Abstract
Exosomes are endosomal-derived nanovesicles released by normal and tumor cells which have been shown to transfer functionally active protein, lipids, mRNAs and miRNAs between cells. Varying in molecular profiles, biological roles, functional roles and protein contents, exosomes have been described as “multi-purpose carriers” playing a role in supporting the survival and growth of tumor cells. The IAP Survivin has been found to be present in tumor exosomes. However, the existence of other IAPs in tumor exosomes is still unknown. Survivin, cIAP1, cIAP2 and XIAP mRNA and protein are differently expressed in a panel of tumor cell lines: DLCL2, HeLa, MCF-7, Panc-1, and PC3. Exosomes were isolated from conditioned media collected from the cells from which RNA and protein were extracted. Our results provide evidence that like Survivin, XIAP, cIAP1 and cIAP2 proteins are found in tumor exosomes. The mRNA expression, however, is differentially expressed across the tumor cell lines. The presence of these bioactive molecules in exosomes may not only serve as warning signals, but also play a role in providing protection to the cancer cells against changes that are constantly occurring in the tumor microenvironment.
Keywords: Exosomes, IAPs, Cancer cells, Tumor microenvironment
Introduction
Exosomes are small membrane vesicles, ranging from 40 to 150 nm in diameter, that are shed from various cell types such as B- and T-lymphocytes, neurons, intestinal epithelial cells, dendritic cells and tumor cells [1–3]. Tumor exosomes, which are constitutively released into the extracellular space, have different molecular profiles, biological roles and molecular contents, giving an indication of the cell of origin, as well as their functional role [4–6]. This has also been shown in vivo, where membrane vesicles isolated from cancer patients’ plasma and neoplastic effusions are characterized by the expression of tumor- specific markers reflecting tumor origin [7–10]. Tumor exosomes have a role in supporting the tumor cells’ survival and growth [11]. The specific roles include evasion of host immunity [12], tissue invasion [13] and neoangiogenesis [14, 15]. Not only do tumor exosomes contain proteins and tumor antigens, but functional mRNA has also been shown to be contained within these microvesicles [7].
The inhibitor of apoptosis (IAP) family of proteins are known to be endogenous caspase inhibitors, where cIAP1, cIAP2 and XIAP directly bind to activated caspase-3, −7 and −9 using their baculorvirus IAP repeat (BIR) domains [16–19]. Survivin, a unique member of the IAP family, contains a BIR domain, but has a multifunctional role in various cellular activities, including regulating mitosis, inhibiting cells from undergoing apoptosis and adapting to stressful environments [20–22]. Survivin’s multifunctional role depends on its subcellular location, where it is found to be localized in the nucleus, mitochondria and cytoplasm [23]. We have shown that an extracellular pool of Survivin also exists, released from cancer cells in exosomes [24]. Upon release and resorption by neighboring cancer cells, these cells become resistant to therapy, rapidly proliferate and acquire an increased invasive potential [25]. In addition to Survivin, we have also recently shown that cIAP1, cIAP2 and XIAP are found in exosomes collected from Panc-1 conditioned media [26].
Here, we evaluate across a panel of cell lines representing five different cancer types and one non-cancer, whether like Survivin, cIAP1, cIAP2 and XIAP are released into the extracellular space via exosomes. We show that though cell type dependent, cIAP1, cIAP2, XIAP and Survivin protein and mRNA are released by exosomes.
Results
Intracellular IAP mRNA and Protein is Differently Expressed in Cancer Cell Lines
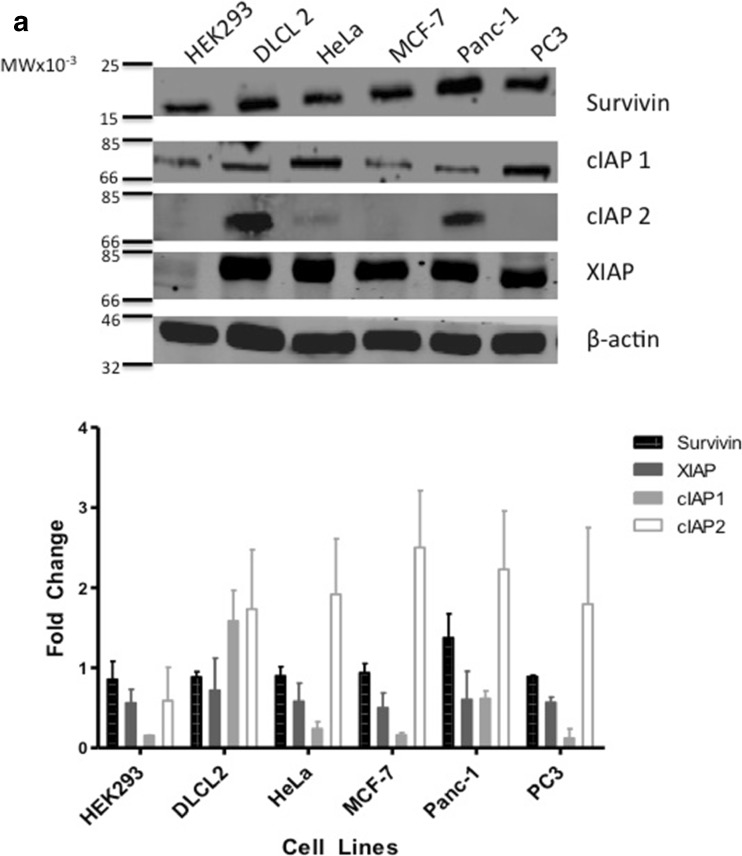
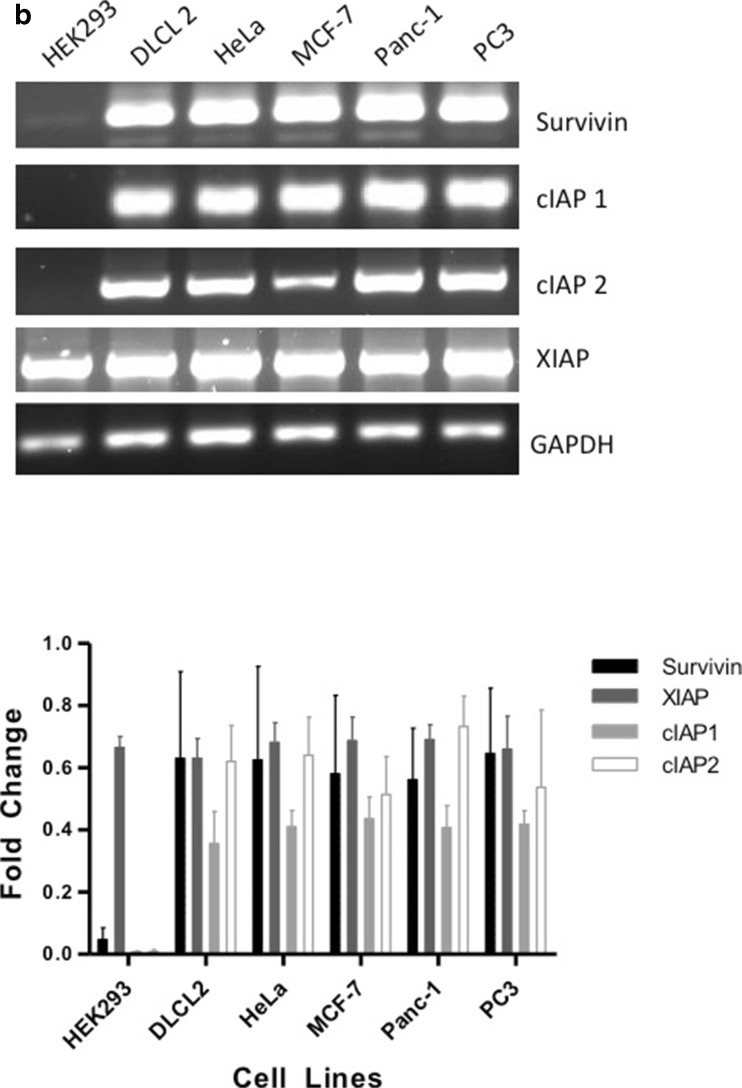
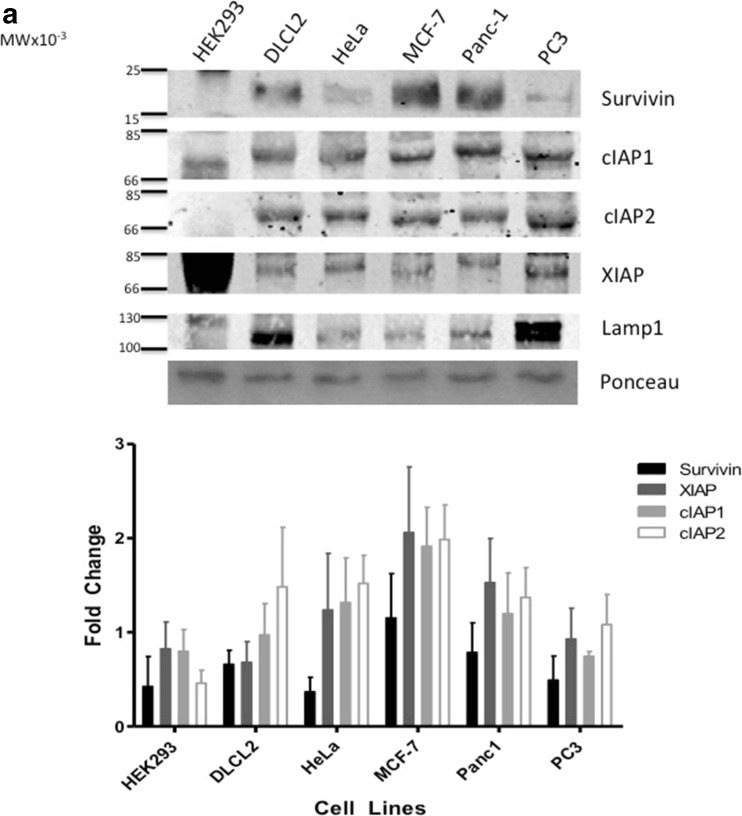
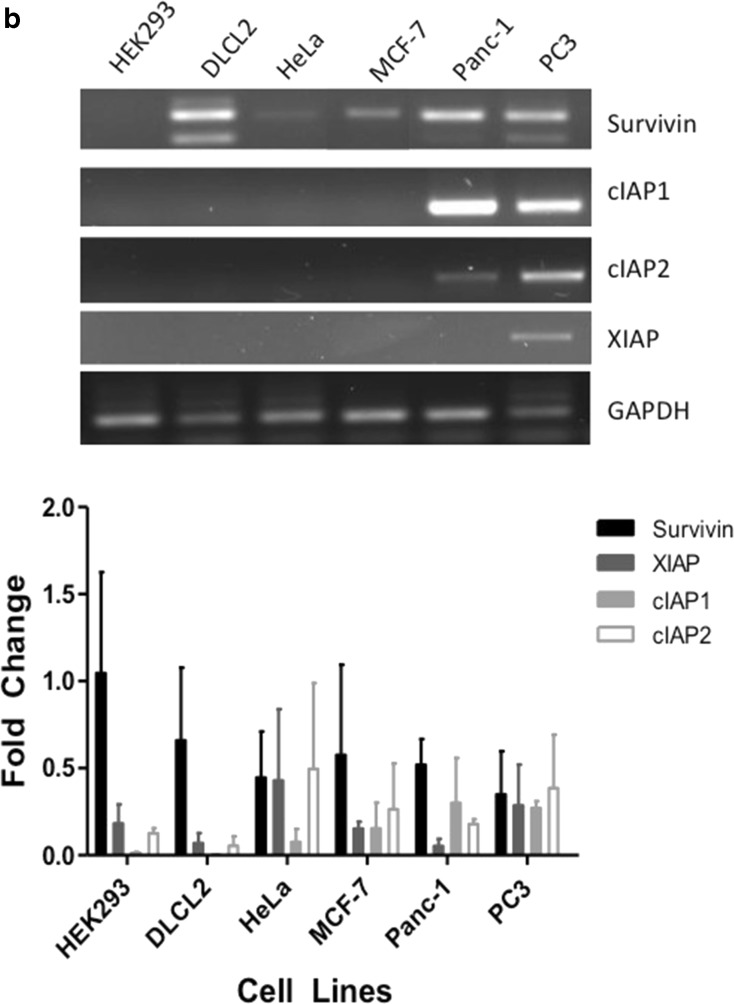
IAPs play an important role in the cancer cell’s ability to resist apoptosis [27]. In this study, we used five different cancer cell lines from various cancer types and one cell line representative of a normal phenotype. All cell lines, including the non- cancer cell line HEK293 displayed a range of IAP protein expression (Fig. 1a). In comparison to the HEK293 cells, which had low levels of Survivin and cIAP1, DLCL2 and Panc-1 cells expressed all four IAPs represented in this study. HeLa, MCF-7 and PC3 cell lines expressed near similar levels of Survivin, cIAP1 and XIAP but did not express cIAP2. In comparison to protein expression, IAP mRNA is equally expressed in all the tumor cell lines. HEK293 cells highly expressed XIAP mRNA and showed low Survivin mRNA expression levels. In contrast, cIAP1 and cIAP2 expression levels were deficient in HEK293 cells (Fig. 1b).
Fig. 1.
Western Blot Analysis of Survivin, cIAP1, cIAP2, XIAP and β-actin taken from non-cancer cell line and nontreated cancer cell lines: Human embryonic kidney cell line (HEK293), Diffuse Large Cell Cleaved (DLCL), cervical (HeLa), breast (MCF-7), pancreatic (Panc-1), and prostate (PC3). a. Antibodies for Survivin, cIAP1, cIAP2, XIAP and β-actin were used for Western blotting cell line-purified protein. b. mRNA was also acquired from the same nontreated non-cancer and cancer cell lines and the varying IAP targets were amplified using PCR. Both Western blots as well as PCR are representative of 2–4 independent experiments
Amount of Exosome Released Depends on Cell Line
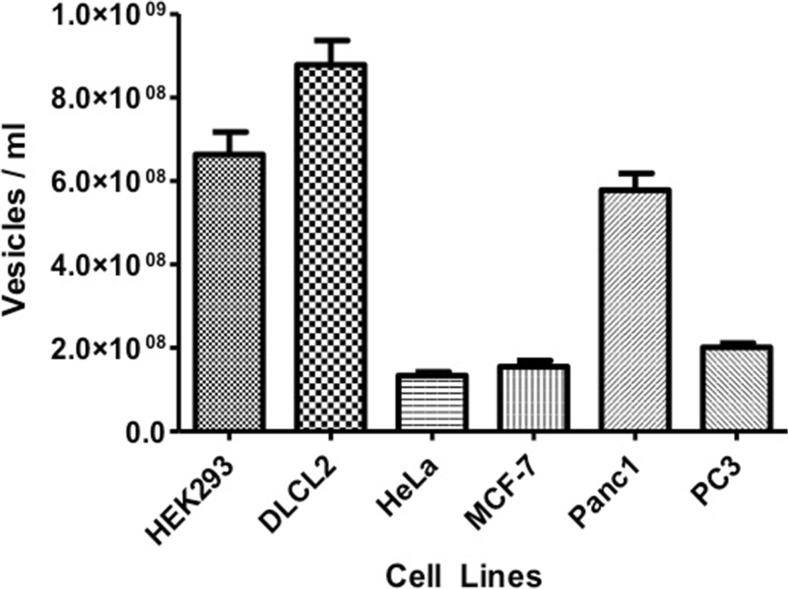
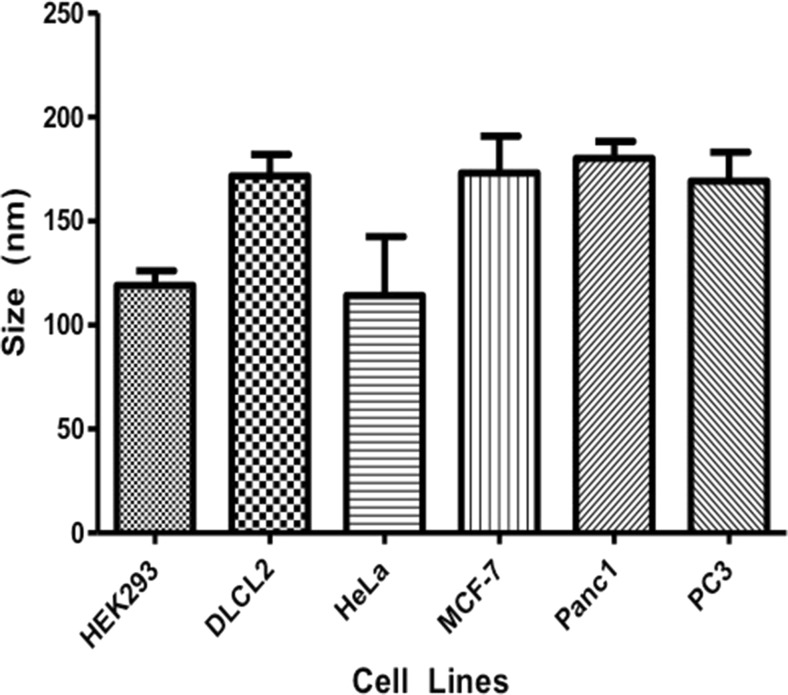
Tumor cells have been shown to constitutively release tumor exosomes (TEX) into the extracellular space [4]. To determine if all cells within our study released exosomes and then further, whether the type of cancer influences the amount of exosomes released, we collected conditioned media from different cancer cell lines. The presence and amount of purified exosomes in vesicle number per mL, were determined by NanoSight showing a diverse difference between the cell lines investigated. Among the cancer cell lines, HeLa, MCF-7 and PC3 released the least amount into the media (Fig. 2). To verify that the vesicles collected were indeed exosomes, the vesicles’ mode average sizes were analyzed using NanoSight’s nanoparticle tracking analysis software. Although a range of vesicle sizes were detected, the majority of the collected vesicles lie within the 100–150 nm size range of exosomes (Fig. 3).
Fig. 2.
Histogram representing concentration of vesicles per ml to quantify exosome numbers. Exosomal contents in conditioned medium from HEK293, DLCL2, HeLa, MCF7, Panc-1, and PC3 cell lines. Data are the mean ± SD of 3 independent experiments in triplicate
Fig. 3.
Mode average size of exosomes isolated using ExoQuick TC™. While there was a range of sizes of vesicles isolated, the mode average size of vesicles falls in the size range of exosomes
IAPs are Present in Exosomes
We have previously shown that Survivin, cIAP1, cIAP2 and XIAP are trafficked into the extracellular space via exosomes [24, 26]. We therefore hypothesized that IAPs would also be exported out of a variety of tumor cells in the same manner. We evaluated the presence of Survivin, cIAP1, cIAP2 and XIAP from isolated exosomes collected from conditioned media by Western blot to determine if these IAPs would be present in exosomes. Across all cell lines, Survivin, along with cIAP1, cIAP2 and XIAP were found in the exosomes of the cell lines evaluated (Fig. 4a).
Fig. 4.
Western Blot Analysis of Survivin, cIAP1, cIAP2, XIAP and Lamp-1 taken from the conditioned medium off of nontreated non-cancer and cancer cell lines: Human embryonic kidney cell line (HEK293), Diffuse Large Cell Cleaved (DLCL), cervical (HeLa), breast (MCF-7), pancreatic (Panc-1), and prostate (PC3). a. Antibodies for Survivin, cIAP1, cIAP2, XIAP and Lamp-1 were used for Western blotting exosome-purified protein. b. mRNA was also acquired from the same nontreated non-cancer and cancer cell lines and the varying IAP targets were amplified using PCR. Both Western blots as well as PCR are representative of 2–4 independent experiments
IAP mRNA are Released into the Extracellular Space by Exosomes
Exosomes serve as vesicles for protein and genetic materials [7]. In addition to verifying the presence of IAP protein in the exosomes, the presence of exosomal IAP mRNA was also investigated in our chosen panel of cell lines. mRNA was extracted from isolated exosomes and PCR was performed. Not all of the cancer cells showed representative abundance of all four IAPs with DLCL2, Panc-1 and PC3 having the greatest variety. HeLa and MCF-7 cells appear to only express survivin mRNA and HEK293 cells do not express any in our hands. Survivin mRNA was found more abundantly than all other IAPs in the cell lines evaluated and PC3 cellular exosomes appear to hold representation from all IAPs investigated (Fig. 4b).
Discussion
The IAP family regulates cell survival and members of this family are often deregulated in cancer, which may be a factor for chemoresistance and treatment failure [28]. In most normal adult tissues, Survivin expression is very low or undetectable [21, 29, 30]. The high levels of Survivin expression in cancer cells have been associated with grim prognosis, disease progression, metastatic dissemination, therapy resistance and an overall dismal disease outcome [21, 29]. The biological characteristics of the tumor, as well as the way the host responds to the tumor also plays a major role on the growth and spread of cancer [25]. Here we show that though there is a consistent cellular expression of IAP mRNA and protein in all cell lines evaluated, there are distinct cell-to-cell differences in the specific expression levels of IAP proteins and mRNAs (Fig. 1). IAP protein expression varies, perhaps reflecting the cell line’s level of therapy resistance and aggressiveness, or at what phase in the cell cycle the cells are in at the time of harvest and purification.
Cancer cells communicate with each other with heterotypic interactions going on between them, the mesenchymal cells that they recruit, and the stromal cells that surround them in the tumor microenvironment. Communication between these components by secretion of various proteins, such as growth factors, ECM-degrading proteinases and chemokines is crucial for the progression, development and maintenance of the tumor [31]. Small membrane vesicles are known to be secreted from tumors [32] and increasing interest and studies to define their role are underway to elucidate the role these vesicles play in cancer development and progression. TEX have been described as “multi-purpose carriers” having vital roles in the communication, protection, progression as well as genetic information exchange with neighboring cells in the tumor microenvironment [33]. Various bioactive molecules have been found packaged within as well as on the TEX, strongly influencing the surrounding environment [34–37] through direct interaction or through trafficking of these molecules into a recipient cell(s). Survivin has a multifunctional role in various cellular activities depending on its subcellular location. We have recently established that Survivin is also found in the extracellular space [25] and exported out of the cancer cells via exosomes [24]. The work described here was to establish whether other IAPs were also exported from cancer cells in a similar fashion. This was accomplished in order to determine the level of similarity or redundancy tumor cells express to overcome the changes or differences in cellular makeup as it relates to intracellular communication.
Exosomes were isolated from conditioned media collected from the panel of cell lines. These samples were analyzed using the NanoSight to determine the presence and amount of purified exosomes (Figs. 2 and 3). Release of TEX can be affected by various changes taking place in the microenvironment, such as chemo-, and radiation stress, as well as the biome they contain [38–40]. Interestingly, chemoresistant cells that have been treated with chemotherapeutic agents show a significant increased secretion of vesicles compared to chemo-sensitive cells [41, 42]. The difference in the amount of exosomes and what resides on their surfaces and in their lumens could be the result of stress, or their response to it or the genetic makeup in the host cell of origin.
Here we show that Survivin, along with cIAP1, cIAP2 and XIAP, are secreted from tumor and non-tumor cells into the extracellular space via exosomes (Fig. 4a). Secretion of IAPs through exosomes and their subsequent uptake by neighboring cells of the tumor microenvironment can serve as a protective strategy from cell death. It could also be a mechanism for these IAPs and other exosomal biomolecules to travel long distances within the body, affecting, stabilizing or manipulating environments far from the primary tumor in order to aid secondary tumor growth and resistance. We have shown that Survivin, when released to the extracellular milieu has the ability to stimulate cellular proliferation, increase resistance and invasive potential [25], and modulate T cells [43]. It may be that the tumor microenvironmental presence of exosome containing biomolecules could play a bigger role in antitumor protections than the cellular modulation of these IAPs, having significant reach beyond that possible for circulating tumor cells.
Genetic material, found in vesicles, has been implicated in furthering tumor growth [7]. Our lab has recently shown that IAP mRNA was found in exosomes isolated from Panc-1 conditioned media [26]. We therefore hypothesized that IAP mRNA is also found in exosomes collected from different tumor cell lines. While Survivin mRNAs were secreted by all the cell lines investigated in this study, cIAP2 and XIAP mRNA are more selectively found in the exosomes from the cell lines observed (Fig. 4b). Interestingly, there appeared from experiment to experiment some variation in the mRNAs found in these exosomes which was not the case with protein. We hypothesize that this variance may be the result of the type of RNA product and its status at the time of capture by the exosome. It may be possible that exosomes package truncated mRNAs as their RNA transcripts undergo a widespread post-transcriptional cleavage. As a result these truncated RNAs provide a more small RNA, regulatory role like a miRNA [44, 45]. Full length IAP mRNA transcripts were also found to be present in exosomes, which may be translated into functional proteins upon reabsorption into recipient cells, as shown by Skog et al. [7]. In addition, the release of these bioactive molecules may not only serve as warning signals to the neighboring cells, but also provide protection against the constant environmental changes in the tumor microenvironment. We propose, like we have with Survivin, that exosomal IAPs may play important biomarker roles in early diagnosis but also in monitoring treatment effects of subjects with cancer.
Materials and Methods
Cell Lines and Cultures
Cervical carcinoma (HeLa), prostate carcinoma (PC3), breast carcinoma (MCF-7), pancreatic carcinoma (Panc-1) and human embryonic kidney (HEK293) cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA). The non-Hodgkin’s lymphoma cell line (DLCL2) was a kind gift from Dr. Ayad Al-Katib (Wayne State University, Detroit, MI). The cells were maintained in DMEM, McCoy’s or RPMI (ATCC, CellGro; Manassas, VA) supplemented with 100U penicillin, 100 μg/ml streptomycin, 10 % fetal bovine serum (FBS: CellGro; Manassas, VA). The cells were grown in a humidified atmosphere at 37 °C of 95 % O2 and 5 % CO2 until 60 % confluent and the medium was changed. The conditioned media was collected after 24 h.
Exosome Isolation
The method for exosome isolation was performed using ExoQuick TC™ (Mountain View, CA). Briefly, CM was collected from the treated cells and centrifuged at 3,000 x g for 15 min. 1 ml of ExoQuick TC™ was mixed with 5 ml of CM and incubated at 4 °C for 12 h. Following incubation, the CM was centrifuged at 1,500 x g for 30 min to pellet exosomes. The pellet was resuspended in the appropriate buffer to isolate RNA or protein to be used for PCR or Western blot analysis. Exosome pellets resuspended in PBS were used for NanoSight analysis.
Verification of Exosome Presence and Exosome Quantification
The instrument NanoSight NS300 (Malvern Instruments Inc., Ranch Cucamonga, CA, USA) was used to confirm the size of the particles present in the exosome isolation fraction. Briefly, the exosomal fraction was diluted 1:1000 with 1X PBS at 23 C and the size dispersion was measured. A video of 30 to 60 s duration was taken with a frame rate of 30 frames/s, and particle movement was analyzed by NTA software (version 2.2, NanoSight). The NTA software is optimized to first identify and then track each particle on a frame-by-frame basis, and its Brownian movement is tracked and measured from frame to frame. The velocity of particle movement is used to calculate particle size by applying the two-dimensional Stokes-Einstein equation. The range of sizes that can be analyzed by NTA depends on the particle type. Exosomes and microvesicles have a low refractive index, and the smallest detectable size using the NTA system is approximately 50 nm. NTA post acquisition settings were optimized and kept constant between samples, and each video was then analyzed to give the mean, mode, and median vesicle size together with an estimate of the concentration.
Western Blots
For total cell Western blot analysis, the cells were harvested and lysed in cell lysis buffer (0.5 % Triton X-100, 300 mM NaCl, 50 mM Tris/HCl, 1 mM PMSF) with sonication. The lysates were centrifuged at 10,000 rpm at 4 °C for 20 min to remove cell debris. For exosomes Western blot analysis, exosomes were solubilized in lysis buffer. Protein concentration was determined using the Micro BCA protein assay (Pierce Chemical; Rockford, IL). A total of 50 μg cellular protein or 30 μg exosome protein was separated using a 10–12 % SDS polyacrylamide gels and transferred onto nitrocellulose membrane (BioRad; Hercules, CA). Blots were immunostained with antibodies against Survivin ((1:500–2000), NOVUS Biologicals, Littleton, CO), cIAP1, cIAP2 and XIAP ((1:500–1000), Cell Signaling, Danvers, MA). β-actin ((1:1000), Cell Signaling) was used as control for cell samples and Lamp-1 ((1:500, BioLegend, San Diego, CA) was used as a loading control for exosome samples. Goat anti-rabbit antibodies (LI-COR Biosciences, Lincoln, NE) were used as secondary antibody. The immunoreactive bands were visualized using the Odyssey imaging system (LI-COR Biosciences).
PCR
Harvested cells and isolated exosomes were resuspended in TRI Reagent® (Molecular Research Center, Cincinnati, OH) and stored at −80 °C until needed. RNA was extracted per the manufacturer’s directions. RNA quantification was performed using NanoDrop 2000c (Thermo Fisher Scientific, Waltham, MA). Reverse transcription of RNA was performed using the First Strand cDNA Synthesis kit (Syd Labs, Inc, Malden, MA). Genomic DNA is eliminated prior to reverse transcription of RNA into cDNA. A total concentration of 100 ng/μl cDNA was utilized to perform PCR reactions using Phusion® Flash High-Fidelity PCR Master Mix (Finnzymes, Thermo Scientific; Pittsburgh, PA). The forward and reverse primers (IDT, San Diego, CA) were designed to detect Survivin, cIAP1, cIAP2 and XIAP genes.
Acknowledgments
The author’s would like to thank the Center for Health Disparities & Molecular Medicine for resource support of this project and the Graduate Students that it involved. The author’s would like to especially thank and acknowledge Malvern and Duncan Griffiths (Product Mananger) for supporting this work with the use of their marvelous NanoSight equipment. Research reported in this publication was supported by the National Institute of Health Disparities and Minority Health of the National Institutes of Health under award numbers p20MD0016321, P20MD006988 and 2R25 GM060507. a National Merit Test Bed (NMTB) award sponsored by the Department of the Army under Cooperative Agreement Number DAMD17-97-2-7016 (NRW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The PI’s personal funds have also contributed to this work.
Financial Support
Funding was obtained from the NCMHD Project EXPORT Program P20MD001632/Project 3 (NRW), P20MD006988 and 2R25GM060507 and from a National Merit Test Bed (NMTB) award sponsored by the Department of the Army under Cooperative Agreement Number DAMD17-97-2-7016 (NRW).
Conflict of interest
All other authors declare no conflict of interest.
References
- 1.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(9):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 2.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107(2):102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Simpson RJ, Lim JWE, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Exp Rev Proteomic. 2009;6(3):267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 4.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 5.Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36:247–254. doi: 10.1385/IR:36:1:247. [DOI] [PubMed] [Google Scholar]
- 6.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 7.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128(7):1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004;31(1):114–121. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 10.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 11.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90(11):1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 12.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67(7):2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 13.Ginestra A, La Placa MD, Saladino F, Cassara D, Nagase H, Vittorelli ML. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18(5A):3433–3437. [PubMed] [Google Scholar]
- 14.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer J Int du Cancer. 2005;113(5):752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 16.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 17.Miller LK. An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol. 1999;9:323–328. doi: 10.1016/S0962-8924(99)01609-8. [DOI] [PubMed] [Google Scholar]
- 18.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 19.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7:602–608. doi: 10.1038/77929. [DOI] [PubMed] [Google Scholar]
- 21.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 22.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 23.Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197:8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 24.Khan S, Jutzy JMS, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S, De Leon M, Langridge WHR, Wall NR. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100:1073–1086. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valenzuela MMA, Castro IV, Gonda A, Osterman CD, Jutzy JMS, Aspe JR, Khan S, Neidigh JW, Wall NR (2015) Cell death in response to antimetabolites directed at ribonucleotide reductase and thymidylate synthase. Onco Targets Ther In Press [DOI] [PMC free article] [PubMed]
- 27.Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darding M, Meier P. IAPs: guardians of RIPK1. Cell Death Differ. 2012;19(1):58–66. doi: 10.1038/cdd.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulators of mitosis and apoptosis and novel targets for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosini G, Adida C, Sirugo G, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 31.Mbeunkui F, Johann DJ., Jr Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63(4):571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieuwland R, Sturk A. Why do cells release vesicles? Thromb Res. 2010;125(Suppl 1):S49–S51. doi: 10.1016/j.thromres.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 36.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 37.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 38.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 39.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM. Senescenceassociated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 42.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 43.Jutzy JM, Khan S, Asuncion-Valenzuela MM, Milford TA, Payne KJ, Wall NR. Tumor-released survivin induces a type-2 t cell response and decreases cytotoxic T cell function, in vitro. Cancer Microenviron: Off J Int Cancer Microenviron Soc. 2013;6(1):57–68. doi: 10.1007/s12307-012-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mercer TR, Dinger ME, Bracken CP, Kolle G, Szubert JM, Korbie DJ, Askarian-Amiri ME, Gardiner BB, Goodall GJ, Grimmond SM, Mattick JS. Regulated post-transcriptional RNA cleavage diversifies the eukaryotic transcriptome. Genome Res. 2010;20(12):1639–1650. doi: 10.1101/gr.112128.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3′-untranslated regions. Biol Direct. 2013;8:12. doi: 10.1186/1745-6150-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]