Abstract
Both platelets and cancer cells display an intimate reciprocal crosstalk resulting in alteration of each other’s properties. Although many past studies have tried to demonstrate effect of platelets on tumour cells, exact role of platelets in carcinogenesis is still not clear. In the above study, we explored the effect of different concentrations of platelet rich plasma (PRP) on viability, proliferation and adhesion of HeLa cells in culture conditions. The above parameters were found to be slightly increased on incubation with lower two concentrations of PRP (4.4 × 105 & 1 × 106 platelets/μl) while a reverse effect was seen at high PRP concentration (2 × 106 lac platelets/μl) especially at 24 h. To further validate that the above effects were due to platelets we repeated the experiments in the presence of antiplatelet drug aspirin (20 mM). On treatment with aspirin alone, the cell viability, proliferation and adhesion were seen to be decreased indicating cytotoxicity of aspirin towards HeLa cells. However, all of the above parameters were found to increase on addition of all PRP concentrations at 24 h. Overall, variations in the number of platelets produced different effects on the cancer cells. Use of aspirin reduced the viability of the cancer cells, but this effect was seen to be partially reversed by all the concentrations of PRP used.
Keywords: Platelets, Adhesion, Cell viability, Proliferation
Introduction
Carcinogenesis is believed to be a multistep process that involves a wide variety of interactions between tumour and its microenvironment, which eventually results in the usage of the host cells for its own survival. Platelets have been known to play a crucial role in cancer metastasis since decades [1, 2]. Both platelets and cancer cells display an intimate reciprocal crosstalk, resulting in the alteration of each other’s properties. Although studies have been done to evaluate effect of platelets on properties of the cancer cells, yet, due to the contradictory results exact role of platelets in modifying the tumour cell properties has not been established [3, 4]. Cancer patients may receive platelets from outside to treat thrombocytopenia and bleeding induced by intensive chemotherapy [5]. Thus, in this study we have evaluated the effect of platelets on viability, proliferation and adhesion of HeLa cells in culture conditions, using different concentrations of platelet rich plasma (PRP) with and without the presence of antiplatelet drug aspirin.
Materials and Methods
HeLa cells obtained from the commercial source were maintained in RPMI 1640 culture medium (Himedia) containing 10 % FCS. For preparation of PRP 5 ml of blood was collected from healthy donors after obtaining written consents, in sodium citrate (0.109 M, 3.2 %) vacutainers under sterile conditions. The blood was centrifuged twice at room temperature (800 and 2000 rpm for 20 min) to obtain pellet containing platelets (PRP) [6]. The platelet count of the PRP was adjusted to 4.4 × 105/μl (P1), 1 × 106 platelets/μl (P2) and 2 × 106 platelets/μl (P3). HeLa cells were treated with various concentrations of PRP (10 μl/105 cells) for 2 and 24 h, with and without aspirin (20 mM, 1 M stock solution in DMSO; Sigma Aldrich). The Cell viability was assayed using trypan blue dye and viable and dead cells were counted under the microscope. Estimation of Cell proliferation was done using MTT and absorbance was recorded spectrophotometrically at 570 nm. Cell adhesion was analysed using crystal violet dye and absorbance was read at 570 nm.
Results
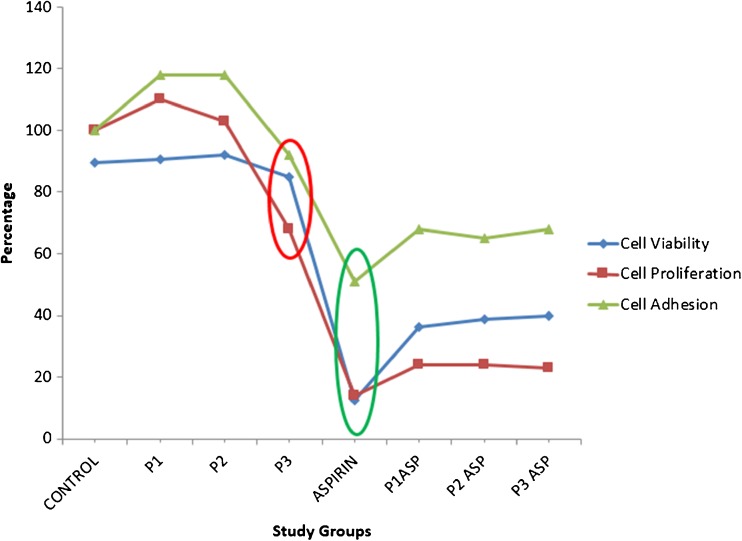
The viability and proliferation of HeLa cells were found to be slightly increased at all PRP concentrations (4.4 × 105, 1 × 106 & 2 × 106 platelets/μl) at 2 h, while at 24 h a mild increase was observed only at lower two concentrations of PRP (Cell viability: control 89.50 % ± 7.68; P1 90.62 % ± 8.12; P2 92.00 % ± 6.98, Proliferation: control 100 %; P1 110 % ± 5.66 P2 103 % ± 2.83). However, at higher concentration (P3) of PRP, both viability (84.75 % ± 19.36) and proliferation (68 % ± 2.12, p-value <0.05, One way Anova) of HeLa cells was found to be reduced (Table 1, Figs. 1 and 2).
Table 1.
Effect on viabilty, proliferation and adhesion of HeLa cells treated with different concentrations of PRP with and without aspirin at 2 and 24 h
| Parameter studied | C | P1 | P2 | P3 | Asp | P1 + Asp | P2 + Asp | P3 + Asp | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | 24 h | 2 h | 24 h | 2 h | 24 h | 2 h | 24 h | 2 h | 24 h | 2 h | 24 h | 2 h | 24 h | 2 h | 24 h | |
| Cell viability | 97 | 89.5 | 98.5 | 90.62 | 99.5 | 92 | 97.5 | 84.75 | 81 | 12.5 | 74.5 | 36.25 | 83.5 | 38.88 | 81 | 39.75 |
| Cell Proliferation | 100 | 100 | 94 | 110 | 92 | 103 | 96 | 68 | 20 | 14 | 25 | 24 | 28 | 24 | 29 | 23 |
| Cell adhesion | 0.45 | 0.85 | 0.45 | 0.87 | 0.49 | 0.87 | 0.46 | 0.78 | 0.52 | 0.43 | 0.54 | 0.58 | 0.56 | 0.55 | 0.54 | 0.58 |
P1 = 4.4 × 105 platelets/μl, P2 = 1 × 106 platelets/μl, P3 = 2 × 106 platelets/μl, Asp = Aspirin 20 mM/l)
Fig. 1.
Line diagram depicting cell viability, adhesion and proliferation of HeLa cells subjected to treatment with PRP and aspirin alone and in combination with each other at 24 h. Note the sharp dip in above parameters upon treatment with aspirin (green circle) and high concentration of PRP (P3) (red circle)
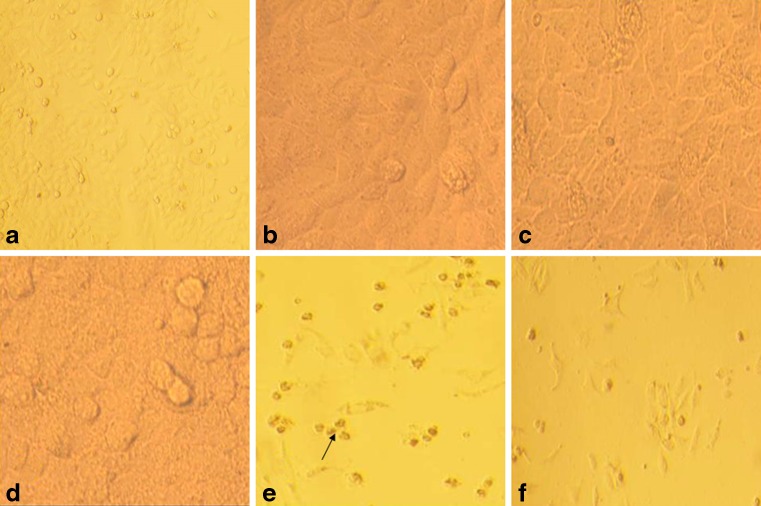
Fig. 2.
Cultured HeLa cells as seen under inverted microscope a Untreated control b, c, d After treatment with different PRP concentrations (4.4 × 105, 1 × 106 and 2 × 106 /μl respectively). Note the film of platelets covering the cells particularly at 2 × 106 platelets / μl. e After treatment with aspirin (20 mM). Cytotoxic effect of aspirin can be seen as rounding off and change in morphology. f After treatment with aspirin and PRP. Note the presence of both viable and dead cells in the later
Further, the cells were incubated with the platelets in the presence of aspirin to confirm that the above effects were due to platelets. Treatment of the HeLa cells with aspirin (20 mM) alone resulted in significant cytotoxicity [cell viability (81.0 % ± 3.61 at 2 h, 12.5 % ± 15.56 at 24 h)& reduction in proliferation (20 % ± 2.03 at 2 and 14 % ± 4.36 at 24 h) at both the time intervals (p-value < 0.05, student t-test). The addition of PRP at all three concentrations however, resulted in an increase in cell viability (36.25 % ± 13.79, 38.87 % ± 11.49 and 39.75 % ± 21.57 at 24 h), and proliferation (25 %±, 28 % ± & 29 % at 2 h; 24 % ± 2.65 &23 % ± 4 at 24 h; p-value < 0.05, One way Anova).
On crystal violet assay, the absorbance at 570 nm was seen to be increased at all PRP concentrations (control 0.447 ± 0.02; 0.453 ± 0.05 with P1, 0.488 ± 0.001 with P2 & 0.463 ± 0.02 with P3) at 2 h however at 24 h it was found to be mildly increased at lower two concentrations (control 0.85; 0.87 ± 0.13 with P1& 0.87 ± 0.02 with P2) and reduced at higher concentration of PRP (0.78 ± 0.05 with P3). On treatment of cells with 20 mM aspirin, the adhesion was seen to be increased at 2 h (0.517 ± 0) but significantly reduced at 24 h (0.436 ± 0.01, p-value < 0.05, student t-test) of incubation. However, on addition of PRP at different concentrations, the adhesion (P1, P2, P3; 0.536 ± .08, 0.564 ± 0.04 & 0.541 ± 0.03at 2 h & 0.581 ± 0.03, 0.548 ± 0.03 & 0.582 ± 0.1at 24 h respectively) of the HeLa cells was found to be increased. These results, though somewhat in parallel to cell viability (as adherence of the cells from the substratum decreased due to increased cell death by aspirin) were not found to be statistically significant.
Discussion
A dual effect of platelets on HeLa cells was noted in our work. Out of the three concentrations of PRP used, the lower two showed mild increase in the viability, proliferation and adhesion of the HeLa cells. However, at higher concentration, a significant inhibitory effect (p-value < 0.05) on the above properties was noted at 24 h. Previous studies have also demonstrated variable effects of platelets on cancer cells. In a study by Hara et al., platelet lysate was shown to sustain proliferation of four malignant cell lines in serum free media [7]. Cho et al. have showed that incubation with platelets increase the proliferation of ovarian cancer cells [8]. A study by Yu et al., concluded that platelets enhanced the adhesion of highly metastatic human hepatoma cells to the extracellular matrix [9]. The effects on the cancer cells may be attributed to release of platelet specific stimulatory factors (VEGF, EGF, FGF, IGF, MMPs, angiopoietin, MMPS-1,2,9), Lysophosphatidic acid, Thromboxane A2, and thrombin), adhesion factors (P-selectin) and cytokines (TGFβ) which have been demonstrated to promote mitogenicity, angiogenesis and invasory phenotype in cancer [10]. More recent studies have shown that platelets release microparticles containing above factors to stimulate proliferation and adhesion in cancer cells [11]. Also, the tumour cells and platelets have been found to exchange RNA transcripts with each other via above microparticles [12].
Though, in some of the previous studies, the platelets have been shown to inhibit the tumour cell growth by impairing their cell cycle a switch in the role of platelets at higher concentrations has not been demonstrated. This effect may probably be attributed to differential release of platelet specific stimulatory and inhibitory factors (Platelet factor-4) at different concentrations of PRP, however, this fact needs to be confirmed by measuring the levels of the above factors in a concentration specific manner. Also, the possible contribution by factors secreted by cancer cells in promoting such a switch cannot be overruled [4, 13, 14].
To confirm that the above effects were due to platelets we repeated the experiments in the presence of antiplatelet drug aspirin. Aspirin, at the concentration used in the study was noted to cause significant cytotoxicity (p-value < 0.05) to HeLa cells. Similar cytotoxicity of aspirin and other NSAIDS has also been described in previous studies in different malignancies like rhabdomyosarcoma, chronic lymphocytic leukemia, cervical cancer etc. The effect has mainly been attributed to inhibition of molecules like COX, ErbB2 etc. and activation of caspase-3 [15–17]. Interestingly, the addition of PRP at all three concentrations was found to partially neutralize the effect of aspirin on viability, proliferation and adhesion profile of the HeLa cells. This may be due to differential expression of COX-1& COX-2 in platelets and more complete inhibition of platelet COX-1rather than COX-2 by aspirin [18, 19]. However, the above finding in our study needs further confirmation with more robust studies assessing the combined effect of different doses of aspirin and PRP on cancer cells.
Conclusion
The above study shows a complex effect of platelets on HeLa cells depending upon the concentration of PRP (number of platelets). The tumour promoting effects of some concentrations of PRP in cancer merit caution in use of PRP for platelet replenishment in cancer patients with thrombocytopenia. Use of aspirin, no doubted, reduced the viability of the cancer cells, but this effect was seen to be reversed to some extent by all the concentrations of PRP that were used. Thus, administration of PRP in cancer patients who are being administered aspirin may require change in dosage of the above drug. However, further studies involving combination of different doses of aspirin and PRP are required for confirmation of the same.
Acknowledgments
Compliance with Ethical Standards
The above work was done after approval from institute ethics committee. A written informed consent was obtained from human volunteers involved in the work. The authors declare that there is no conflict of interest involved.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013;24:393–400. doi: 10.1016/j.ejim.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet—cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143:819–26. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menter DG, Sloane BF, Steinert BW, Onoda J, Craig R, Harkins C, et al. Platelet enhancement of tumor cell adhesion to subendothelial matrix: role of platelet cytoskeleton and platelet membrane. J Natl Cancer Inst. 1987;79:1077–90. [PubMed] [Google Scholar]
- 4.Wang Y, Zhang H. Platelet-induced inhibition of tumor cell growth. Thromb Res. 2008;123:324–30. doi: 10.1016/j.thromres.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, et al. American society of clinical oncology. Platelet transfusion for patients with cancer: clinical practice guidelines of the American society of clinical oncology. J Clin Oncol. 2001;19:1519–38. doi: 10.1200/JCO.2001.19.5.1519. [DOI] [PubMed] [Google Scholar]
- 6.Nagata MJ, Messora MR, Furlaneto FA, Fucini SE, Bosco AF, Garcia VG, et al. Effectiveness of two methods for preparation of autologous platelet-rich plasma: an experimental study in rabbits. Eur J Dent. 2010;4:395–402. [PMC free article] [PubMed] [Google Scholar]
- 7.Hara Y, Steiner M, Baldini MG. Platelets as a source of growth-promoting factor(s) for tumor cells. Cancer Res. 1980;40:1212–6. [PubMed] [Google Scholar]
- 8.Cho MS, Bottsford-Miller J, Vasquez HG, Stone R, Zand B, Kroll MH, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120:4869–72. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Zhou XD, Liu YK, Ren N, Chen J, Zhao Y. Platelets promote the adhesion of human hepatoma cells with a highly metastatic potential to extracellular matrix protein: involvement of platelet P-selectin and GP IIb-IIIa. J Cancer Res Clin Oncol. 2002;128:283–7. doi: 10.1007/s00432-002-0325-6. [DOI] [PubMed] [Google Scholar]
- 10.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–60. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 11.Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov Med. 2009;8:237–41. [PubMed] [Google Scholar]
- 12.Nilsson RJ, Balaj L, Hulleman E, van Rijn S, Pegtel DM, Walraven M, et al. Blood platelets contain tumor-derived RNA biomarkers. Blood. 2011;118:3680–3. doi: 10.1182/blood-2011-03-344408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Du J, Hou J, Jiang H, Zou J. Platelet factor-4 and its p17-70 peptide inhibit myeloma proliferation and angiogenesis in vivo. BMC Cancer. 2011;11:261. doi: 10.1186/1471-2407-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang P, Cheng SH, Cheng CK, Lau KM, Lin SY, Chow EYD, et al. Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica. 2013;98:288–295. doi: 10.3324/haematol.2012.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Nimer SMM, Hameed HG, Mahmood MM. Antiproliferative effects of aspirin and diclofenac against the growth of cancer and fibroblast cells: In vitro comparative study. Saudi Pharm J. 2015 doi: 10.1016/j.jsps.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang S, Sun Z, He Q, Yan F, Wang Y, Zhang J. Aspirin inhibits ErbB2 to induce apoptosis in cervical cancer cells. Med Oncol. 2010;27:379–387. doi: 10.1007/s12032-009-9221-0. [DOI] [PubMed] [Google Scholar]
- 17.Bellosillo B, Piqué M, Barragán M, Castaño E, Villamor N, Colomer D, et al. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92(4):1406–1414. [PubMed] [Google Scholar]
- 18.Guthikonda S, Lev EI, Patel R, DeLao T, Bergeron AL, Dong JF, et al. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J Thromb Haemost. 2007;5:490–6. doi: 10.1111/j.1538-7836.2007.02387.x. [DOI] [PubMed] [Google Scholar]
- 19.Dibra HK, Brown JE, Hooley P, Nicholl ID. Aspirin and alterations in DNA repair proteins in the SW480 colorectal cancer cell line. Oncol Rep. 2010;24(1):37–46. doi: 10.3892/or_00000826. [DOI] [PubMed] [Google Scholar]