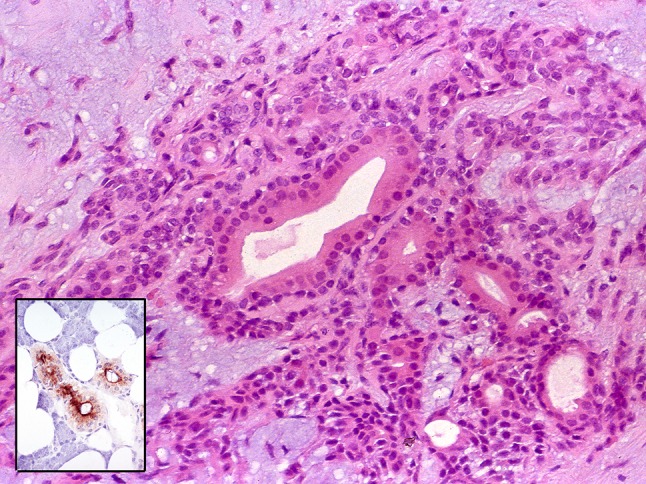
PA with luminal structures resembling striated ducts. The inset shows normal striated ducts with adluminally localized prostate specific antigen
Basal/Myoepithelial Differentiation or Epithelial–Mesenchymal Transition in PA?
Non-luminal, cohesive, cuboidal cells in mono-layered, palisaded arrangements resembling those of the basal layer of stratified epithelia can be seen in PA (Fig. 12).
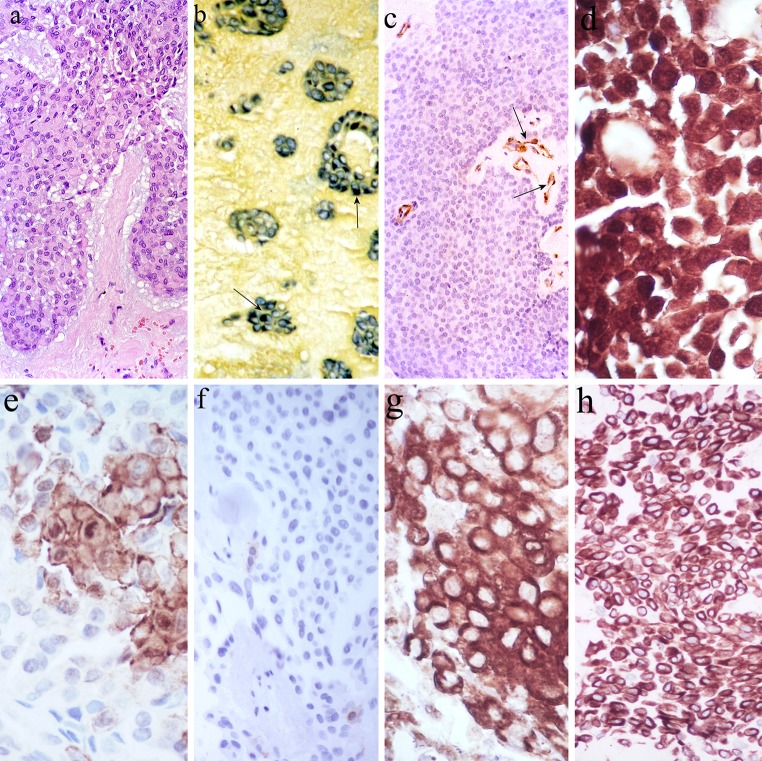
Single or multiple layers of non-luminal, spindled or clear cells that are fraying off into the stroma (Figs. 2a, 3a), where they then assume angular, stellate or plasmacytoid phenotypes are far more common. They have been interpreted as neoplastic myoepithelia or modified myoepithelia (see “Morphological perception of PA” above) [10–12, 59], in accordance with the idea of disordered site-specific differentiation in neoplasia. The selective staining of various numbers of these cells by milling dyes [60] and immunohistochemistry for S-100 protein, p63, α-smooth muscle actin (α-SMA), caldesmon, calponin, and cytokeratin (CK) 14 (Figs. 16, 17) [10, 21, 61–65] appeared to reinforce such an interpretation. However, S-100 protein is not a marker of normal salivary myoepithelial cells (Fig. 16, inset) [66]; CK14 may be luminally expressed (Fig. 18) [63]; combined electron microscopy and stereology showed that typical myoepithelial cells are rare in PA [67, 68]; the subpopulation of α-SMA (+) cells may not be extensive therein [64]; plasmacytoid cells do not express α-SMA [69, 70]. Special staining and immunohistochemical features of the filament-laden and likely effete, plasmacytoid cells are shown in Fig. 19.
Fig. 16.
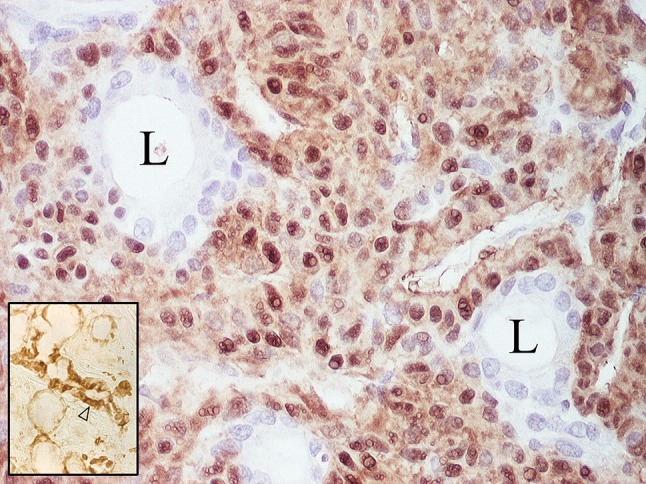
Nuclear and cytoplasmic S-100 protein immunoreactivity of largely cohesive, non-luminal tumor cells. L lumina. The inset shows minor salivary gland where expression of the protein is confined to demilunes and ductal cells (arrowhead); the demilunar staining accounts for the perception of S-100 protein as a myoepithelial marker
Fig. 17.
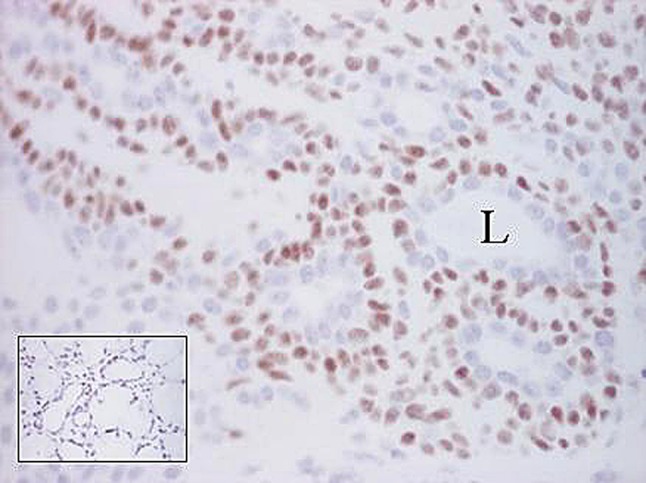
Nuclear p63 immunoreactivity of largely cohesive, non-luminal tumor cells. L Lumen. The inset shows normal salivary acini with stained nuclei at their periphery, but it is difficult to ascribe them to myoepithelial cells
Fig. 18.
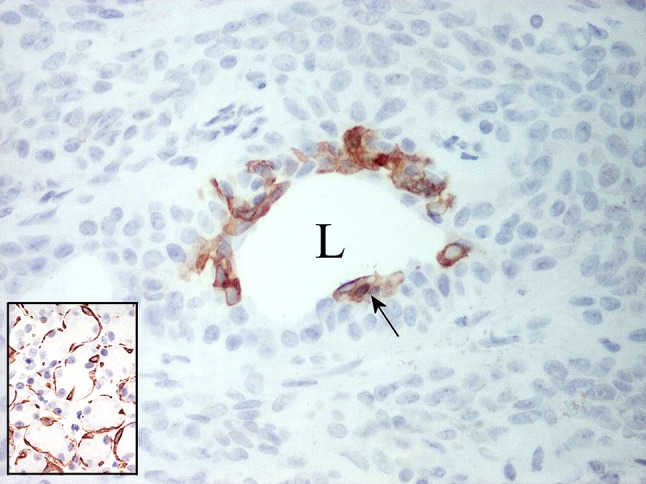
Expression of CK14 in luminal tumor cells. L Lumen. The inset shows normal salivary acini embraced by strongly stained myoepithelial cells; this accounts for the popular perception of CK14 as a myoepithelial marker
Fig. 19.
Plasmacytoid cells (a). They are variously stained with a milling dye (tannic acid—phosphomolybdic acid—amidoblack; b arrows), and are α-SMA (−) (c, stained vessels are arrowed), S-100 protein (+) (d), CK/5/6 (−/+) (e), CK14 (−) (f), vimentin (+) (g) and WT1 (+) (h)
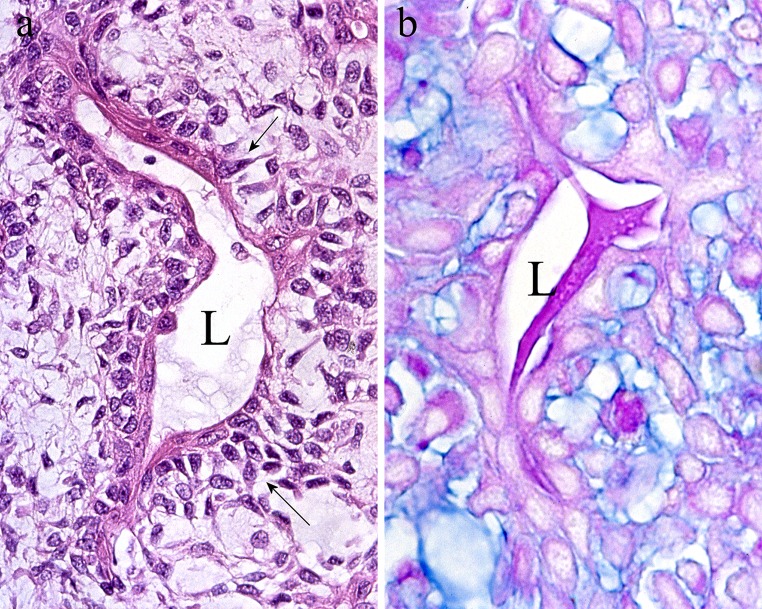
It has been previously noticed that Masson favoured mesenchymatous transformation as a feature of PA. Possibly, his investigations on Wilms tumor, where similar transformations occur, influenced this, and the analogies between his diagrams of the microstructure of PA and Wilms tumor (Figs. 3.18/Plate IV and 11/Chart 1, respectively) are of interest [5, 71]. The process has been later described as mesenchymalisation or stromalisation attributable to activation of dormant mesenchymal genes in tumor epithelial cells [18]. Figure 20 indicates that its early stages are recognized as decreased intercellular cohesion in epithelial luminal structures and accumulation of newly synthesised glycosaminoglycan therein. This has been electron-microscopically confirmed [18] and to some extent is a feature of other salivary adenomas [72]. Via the process, formerly polarized tumor epithelial cells lose cell adhesion molecules (E-cadherin) and secrete matrix [19, 73]. Eventually, they separate and disperse in copious myxoid stroma (Fig. 2a) where they simulate primitive mesenchyme or “swarming bees” and express α5-integrin and fibroblastic and chondrocytic collagens (types I–III) [74, 75]. Electron microscopy showed that the decreased intercellular cohesion involves both luminal and non-luminal cells [76], while immunohistochemistry showed similar numbers of Ki67 (+) cycling cells in luminal and non-luminal arrangements [77]. These findings allow tumor cells in PA to be regarded as a continuum. Although often overshadowed by stroma, the luminal structures are principal components of this tumor, which is in keeping with the microstructure of benign tumors of exocrine glands.
Fig. 20.
a The lining of a luminal structure (L) shows variably widened intercellular spaces (arrows), which indicates decreased cell cohesion. b Adjacent section stained by AB pH 2.5-PAS shows Alcianophilic, sky blue glycosaminoglycan in the intercellular spaces. Glycoproteins staining royal blue are present in the lumen (L)
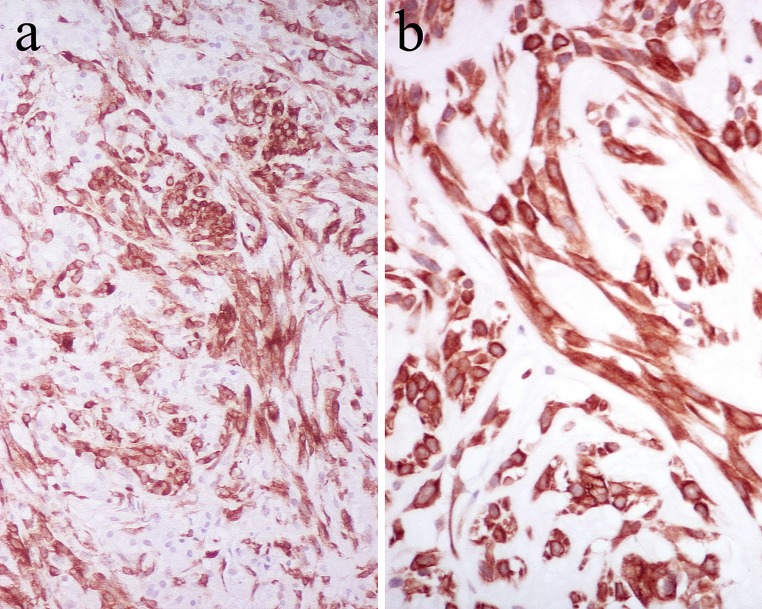
Mesenchymatous transformation/stromalisation as described above is within the range of epithelial–mesenchymal transition (EMT) [78]. Immunohistochemistry neatly highlights the observation that much of the tumor parenchyma in PA shows transitional, epithelial, and mesenchymal phenotypes (Fig. 21) [79]; in situ hybridization localizes aggrecan (chondroitin sulfate proteoglycan 1) and CK14 mRNAs in luminal cells of epithelial phenotype and non-luminal cells of mesenchymal phenotype, respectively [79, 80]. Further support for EMT in PA is given by the variable immunohistochemical localization of transforming growth factor (TGF)-β isoforms in luminal and non-luminal tumor cells [81], for TGF-β effects EMT [82]. EMT would also account for the hyalinisation/collagenous structures, elastosis and cartilaginous (Figs. 2b, 6), osseous, myoid (smooth muscular) and adipocytic (Fig. 22) [83, 84] phenotypes of PA; this explains its complex microstructure in turn. In this context, assessing expression of Snail1 (a protein that influences EMT via transcriptional repression of E-cadherin) in PA would be of interest.
Fig. 21.
Spindled, angular non-luminal cells staining for α-SMA (a) and CK7 (b)
Fig. 22.
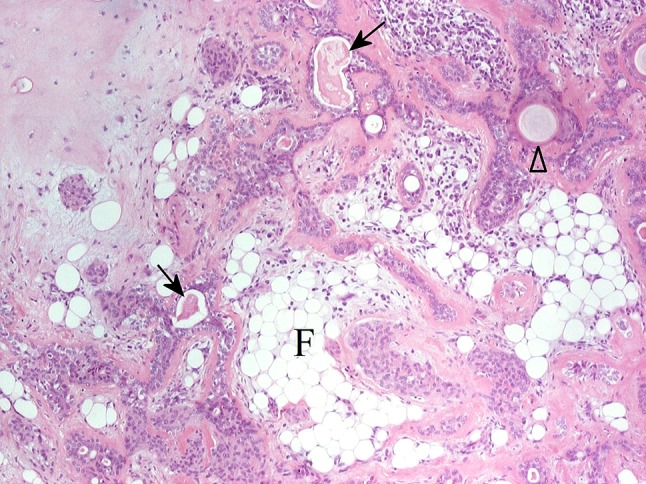
Lipometaplasia or adipose EMT in PA? Collections of adipocytes (F), luminal structures (arrows), pearly keratinisation (arrowhead) and chondroid stroma (upper left part of the picture) are seen
EMT as a feature of PA is certainly appealing, but the notion of neoplastic or modified myoepithelia seems anchored and reinforced by the argument that PA does not originate in exocrine pancreas where myoepithelial cells are absent [85]. Langman et al. [86] noted that calponin (+) and p63 (+) non-luminal cells in PA co-express Wilms tumor 1 protein (WT1), and suggested WT1 as a myoepithelial marker. Interestingly, these authors did not record any WT1 immunoreactivity in normal salivary myoepithelial cells [86]. A recent investigation confirmed this absence and suggested that WT1 (+) cells in PA undergo EMT (Fig. 23) [87]. This would be consistent with the role of the WT1 gene in influencing epithelial or mesenchymal status [88]. When particular macromolecules are not expressed in normal cells, caution should be exerted before immunoreactivities of purported pathological analogues are interpreted as markers of those cells. The trend of discovering novel myoepithelial markers is still upwards, podoplanin being a recent example [21].
Fig. 23.
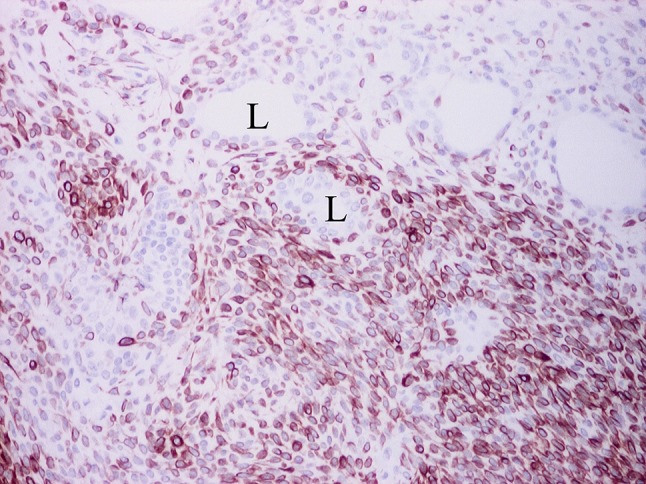
Sheets of variably dyscohesive, non-luminal cells strongly stained for the WT1 antigen, stream from luminal structures (L) with unstained, luminal cells. The unstained nuclei of the non-luminal cells can be seen
It may be argued that EMT and neoplastic or modified myoepithelia are not mutually exclusive. Loss of intercellular cohesion and cytoplasmic accumulation of myofibrils would be expected in a tumor cell undergoing myoid EMT, which would thus qualify as a neoplastic myoepithelial or modified myoepithelial cell (Fig. 21) [87]. EMT allows, however, a broader perspective and an explanation of non-myoid cell phenotypes independent of an intervening phase of modified myoepithelium.
Although outside the scope of this article, “myoepitheliomas” have been considered as members of the PA family [89]. It is likely that “myoepitheliomas” are PAs featuring widespread myoid EMT that eventually results in “depletion” of luminal structures.
Heterotopic Differentiation in PA
Except for the innate lines of differentiation discussed above, the ectodermal origin of salivary glands would account for the occasional finding in PA of structures resembling abortive hair follicles (Fig. 24). Luminal cells showing apocrine features (Fig. 25) and collections of sebocytes are also seen. Apocrine and sebaceous phenotypes in salivary tissues have been recently reviewed [14].
Fig. 24.
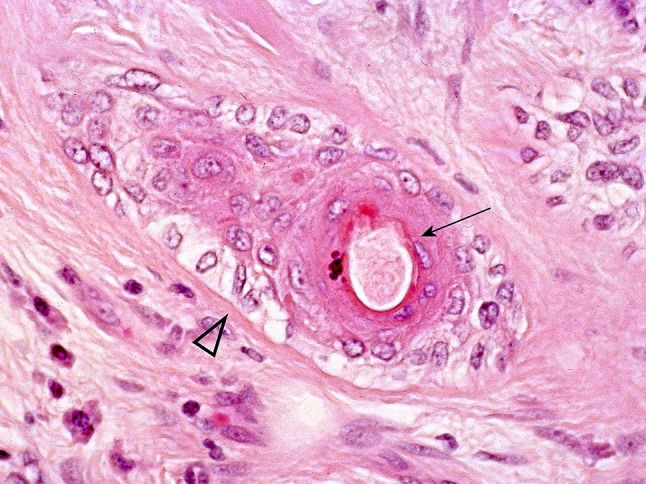
Hair follicle in PA? “Normal” associations of “hair shaft” (arrow) and “outer root sheath” (arrowhead)
Fig. 25.
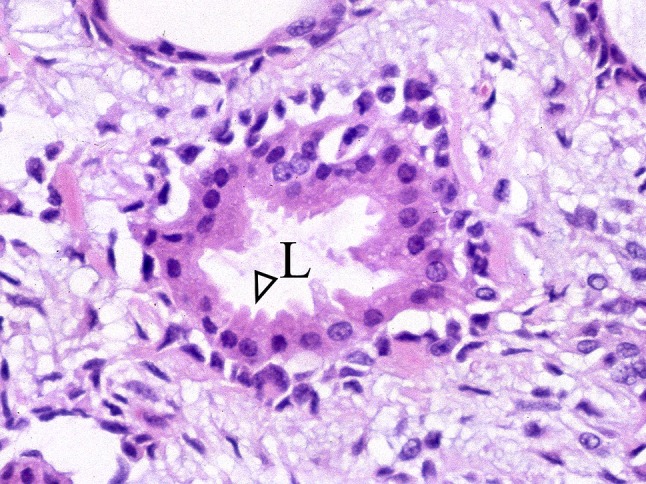
Apocrine-like cells showing intraluminally bulging apices (arrowhead). L Lumen
Absorption, Lysosomal Activities, and Calcification in PA
Although absorption and processing of luminal material is a form of heterophagy [3], little attention has been paid to lysosomal activities in salivary neoplasia. In normal glands, such activities also effect post-translational modifications of secretory glycoproteins, whereas autophagy eliminates redundant secretory granules and is significant in the formation of microliths [3, 14, 41].
The use of special stains shows lipofuscin, an end product of phagy, in luminal and plasmacytoid cells of PA [90, 91]. Later electron-microscopic and cytochemical investigations demonstrated lysosomes and lysosomal enzyme activity in luminal cells of PA [92]. A recent immunohistochemical investigation showed expression of CD63, a glycoprotein of lysosomal membranes [93], in apices of luminal cells and in tumor mucous cells (Fig. 26) [94]. While lysosomal activities in luminal or mucous cells could reflect absorption and/or autophagy of secretory granules, those in plasmacytoid cells may relate to remodelling of cytoskeleton (see below).
Fig. 26.
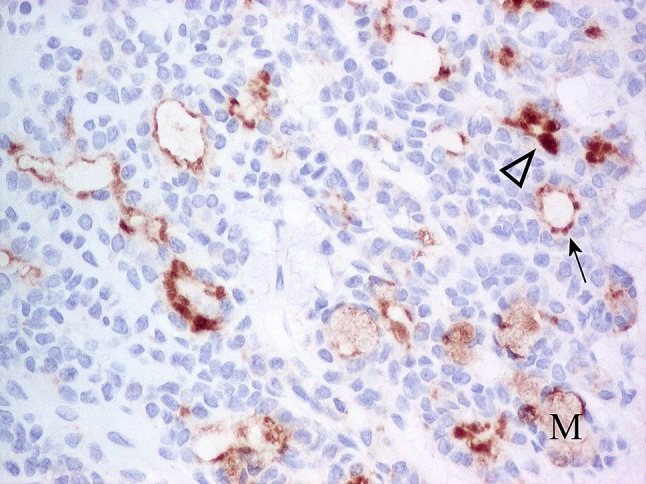
Luminal cells of PA showing moderate to strong, adluminal CD63 immunoreactivity. The extent of staining varies from cytoplasmic, adluminal rims (arrow) to apical (arrowhead). Mucous cells (M) show weak, cytoplasmic, diffuse immunoreactivity. Non-luminal cells are unstained
Autophagy of secretory granules containing calcium would account for the formation of microliths in PA (Fig. 27) [14, 40]. Absorption/processing of luminal material may result in “apocrine” phenotypes of luminal cells [14, 72].
Fig. 27.
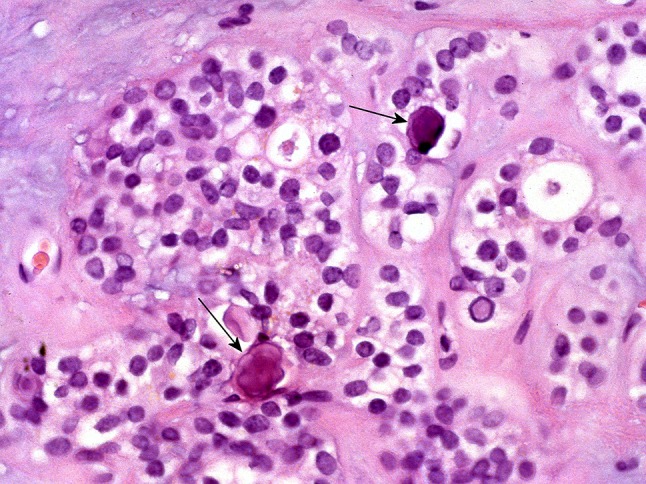
Cellular aggregates interspersed with microliths (arrows)
Adapting to Microenvironment: Metaplasia and Remodelling of Cytoskeleton in PA
Although keratin pearls are unusual in PA (Fig. 22), aggregates of squamous (epidermoid) cells are common (Fig. 28) and conventionally attributed to metaplasia. Apocrine, sebaceous, mucous and oncocytic cells and lipomatous or osseous areas may also reflect metaplasia (Figs. 29, 30). The squamous cells usually express CKs of higher molecular weight [63]; sebaceous and oncocytic cells have been recently reviewed [14]; and bone morphogenetic proteins, variously expressed in luminal and non-luminal tumor cells may influence metaplastic ossification [95].
Fig. 28.
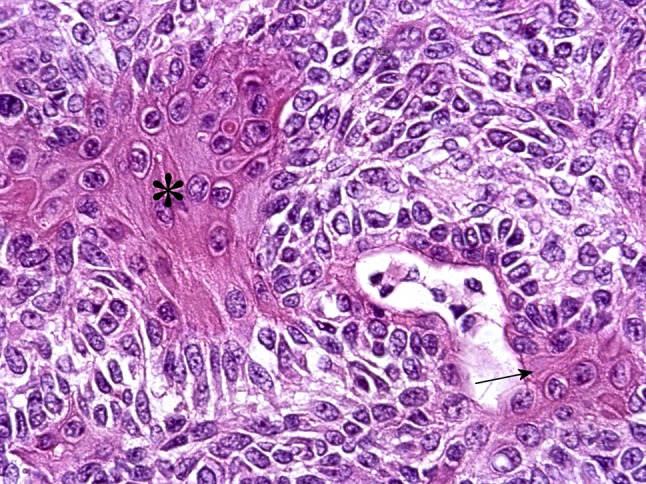
Squamous, non-luminal (asterisk) and luminal (arrow) cells in PA
Fig. 29.
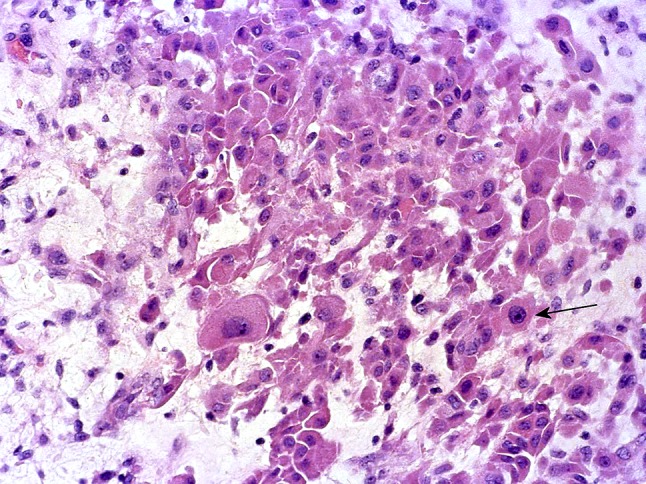
Oncocytic cells in PA, one of which shows “atypical” nucleus (arrow)
Fig. 30.
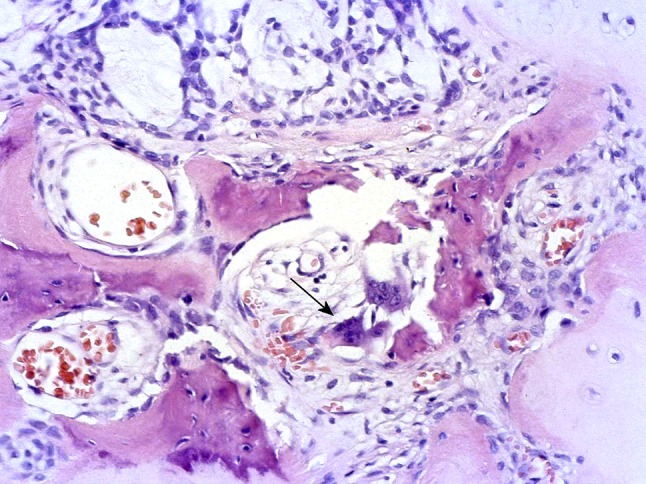
Osseous metaplasia or EMT in PA? Typical tumor is seen at the upper and right parts of the picture. The arrow indicates osteoclasts
The squamatisation/keratinisation processes in PA provide an opportunity for commenting on the CK profile of tumor cells. The likely trend is that luminal cells express basic CKs of low molecular weight, for instance CK7, whereas non-luminal cells express acidic CKs of high molecular weight, for instance CK14 [63], and may reflect particular lines of differentiation [64]. Variations are, however, possible [63] and the often complex patterns can be alternatively explained as indicative of a plastic cytoskeleton adapting to an altered microenvironment. Remodelling of the cytoskeleton to withstand increased luminal pressure could explain the staining of luminal cells for CK14 (Fig. 18) [63], and the occasional finding of squamatized luminal cells (Fig. 28). Such remodelling is also a feature of other salivary adenomas [72], with luminal squamatisation reinforcing consideration of tumor cells as a continuum. Expression of HSP27 in non-luminal, cohesive or non-cohesive, squamous and plasmacytoid cells of PA has been reported [94]. It has been suggested that expression of HSP27 in salivary tissues is associated with remodelling of the cytoskeleton [96]. This may reflect cellular adaptation, disturbance, or differentiation. Expression of HSP27 in non-luminal cells (Fig. 31) can be reconciled with remodelling of cytoskeleton to provide mechanical support of luminal cells [31, 32] and enable adjustment to microenvironmental changes effected by copious or reorganised matrix. Excessive cytoplasmic accumulation of intermediate filaments, indicative of cellular disturbance and effecting cytoplasmic hyalinisation [28, 32], would account for the immunoreactivity of plasmacytoid cells.
Fig. 31.
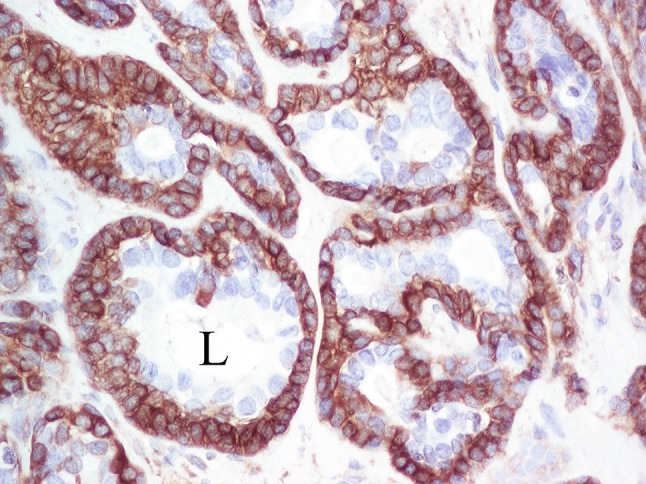
Selective, strong, cytoplasmic, diffuse HSP27 immunoreactivity of cohesive non-luminal basal cells. Note the unstained luminal cells and nuclei of basal cells. L lumen
Finally, response to increased luminal pressure may account for the occasional mucous cells in PA that produce sulfated glycoproteins [33].
Organogenesis in PA
Occasionally, mucous and seromucous tumor cells are arranged in structures resembling acini [33], whereas serous tumor cells capping luminal structures simulate demilunes (Fig. 32). Possibly they correspond with Willis’ “nearly normal-looking salivary tissue” [6]. Figure 33 shows what could be highly organized, tumor parenchyma of branching ductal and terminal acinar segments, although the possibility of atrophic salivary glands trapped within the growing tumor cannot be excluded.
Fig. 32.
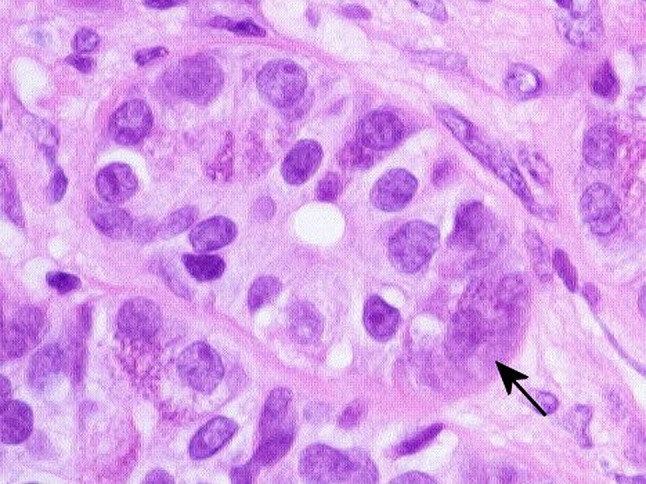
Serous-like cell in a demilunar arrangement (arrow)
Fig. 33.
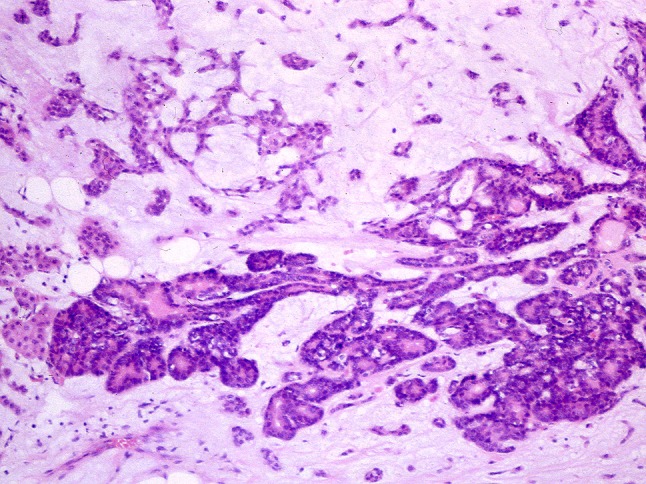
Organogenesis or atrophic salivary parenchyma trapped within a PA?
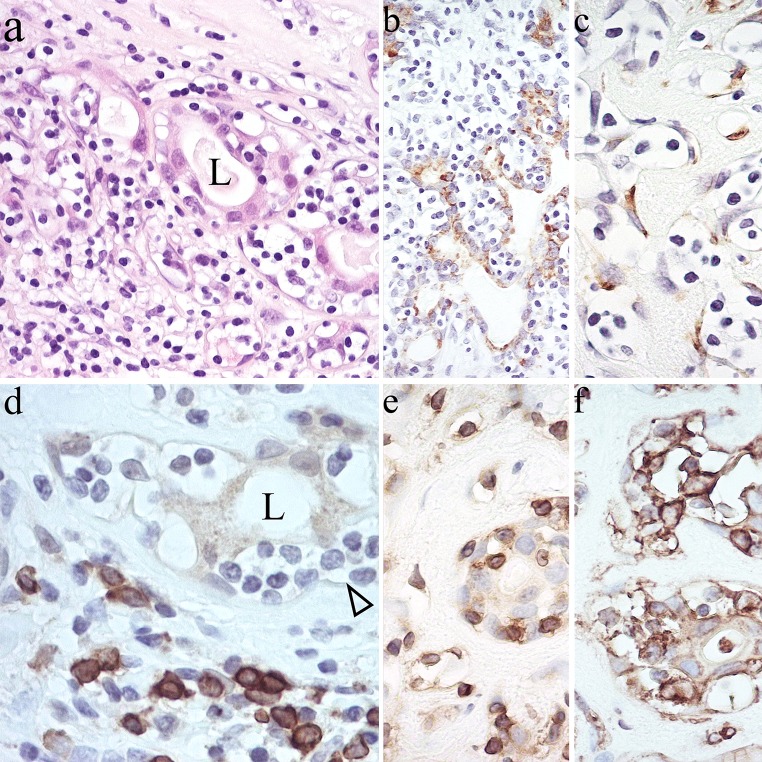
A very rare feature of PA is shown in Fig. 34. It has been interpreted as the neoplastic counterpart of salivary analogues of mucosa-associated lymphoid tissue (MALT; see [14]), although the small lymphocytes “homing” to luminal structures appear to be T-cells rather (Fig. 34e) than B-cells (Fig. 34d). HLA-DR in PA is usually associated with dendritic non-epithelial stromal cells [97], hence, its expression in tumor cells (Fig. 34f) seems linked to “homing”.
Fig. 34.
MALT-like arrangements in PA? The “homing” lymphocytes are affected by retraction artefact, which results in a “clear” appearance (a–d). They are interspersed between luminal CAM5.2 (+) (b) and non-luminal α-SMA (+) cells (c); and are CD79A (−) (d), CD3 (+) (e) and HLA-DR (+) (f). L lumen
Conclusion
The standard texts on the histology of PA are detailed [4, 16, 27, 89, 98] and reviews of advances in salivary pathology ignore PA or are concerned with genetics rather than morphology [65, 99]. It is only hoped that those texts are reviewed in conjunction with this article. The “functional” approach allows perceiving PA free from the restraints of histogenetic considerations; envisaging expansion of a cellular population concerned with forming lumina/polarized secretion, and affected by various genotypic derepression/phenotypic responses to altered microenvironment; defining PA as “a benign epithelial tumor that is characterised by variable epithelial–mesenchymal transition, secretion/differentiation and metaplasia”; planning of further research to address specific questions. Increased understanding of luminal cells may be more gratifying (Fig. 35) [100], than a quest for “myoepithelial” markers.
Fig. 35.
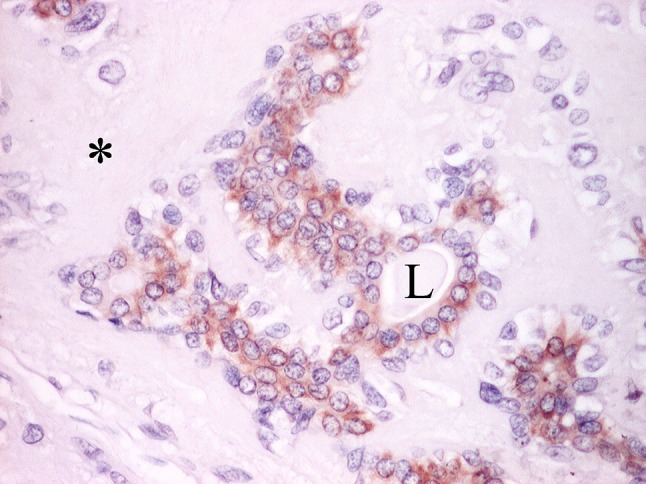
Biphasic FANCD2 immunoreactivity in PA. Luminal cells show moderate to strong cytoplasmic staining. Non-luminal cells and stroma (asterisk) are unstained. L lumen
Footnotes
This article was written by members and invitees of the International Head and Neck Scientific Group (http://www.IHNSG.com).
References
- 1.Harrison JD. Minor salivary glands of man: enzyme and mucosubstance histochemical studies. Histochem J. 1974;6(6):633–647. doi: 10.1007/BF01011504. [DOI] [PubMed] [Google Scholar]
- 2.Triantafyllou A, Fletcher D, Scott J. Morphological phenotypes and functional capabilities of submandibular parenchymal cells of the ferret investigated by protein, mucosubstance and enzyme histochemistry. Histochem J. 1999;31(12):789–796. doi: 10.1023/A:1003902120220. [DOI] [PubMed] [Google Scholar]
- 3.Hand AR. Functional ultrastructure of the salivary glands. In: Sreebny LM, editor. The salivary system. Boca Raton: CRC Press; 1987. pp. 43–67. [Google Scholar]
- 4.Foote FW, Jr, Frazell EL. Tumors of the major salivary glands. Cancer. 1953;6(6):1065–1133. doi: 10.1002/1097-0142(195311)6:6<1065::AID-CNCR2820060602>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Masson P. Human tumors. Histology, diagnosis and technique. 2. Detroit: Wayne State University Press; 1970. [Google Scholar]
- 6.Willis RA. Pathology of tumours. 4. London: Butterworths; 1967. [Google Scholar]
- 7.Seifert G, Langrock I, Donath K. Pathomorphologische Subklassifikation der pleomorphen Speichedrüsenadenome. Analyse von 310 pleomorphen Parotisadenomen. HNO. 1976;24(12):415–426. [PubMed] [Google Scholar]
- 8.Seifert G, Rieb H, Donath K. Klassifikation der Tumoren der kleinen Speicheldrüsen Pathohistologische Analyse von 160 Tumoren. Laryngol Rhinol Otol (Stuttg) 1980;59(7):379–400. doi: 10.1055/s-2007-1008874. [DOI] [PubMed] [Google Scholar]
- 9.Zbären P, Vander Poorten V, Witt RL, Woolgar JA, Shaha AR, Triantafyllou A, Takes RP, Rinaldo A, Ferlito A. Pleomorphic adenoma of the parotid: formal parotidectomy or limited surgery? Am J Surg. 2013;205(1):109–118. doi: 10.1016/j.amjsurg.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Erlandson RA, Cardon-Cardo C, Higgins PJ. Histogenesis of benign pleomorphic adenoma (mixed tumor) of the major salivary glands. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1984;8(11):803–820. doi: 10.1097/00000478-198411000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Dardick I, van Nostrand AW, Phillips MJ. Histogenesis of salivary gland pleomorphic adenoma (mixed tumor) with an evaluation of the role of the myoepithelial cell. Hum Pathol. 1982;13(1):62–75. doi: 10.1016/S0046-8177(82)80140-8. [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Tsukitani K, Ninomiya T, Okada Y. Various expressions of modified myoepithelial cells in salivary pleomorphic adenoma. Immunohistochemical studies. Pathol Res Pract. 1987;182(5):632–646. doi: 10.1016/S0344-0338(87)80005-5. [DOI] [PubMed] [Google Scholar]
- 13.Dardick I, van Nostrand AW, Jeans MT, Rippstein P, Edwards V. Pleomorphic adenoma, I: ultrastructural organization of “epithelial” regions. Hum Pathol. 1983;14(9):780–797. doi: 10.1016/S0046-8177(83)80301-3. [DOI] [PubMed] [Google Scholar]
- 14.Triantafyllou A, Hunt JL, Devaney KO, Ferlito A. A perspective of comparative salivary and breast pathology. Part I: microstructural aspects, adaptations and cellular events. Eur Arch Otorhinolaryngol. 2014;271(4):647–663. doi: 10.1007/s00405-013-2488-y. [DOI] [PubMed] [Google Scholar]
- 15.Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161–220. doi: 10.1007/978-3-662-30514-0_3. [DOI] [PubMed] [Google Scholar]
- 16.Thackray AC, Lucas RB. Tumors of the major salivary glands. Atlas of tumor pathology, 2nd series, fascicle 10. Washington, DC: Armed Forces Institute of Pathology; 1974.
- 17.Merino MJ, LiVolsi VA. Pleomorphic adenomas of the parotid gland resembling mesenchymal tumors. Oral Surg Oral Med Oral Pathol. 1977;44(3):405–410. doi: 10.1016/0030-4220(77)90410-8. [DOI] [PubMed] [Google Scholar]
- 18.Harrison JD, Auger DW. Mucosubstance histochemistry of pleomorphic adenoma of parotid and submandibular salivary glands of man: light and electron microscopy. Histochem J. 1991;23(7):293–302. doi: 10.1007/BF01044960. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M, Takata T, Kudo Y, Sato S, Ogawa I, Wakida K, Uchida T, Nikai H. Biosynthesis of glycosaminoglycans and aggrecan by tumor cells in salivary pleomorphic adenoma: ultrastructural evidence. J Oral Pathol Med. 1999;28(10):442–450. doi: 10.1111/j.1600-0714.1999.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 20.Kusafuka K, Ishiwata T, Sugisaki Y, Takemura T, Kusafuka M, Hisha H, Ikehara S. Lumican expression is associated with the formation of mesenchyme-like elements in salivary pleomorphic adenomas. J Pathol. 2004;203(4):953–960. doi: 10.1002/path.1599. [DOI] [PubMed] [Google Scholar]
- 21.Tsuneki M, Maruyama S, Yamazaki M, Essa A, Abé T, Babkair HA, Ahsan MS, Cheng J, Saku T. Podoplanin is a novel myoepithelial cell marker in pleomorphic adenoma and other salivary gland tumors with myoepithelial differentiation. Virchows Arch. 2013;462(3):297–305. doi: 10.1007/s00428-012-1359-z. [DOI] [PubMed] [Google Scholar]
- 22.Hirokawa M, Tamura M, Horiguchi H, Wakatsuki S, Sano T. Abnormal venous structures in salivary gland tumors: vasculature characteristics of pleomorphic adenoma. APMIS. 2001;109(9):625–630. doi: 10.1034/j.1600-0463.2001.d01-184.x. [DOI] [PubMed] [Google Scholar]
- 23.Swelam W, Ida-Yonemochi H, Maruyama S, Ohshiro K, Cheng J, Saku T. Vascular endothelial growth factor in salivary pleomorphic adenomas: one of the reasons for their poorly vascularized stroma. Virchows Arch. 2005;446(6):653–662. doi: 10.1007/s00428-005-1219-1. [DOI] [PubMed] [Google Scholar]
- 24.Faur AC, Lazar E, Cornianu M. Vascular endothelial growth factor (VEGF) expression and microvascular density in salivary gland tumours. APMIS. 2014;122(5):418–426. doi: 10.1111/apm.12160. [DOI] [PubMed] [Google Scholar]
- 25.Garrett JR. The effects of certain experimental procedures on the innervation of cat salivary glands and their relation to human pathology. Ph.D. Thesis. London: King’s College Hospital; 1965.
- 26.Hayashi Y, Deguchi H, Nakahata A, Kurashima C, Hirokawa K. Immunopathological study of neuropeptide expression in human salivary gland neoplasms. Pathobiology. 1990;58(4):212–220. doi: 10.1159/000163587. [DOI] [PubMed] [Google Scholar]
- 27.Ellis GL, Auclair P. Tumors of the salivary glands. Atlas of tumor pathology. 3rd series. Fascicle 17. Washington, DC: Armed Forces Institute of Pathology; 1996.
- 28.Dardick I, Van Nostrand AW, Jeans MT, Rippstein P, Edwards V. Pleomorphic adenoma, II: ultrastructural organization of “stromal” regions. Hum Pathol. 1983;14(9):798–809. doi: 10.1016/S0046-8177(83)80302-5. [DOI] [PubMed] [Google Scholar]
- 29.Seifert G. Histological typing of salivary gland tumours of the salivary glands. 2. Berlin: Springer; 1991. [Google Scholar]
- 30.Eveson JW, Kusafuka K, Stenman G, Nagao T. Pleomorphic adenoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 254–258. [Google Scholar]
- 31.Hayward SW, Brody JR, Cunha GR. An edgewise look at basal epithelial cells: three-dimensional views of the rat prostate, mammary gland and salivary gland. Differentiation. 1996;60(4):219–227. doi: 10.1046/j.1432-0436.1996.6040219.x. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher D, Triantafyllou A, Scott J. Innervation and myoepithelial arrangements in the submandibular salivary gland of ferret investigated by enzyme, catecholamine and filament histochemistry. Arch Oral Biol. 1999;44(12):1035–1043. doi: 10.1016/S0003-9969(99)00096-5. [DOI] [PubMed] [Google Scholar]
- 33.Triantafyllou A. Acinar phenotypes in salivary pleomorphic adenoma: unusual differentiation or disordered functional activity? Pathol Res Pract. 2001;197(11):743–751. doi: 10.1078/0344-0338-00153. [DOI] [PubMed] [Google Scholar]
- 34.Tandler B, Erlandson RA. Giant mitochondria in a pleomorphic adenoma of the submandibular gland. Ultrastruct Pathol. 1983;4(1):85–96. doi: 10.3109/01913128309140575. [DOI] [PubMed] [Google Scholar]
- 35.Prasad KN, Carvalho E, Edwards-Prasad J, La Rosa FG, Kumar S, Kim JH, Meyers A, Kentroti S. Establishment of human parotid pleomorphic adenoma cells in culture: morphological and biochemical characterization. In Vitro Cell Dev Biol Anim. 1994;30A(5):312–320. doi: 10.1007/BF02631452. [DOI] [PubMed] [Google Scholar]
- 36.Steiner MG, Kuhel WI, Carew JF, Huo J, Hoda SA, Staiano-Coico L, Schley WS. Characterization of novel cell lines from pleomorphic adenomas of the parotid gland established in a collagen gel system. Laryngoscope. 1997;107(5):654–660. doi: 10.1097/00005537-199705000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Scott J, Liu P, Smith PM. Morphological and functional characteristics of acinar atrophy and recovery in the duct-ligated parotid gland of the rat. J Dent Res. 1999;78(11):1711–1719. doi: 10.1177/00220345990780110801. [DOI] [PubMed] [Google Scholar]
- 38.Dawson LJ, Field EA, Harmer AR, Smith PM. Acetylcholine-evoked calcium mobilization and ion channel activation in human labial gland acinar cells from patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2001;124(3):480–485. doi: 10.1046/j.1365-2249.2001.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison JD, Auger DW. Ultrastructural morphology of secretory granules in pleomorphic adenoma of human parotid and submandibular salivary glands. Arch Oral Biol. 1989;34(9):759–761. doi: 10.1016/0003-9969(89)90083-6. [DOI] [PubMed] [Google Scholar]
- 40.Harrison JD, Triantafyllou A, Baldwin D, Schäfer H. Histochemical and biochemical determination of calcium in pleomorphic adenoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(2):123–125. doi: 10.1007/BF02915104. [DOI] [PubMed] [Google Scholar]
- 41.Hand AR. The secretory process of salivary glands and pancreas. In: Riva A, Motta PM, editors. Ultrastructure of the extraparietal glands of the digestive tract. Boston: Kluwer; 1990. pp. 1–17. [Google Scholar]
- 42.Warner TF, Seo IS, Azen EA, Hafez GR, Zarling TA. Immunocytochemistry of acinic cell carcinomas and mixed tumors of salivary glands. Cancer. 1985;56(9):2221–2227. doi: 10.1002/1097-0142(19851101)56:9<2221::AID-CNCR2820560915>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Mitani H, Murase N, Mori M. Immunohistochemical demonstration of lysozyme and lactoferrin in salivary pleomorphic adenomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(4):257–265. doi: 10.1007/BF02899090. [DOI] [PubMed] [Google Scholar]
- 44.Mannweiler S, Beham A, Langner C. MUC1 and MUC2 expression in salivary gland tumors and in non-neoplastic salivary gland tissue. APMIS. 2003;111(10):978–984. doi: 10.1034/j.1600-0463.2003.1111010.x. [DOI] [PubMed] [Google Scholar]
- 45.Gusterson BA, Lucas RB, Ormerod MG. Distribution of epithelial membrane antigen in benign and malignant lesions of the salivary glands. Virchows Arch A Pathol Anat Histol. 1982;397(2):227–233. doi: 10.1007/BF00442392. [DOI] [PubMed] [Google Scholar]
- 46.Vugman I, Hand AR. Quantitative immunocytochemical study of secretory protein expression in parotid glands of rats chronically treated with isoproterenol. Microsc Res Tech. 1995;31(2):106–117. doi: 10.1002/jemt.1070310203. [DOI] [PubMed] [Google Scholar]
- 47.Tandler B, Phillips CJ. Organic secretion by striated ducts. Eur J Morphol. 2000;38(4):233–236. doi: 10.1076/ejom.38.4.0233. [DOI] [PubMed] [Google Scholar]
- 48.Schachter M, Perret MW, Moriwaki C, Rodrigues JA. Localization of kallikrein in submandibular gland of cat, guinea pig, dog, and man by the immunoperoxidase method. J Histochem Cytochem. 1980;28(12):1295–1300. doi: 10.1177/28.12.7014710. [DOI] [PubMed] [Google Scholar]
- 49.Garrett JR, Harrison JD, Kidd A, Kyriacou K, Smith RE. Kallikrein-like activity in human salivary glands and colon, including mast cells. J Physiol. 1982;334:78P. [Google Scholar]
- 50.Clements JA, Willemsen NM, Myers SA, Dong Y. The tissue kallikrein family of serine proteases: functional roles in human disease and potential as clinical biomarkers. Crit Rev Clin Lab Sci. 2004;41(3):265–312. doi: 10.1080/10408360490471931. [DOI] [PubMed] [Google Scholar]
- 51.Henwood T. Prostate-specific antigen and the salivary gland. Histopathology. 1998;32(5):478. doi: 10.1046/j.1365-2559.1998.0358b.x. [DOI] [PubMed] [Google Scholar]
- 52.van Krieken JH. Prostate marker immunoreactivity in salivary gland neoplasms. A rare pitfall in immunohistochemistry. Am J Surg Pathol. 1993;17(4):410–414. doi: 10.1097/00000478-199304000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Fan CY, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000;24(4):579–586. doi: 10.1097/00000478-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Weinreb I, Simpson RH, Skálová A, Perez-Ordoňez B, Dardick I, Chetty R, Hunt JL. Ductal adenomas of salivary gland showing striated duct differentiation (‘striated duct adenoma’): a report of six cases. Histopathology. 2010;57(5):707–715. doi: 10.1111/j.1365-2559.2010.03682.x. [DOI] [PubMed] [Google Scholar]
- 55.Darling MR, Tsai S, Jackson-Boeters L, Daley TD, Diamandis EP. Human kallikrein 8 expression in salivary gland tumors. Head Neck Pathol. 2008;2(3):169–174. doi: 10.1007/s12105-008-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darling MR, Hashem NN, Zhang I, Mohamed AB, Fung K, Kwan K, Mara TW, Daley TD, Diamandis EP. Kallikrein-related peptidase 10 expression in salivary gland tissues and tumours. Int J Biol Markers. 2012;27(4):e381–e388. doi: 10.5301/JBM.2012.10373. [DOI] [PubMed] [Google Scholar]
- 57.Darling MR, Jackson-Boeters L, Daley TD, Diamandis EP. Human kallikrein 13 expression in salivary gland tumors. Int J Biol Markers. 2006;21(2):106–110. doi: 10.1177/172460080602100206. [DOI] [PubMed] [Google Scholar]
- 58.Hashem NN, Mara TW, Mohamed M, Zhang I, Fung K, Kwan KF, Daley TD, Diamandis EP, Darling MR. Human kallikrein 14 (KLK14) expression in salivary gland tumors. Int J Biol Markers. 2010;25(1):32–37. doi: 10.1177/172460081002500105. [DOI] [PubMed] [Google Scholar]
- 59.Buchner A, David R. Hansen LS “Hyaline cells” in pleomorphic adenoma of salivary gland origin. Oral Surg Oral Med Oral Pathol. 1981;52(5):506–512. doi: 10.1016/0030-4220(81)90363-7. [DOI] [PubMed] [Google Scholar]
- 60.Nikai H, el-Bardaie AM, Takata T, Ogawa I, Ijuhin N. Histologic evaluation of myoepithelial participation in salivary gland tumors. Int J Oral Maxillofac Surg. 1986;15(5):597–605. doi: 10.1016/S0300-9785(86)80066-7. [DOI] [PubMed] [Google Scholar]
- 61.Crocker J, Jenkins R, Campbell J, Fuggle WJ, Shah VM. Immunohistochemical demonstration of S-100 protein in salivary gland neoplasms. J Pathol. 1985;146(2):115–121. doi: 10.1002/path.1711460206. [DOI] [PubMed] [Google Scholar]
- 62.Zarbo RJ, Regezi JA, Batsakis JG. S-100 protein in salivary gland tumors: an immunohistochemical study of 129 cases. Head Neck Surg. 1986;8(4):268–275. doi: 10.1002/hed.2890080406. [DOI] [PubMed] [Google Scholar]
- 63.Draeger A, Nathrath WB, Lane EB, Sundström BE, Stigbrand TI. Cytokeratins, smooth muscle actin and vimentin in human normal salivary gland and pleomorphic adenomas. Immunohistochemical studies with particular reference to myoepithelial and basal cells. APMIS. 1991;99(5):405–415. doi: 10.1111/j.1699-0463.1991.tb05169.x. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa Y, Toyosawa S, Ishida T, Ijuhin N. Keratin 14 immunoreactive cells in pleomorphic adenomas and adenoid cystic carcinomas of salivary glands. Virchows Arch. 2000;437(1):58–68. doi: 10.1007/s004280000186. [DOI] [PubMed] [Google Scholar]
- 65.Cheuk W, Chan JK. Advances in salivary gland pathology. Histopathology. 2007;51(1):1–20. doi: 10.1111/j.1365-2559.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 66.Dardick I, Stratis M, Parks WR, DeNardi FG, Kahn HJ. S-100 protein antibodies do not label normal salivary gland myoepithelium. Histogenetic implications for salivary gland tumors. Am J Pathol. 1991;138(3):619–628. [PMC free article] [PubMed] [Google Scholar]
- 67.Chisholm DM, Waterhouse JP, Kraucunas E, Sciubba JJ. A quantitative ultrastructural study of the pleomorphic adenoma (mixed tumor) of human minor salivary glands. Cancer. 1974;34(5):1631–1641. doi: 10.1002/1097-0142(197411)34:5<1631::AID-CNCR2820340511>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 68.Palmer RM, Lucas RB, Langdon JD. Ultrastructural analysis of salivary gland pleomorphic adenoma, with particular reference to myoepithelial cells. Histopathology. 1985;9(10):1061–1076. doi: 10.1111/j.1365-2559.1985.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 69.Franquemont DW, Mills SE. Plasmacytoid monomorphic adenoma of salivary glands. Absence of myogenous differentiation and comparison to spindle cell myoepithelioma. Am J Surg Pathol. 1993;17(2):146–153. doi: 10.1097/00000478-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Ogawa Y, Kishino M, Atsumi Y, Kimoto M, Fukuda Y, Ishida T, Ijuhin N. Plasmacytoid cells in salivary-gland pleomorphic adenomas: evidence of luminal cell differentiation. Virchows Arch. 2003;443(5):625–634. doi: 10.1007/s00428-003-0890-3. [DOI] [PubMed] [Google Scholar]
- 71.Masson P. The role of the neural crests in the embryonal adenosarcomas of the kidney. Am J Pathol. 1938;33(1):1–32. [Google Scholar]
- 72.Triantafyllou A, Coulter P, Scott J. Phenotypes in canalicular adenoma of human minor salivary glands reflect the interplay of altered secretory product, absent neuro-effector relationships and the diversity of the microenvironment. Histopathology. 1999;35(6):502–516. doi: 10.1046/j.1365-2559.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 73.Economopoulou P, Hanby A, Odell EW. Expression of E-cadherin, cellular differentiation and polarity in epithelial salivary neoplasms. Oral Oncol. 2000;36(6):515–518. doi: 10.1016/S1368-8375(00)00043-9. [DOI] [PubMed] [Google Scholar]
- 74.Franchi A, Santoro R, Paglierani M, Bondi R. Immunolocalization of alpha 2, alpha 5, and alpha 6 integrin subunits in salivary tissue and adenomas of the parotid gland. J Oral Pathol Med. 1994;23(10):457–460. doi: 10.1111/j.1600-0714.1994.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 75.Neureiter D, Böhmer J, Kirchner T, Aigner T. Pleomorphic adenomas of the parotid express different mesenchymal phenotypes: demonstration of matrix gene expression products characteristic of the fibroblastic and chondrocytic cell lineages. Histopathology. 1999;35(4):373–379. doi: 10.1046/j.1365-2559.1999.00762.x. [DOI] [PubMed] [Google Scholar]
- 76.Harrison JD, Auger DW. Ultrastructural observations on luminal structures of pleomorphic adenoma of parotid and submandibular salivary glands of man. Virchows Archiv A Pathol Anat. 1989;415(6):559–563. doi: 10.1007/BF00718650. [DOI] [PubMed] [Google Scholar]
- 77.Norberg L, Stratis M, Dardick I. Quantitation and localization of cycling tumor cells in pleomorphic adenomas and myoepitheliomas: an immunohistochemical analysis. J Oral Pathol Med. 1997;26(3):124–128. doi: 10.1111/j.1600-0714.1997.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 78.Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Aigner T, Neureiter D, Völker U, Belke J, Kirchner T. Epithelial–mesenchymal transdifferentiation and extracellular matrix gene expression in pleomorphic adenomas of the parotid salivary gland. J Pathol. 1998;186(2):178–185. doi: 10.1002/(SICI)1096-9896(1998100)186:2<178::AID-PATH161>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 80.Su L, Morgan PR, Harrison DL, Waseem A, Lane EB. Expression of keratin mRNAs and proteins in normal salivary epithelia and pleomorphic adenomas. J Pathol. 1993;171(3):173–181. doi: 10.1002/path.1711710305. [DOI] [PubMed] [Google Scholar]
- 81.Kusafuka K, Yamaguchi A, Kayano T. Takemura T Immunohistochemical localization of members of the transforming growth factor (TGF)-beta superfamily in normal human salivary glands and pleomorphic adenomas. J Oral Pathol Med. 2001;30(7):413–420. doi: 10.1034/j.1600-0714.2001.300706.x. [DOI] [PubMed] [Google Scholar]
- 82.Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-beta signaling during epithelial–mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179(1–2):11–12. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- 83.Seifert G, Donath K, Schäfer R. Lipomatous pleomorphic adenoma of the parotid gland. Classification of lipomatous tissue in salivary glands. Pathol Res Pract. 1999;195(4):247–252. doi: 10.1016/S0344-0338(99)80042-9. [DOI] [PubMed] [Google Scholar]
- 84.Haskell HD, Butt KM, Woo SB. Pleomorphic adenoma with extensive lipometaplasia: report of three cases. Am J Surg Pathol. 2005;29(10):1389–1393. doi: 10.1097/01.pas.0000168509.47243.4b. [DOI] [PubMed] [Google Scholar]
- 85.Garrett JR, Lenninger S, Ohlin P. Concerning possible contractile mechanisms in the pancreas—myoepithelial cells. Experientia. 1970;26(7):741. doi: 10.1007/BF02232518. [DOI] [PubMed] [Google Scholar]
- 86.Langman G, Andrews CL, Weissferdt A. WT1 expression in salivary gland pleomorphic adenomas: a reliable marker of the neoplastic myoepithelium. Mod Pathol. 2011;24(2):168–174. doi: 10.1038/modpathol.2010.190. [DOI] [PubMed] [Google Scholar]
- 87.Leader R, Kaur Deol-Poonia R, Sheard J, Triantafyllou A. Immunohistochemical localisation of WT1 in epithelial salivary tumours. Pathol Res Pract. 2014. doi:10.1016/j.prp.2014.06.029. [DOI] [PubMed]
- 88.Hohenstein P, Hastie ND. The many facets of the Wilms’ tumour gene, WT1. Hum Mol Genet. 2006;15(Spec No 2):R196–R201. doi: 10.1093/hmg/ddl196. [DOI] [PubMed] [Google Scholar]
- 89.Cheuk W, Chan JK. Salivary gland tumors. In: Fletcher CDM, editor. Diagnostic histopathology of tumors. 3rd ed. Philadelphia: Churchill Livingstone Elsevier; 2007. p. 239–325.
- 90.Buchner A, David R. Lipofuscin in salivary glands in health and disease. Oral Surg Oral Med Oral Pathol. 1978;46(1):79–86. doi: 10.1016/0030-4220(78)90441-3. [DOI] [PubMed] [Google Scholar]
- 91.Matsumoto Y. Lipofuscin pigmentation in pleomorphic adenoma of the palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(3):299–302. doi: 10.1067/moe.2001.116820. [DOI] [PubMed] [Google Scholar]
- 92.Harrison JD, Auger DW. Ultrastructural cytochemistry of phosphatases in the ductal component of pleomorphic adenoma of human parotid and submandibular salivary glands. Histochem J. 1982;14(5):703–711. doi: 10.1007/BF01033619. [DOI] [PubMed] [Google Scholar]
- 93.Metzelaar MJ, Wijngaard PL. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991;266(5):3239–3245. [PubMed] [Google Scholar]
- 94.Ruggles N, Triantafyllou A. Lysosomal activities and cellular homeostasis in epithelial tumours of salivary glands: an immunohistochemical investigation. J Pathol. 2013;231(S1):S34. [Google Scholar]
- 95.Hatakeyama S, Satoh M, Yoshimura N, Otsu T. Immunocytochemical localization of bone morphogenetic proteins (BMPs) in salivary gland pleomorphic adenoma. J Oral Pathol Med. 1994;23(5):232–236. doi: 10.1111/j.1600-0714.1994.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 96.Vanmuylder N, Evrard L, Daelemans P, Douroy N. Chaperones in the parotid gland: localization of heat shock proteins in human adult salivary glands. Cells Tissues Organs. 2000;167(2–3):199–205. doi: 10.1159/000016782. [DOI] [PubMed] [Google Scholar]
- 97.Thrane PS, Roop DR, Sollid LM, Huitfeldt HS, Brandtzaeg P. Two-colour immunofluorescence marker study of pleomorphic adenomas. Histochemistry. 1990;93(5):459–468. doi: 10.1007/BF00266401. [DOI] [PubMed] [Google Scholar]
- 98.Dardick I. Color atlas/text of salivary gland tumor pathology. New York: Igaku-Shoin; 1996. [Google Scholar]
- 99.Simpson RHW, Di Palma S. Selected recent advances in the pathology of salivary neoplasms. Diagn Histopathol. 2010;16(6):276–286. doi: 10.1016/j.mpdhp.2010.03.007. [DOI] [Google Scholar]
- 100.Wilson JB, Madhavan A, Risk JM, Triantafyllou A. Expression of FANCD2 in epithelial tumours of salivary glands in man: an immunohistochemical investigation. J Pathol. 2012;228(S1):S28. [Google Scholar]