Abstract
Traditionally, anticancer chemotherapy has been generally considered to be strongly immunosuppressive. However, increasing evidence suggests that certain chemotherapeutic agents rely on the induction of antitumor immune responses, in both experimental animal models and patients with cancer. Many of these chemotherapeutic agents exert immunogenic effects via the induction and release of immunostimulatory “danger” signals from dying cancerous cells when used in low doses. New data suggests that several common chemotherapeutic agents may also display direct stimulating effects on immune cells even when applied in ultra-low concentrations (chemoimmunomodulation). Importantly, it is becoming clear that both immune effector cells and immune regulatory cells can be targeted by various chemotherapeutic agents to produce favorable antitumor immune responses. Therefore, utilizing cancer drugs to enhance host antitumor immunity should be considered a feasible therapeutic approach; and recent characterization of the immunomodulatory mechanisms of anticancer chemotherapy using both new and traditional cytotoxic agents suggests that combinations of these approaches with “classical” immunomodulatory agents could lead to a viable new therapeutic paradigm for the treatment of cancer.
Keywords: Chemotherapy, Low dose chemotherapy, Chemoimmunomodulation, Tumor immunoenvironment, Immunosuppression, Immunotherapy, Cancer therapy, Immune regulators
Introduction
Cancer remains the second most common cause of death in the United States, accounting for nearly 1 of every 4 deaths in this country. More than 1.6 million new cancer cases are expected to be diagnosed in 2013; and almost 600,000 Americans are projected to die of cancer, equivalent to about 1,600 people per day [1]. Since the advent of the age of systemic cancer drug therapy, treatment strategies have been dominated by the use of cytotoxic chemotherapeutic agents for the majority of cancer types. From 1948, when Farber et al. introduced aminopterin, the first chemotherapeutic agent, more than 100 such agents have come into use in clinical practice [2]. While significant advances have been made since that time, such as the development of novel classes of drugs and the use of combinatorial therapies, most drug regimens continue to be based on the traditional, maximum tolerated dose (MTD) regimen. While such a strategy has found success in the treatment of various neoplasms, MTD drug therapy is associated with significant morbidity such as myelosuppression, neurotoxicity and damage to the gut mucosa and hair follicles. Treating cancer with cytotoxic drugs is also limited by the inherent genetic instability of cancerous cells, which results in the expansion of drug-resistant cancer mutants and the acquired resistance to chemotherapeutic agents. As such, future advances in the pharmacological treatment of cancer will require an alternative strategy for targeting this group of more than 200 diseases (Table 1).
Table 1.
Examples of primary targets of chemotherapeutic approaches
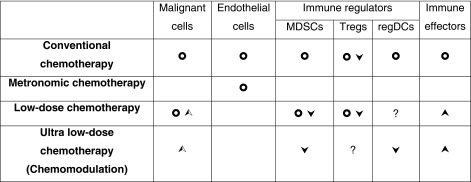
Chemotherapeutic approaches were grouped based on the reported doses in relation to Maximum Tolerated Dose (MTD). Conventional chemotherapy, ~MTD; Metronomic chemotherapy, repeated ~1/3-1/5 MTD; Low-dose chemotherapy, single or short-term ~1/3–1/10 MTD; Chemomodulation, ~1/10–1/30 MTD. Symbols:  , cell death;
, cell death;  , functional down-regulation;
, functional down-regulation;  , functional up-regulation;
, functional up-regulation;  , increased immunogenicity; ?, unproven effects
, increased immunogenicity; ?, unproven effects
Metronomic Chemotherapy
Over the past decade, a new paradigm has emerged in the pharmacological treatment of neoplastic disease termed “metronomic chemotherapy,” which involves the frequent administration of chemotherapeutic drugs at concentrations 3–10 times below the established MTD without breaks in dosing schedule for prolonged periods of time. For example, by using a dosing schedule of cyclophosphamide in the murine lung cancer and leukemia models that provided more sustained apoptosis of endothelial cells within the vascular bed of a tumor, it was shown that a chemotherapeutic agent can more effectively control tumor growth in mice, regardless of whether the tumor cells are drug resistant [3]. In the neuroblastoma xenograft model, continuous treatment with low doses of vinblastine resulted in significant xenograft regression, diminished tumor vascularity and direct inhibition of angiogenesis [4].
Such metronomic drug regimens offer the possibility to provide significant relief of the burden of disease while avoiding the significant morbidity encountered with higher dosing. The potential of metronomic chemotherapy was revealed in animal models and the efficacy of this approach has been confirmed in the clinic [5]. Although phase III evidence of the efficacy of this type of therapy is still several years away, evidence from phase II trials suggests that metronomic chemotherapy as an interesting alternative for either primary systemic therapy or maintenance therapy is safe and can be clinically beneficial in a broad range of tumors [6]. As opposed to targeting rapidly-dividing tumor cells with MTD therapy, metronomic therapy regimens primarily exert their effects on the vascular endothelial cells of the tumor, which are more sensitive to low concentrations of chemotherapeutic drugs than normal host or tumor cells [7]. When combined with angiogenesis inhibitors such as Bevacizumab (Avastin®, a recombinant humanized monoclonal antibody that neutralizes the biological activity of human VEGF), metronomic chemotherapy may have significant clinical utility even in drug-resistant cancers [8]. However, it is important to note that tumor endothelial cells (TEC) quite differ from normal endothelial cells in many parameters, including cell proliferation, migration, gene profile and responses to growth factors and several chemotherapeutic drugs [9]. TEC may be genetically abnormal and might acquire drug resistance. For instance, TEC have been shown to be resistance to paclitaxel through greater mRNA expression of multidrug resistance 1, which encodes P-glycoprotein, as compared with normal endothelial cells [10]. Interestingly, high levels of vascular endothelial growth factor in the tumor microenvironment may be responsible for upregulated P-glycoprotein expression and the acquisition of drug resistance by TEC in the tumor milieu.
Low Dose Chemotherapy
More recently, there has been increasing interest in the application of traditional chemotherapeutic agents for the purpose of manipulating the immune system to produce favorable antitumor immune responses using low-dose chemotherapy (“immunogenic” chemotherapy) [11] or even ultra-low doses of chemotherapeutic agents, a term coined “chemoimmunomodulation” [2]. It is clear that there is significant interaction between chemotherapeutic drugs, dying tumor cells, and local immune cells that have various effects on tumor immunogenicity, and many of these effects have been previously described using traditional, MTD drug regimen strategies [12]. These include both immunogenic effects, such as paclitaxel and doxorubicin enhancing antigen-specific Th1 immune responses and tumorigenic effects such as prolonged T cell and B cell depletion [13, 14]. However, recent observations suggest that low and ultra-low doses of these drugs may also exert profound effects on tumor immunogenicity. Such a strategy has the dual benefits of utilizing readily-available pharmacological agents at doses not large enough to induce significant morbidity while allowing for the potential to overcome some of the barriers to successful implementation of tumor immunotherapy, such as tumor-induced systemic and local immunosuppression that allows for immune escape [15]. For instance, it has been recently reported that while being effective as a chemotherapeutic agent, paclitaxel (Taxol) in high doses is neurotoxic, specifically targeting sensory innervations. However, low doses of paclitaxel are devoid of neuronal toxicity and thus can be safely used in a chemomodulation mode [16]. While the possible applications and mechanisms involved with using common chemotherapeutic drugs in low and ultra-low doses to produce favorable immunogenic effects have only recently begun to be described, here we briefly review the current state of this field.
Enhancement of Antigen-Presentation by Chemotherapeutic Drugs
Several studies have demonstrated that various antineoplastic drugs can indirectly activate local antigen presenting cells and increase the immunogenicity of the tumor. For example, anthracyclines and oxaliplatin exert pro-immunogenic properties by increasing preapoptotic translocation of calreticulin on the tumor cell surface, causing post-apoptotic release of the chromatin-binding protein high-mobility group B1 (HMGB1), and increasing extracellular release of adenosine triphosphate (ATP). These molecules act in concert to promote presentation of tumor antigens by dendritic cells (DCs) through activation of CD91, Toll-like receptor (TLR)-4, and purinergic P2 receptors [17–19]. Therefore, many chemotherapeutic agents (mitoxantrone, anthracyclines, oxaliplatin, and cyclophosphamide) are categorized as type I immunogenic cell death (ICD) inducers that primarily target cytosolic proteins, plasma membranes, or nucleic proteins [20]. Surface exposure of intracellular chaperones such as calreticulin, heat-shock protein 90 (HSP90) and HSP70 is crucial for the immunogenicity of dying cancer cells [21]. These chaperones have been reported to bind to various receptors on immune cells, like CD91 and certain scavenger receptors [21]. Pre-apoptotic calreticulin seems to be an important mediator of dying cell’s immunogenicity, by acting as a potent pre-apoptotic ‘eat me’ signal that assists in phagocytic uptake of dying cancer cells [22]. Moreover, calreticulin can incite the production of both IL-6 and TNF-α from DCs and facilitate Th17 polarization [23]. Similarly, HSP90 has been demonstrated to be a crucial mediator of immunogenicity [24]. During anti-cancer DC vaccination based on immunogenic cell death, HSP90 correlates well with the ability of patients to respond to vaccination [25]. The role of HSP70 in immunogenic cell death has not been strongly elucidated; however, HSP70 might favor nitric oxide (NO) production from innate immune cells [21, 25]. In terms of capacity to mediate phagocytosis of dying cells, the presence of calreticulin correlates better with increased phagocytosis of dying cancer cells than either HSP70/90 [26]. Furthermore, unlike the release of HMGB1 and ATP, calreticulin could be one of the determinants that distinguish between immunogenic and non-immunogenic cell death [17].
Recently, it has been shown that exposure of DCs to low doses of several classes of chemotherapeutic agents may also exert direct effects on their function. When administered in low concentrations, the antimicrotubule drug vinblastine has been demonstrated to induce the phenotypic and functional maturation of both human and murine DCs by increasing expression of the costimulatory molecules CD40, CD80 and CD86, as well as MHCII, IL-1 and IL-6, significantly augmenting their capacity to stimulate T cells [27]. It has also been shown that the antineoplastic chemotherapy drugs paclitaxel, doxorubicin, mitomycin C and methotrexate enhance the ability of DCs to present antigen to antigen-specific T cells when given in ultra-low, non-cytotoxic concentrations [28]. This ability to augment DC antigen presentation was shown to be mediated by autocrine or paracrine IL-12 signaling, as DCs from IL-12 knockout mice did not display this increased ability to activate T cells. It also appears that paclitaxel, methotrexate and doxorubicin given in ultra-low, non-cytotoxic concentrations may also directly augment the phagocytic ability of DCs in vitro by regulating the activity of the small Rho GTPases Rac1/2, RhoA and RhoE in murine DCs [29]. While many of the mechanisms involved in altering antigen processing and presentation are only beginning to be described, it is clear that common antineoplastic agents can be utilized in clinically-relevant ways that do not rely on their traditional cytotoxic or cytostatic mechanisms.
Several pre-clinical human studies have confirmed the effects of low-dose chemotherapeutic agents on the immune system reported in mice. For example, the effects of ultra-low noncytotoxic concentrations of different classes of chemotherapeutic agents on human DCs in vitro have recently been characterized [30]. DCs treated with antimicrotubule agents vincristine, vinblastine and paclitaxel or with antimetabolites 5-aza-2-deoxycytidine and methotrexate showed increased expression of CD83 and CD40 molecules. Expression of CD80 on DCs was also stimulated by vinblastine, paclitaxel, azacytidine, methotrexate and mitomycin C used in extra low, nontoxic concentrations. Furthermore, 5-aza-2-deoxycytidine, methotrexate and mitomycin C increased the ability of human DCs to stimulate the proliferation of allogeneic T lymphocytes. Thus, these data demonstrate that in ultra-low noncytotoxic concentrations chemotherapeutic agents do not induce apoptosis of human DCs, but directly enhance DC maturation and function. The authors concluded that modulation of human DCs by noncytotoxic concentrations of antineoplastic drugs, i.e. chemomodulation, might represent a novel approach for up-regulating the functional activity of resident DCs in the tumor microenvironment or improving the efficacy of DCs prepared ex vivo for subsequent vaccinations [30].
Of special interest is the fact that certain chemotherapeutic drugs might also alter the immunogenicity of tumor cells when used in ultra-low noncytotoxic concentrations. Recently, Kaneno et al. reported that the treatment of human tumor cell lines with ultra-low noncytotoxic concentrations of paclitaxel and doxorubicin did not induce cell death, but instead changed the immunogenicity of tumor cells by increasing expression of genes and proteins associated with antigen processing, including calmodulin, LMP2, LMP7, TAP1 and tapasin [31]. The biological significance of the modulation of antigen processing and presentation proteins in tumor cells by ultra-low nontoxic concentrations of chemotherapeutic drugs was revealed when non-treated and treated tumor cells were used as a source of tumor antigens for the generation of tumor-specific cytotoxic T cells (CTLs) in vitro. The results demonstrated that (i) DCs that engulf tumor cells pretreated with noncytotoxic concentrations of chemotherapeutic agents induced CTLs with a higher cytotoxic potential than DCs loaded with non-treated tumor cells, and (ii) CTLs induced by tumor lysate-pulsed DCs killed live tumor cells more efficiently if these tumor cells were pretreated with extra low noncytotoxic concentrations of chemotherapeutic drugs [31]. These results, thus demonstrate that chemomodulation of human tumor cells with noncytotoxic concentrations of chemotherapeutic agents increases tumor immunogenicity and results in the generation of more efficient DC vaccines and CTLs, which can be used for cell-based anticancer immunotherapies.
Low-dose chemotherapy may also favorably alter the tumor microenvironment to enhance the migration and the antitumor function of infiltrating DCs. Zhong et al. reported that administration of a single dose of low-dose paclitaxel resulted in increased local expression of monocyte chemoattractant protein 1 (MCP-1) at the tumor site [32]. With co-administration of low-dose paclitaxel followed by a DC vaccine, increases in both MCP-1 and IFN-inducible protein 10 (ID-10), as well as a decrease in IL-1α were observed at the tumor site, which was associated with significant inhibition of tumor growth. Importantly, this study showed that this pre-treatment with low-dose paclitaxel also abrogated the ability of the tumor environment to arrest the DCs in an immature state and allowed for their activation of tumor-specific T cells, as measured by IFN-γ production.
Increased development of tumor antigen-specific immunity by ultra-low doses of chemotherapeutic agents in vivo has been recently reported. For example, the effect of paclitaxel applied in ultra-low, non-cytotoxic doses (3 weekly injections) on the efficiency of immunization with the peptide derived from tyrosinase related protein (TRP)-2 as a model melanoma antigen was assessed in mice [33]. Using an IFN-γ ELISPOT assay, it was found that administration of 1 mg paclitaxel/kg in combination with the peptide vaccination strongly increased the frequencies of TRP-2 specific T cells as compared to levels due to the vaccination alone. This was associated with a significant decrease in the levels of Treg cells and immature myeloid cells. Such impairments of potential immunosuppressive cells were found to correlate with a strong increase in the amount of effector CD8+ and CD4+ T cells. Furthermore, in paclitaxel-treated mice, a significant augmentation of NK cell numbers and their ability to produce IFN-γ were observed. In addition, the level of NKT cells was also increased [33]. These data suggest that paclitaxel applied in ultra-low, non-cytotoxic doses may potentially enhance the efficacy of antitumor vaccinations by neutralizing immunosuppressive Treg and MDSC populations and stimulating immune effectors.
Treg/MDSC Modulation by Chemotherapeutic Drugs
One of primary key mechanisms by which tumors escape immune surveillance is by the local accumulation of suppressor T cells in the tumor microenvironment. This heterogeneous population includes cells from both the CD4+ and CD8+ T cell compartments, although the most prominent cell type is CD4+ and uniquely expresses the forkhead transcription factor FoxP3 and are known as regulatory T (Treg) cells [34]. These Tregs may be recruited to the tumor site or induced locally by tumor factors and exert potent immunosuppressive effects through copious production of IL-10 and transforming growth factor-β (TGF-β) leading to poor antitumor immune responses [35–37]. There is great interest in abrogating the immunosuppressive effects of these cells, as it has been shown that depletion of Treg cells has the potential to restore antitumor immunity and prevent tumor development [38, 39].
There is evidence to suggest that immunosuppressive Treg cells may be susceptible to traditional antineoplastic pharmacological agents when given in low doses. For instance, in rat glioma model, low-dose temozolomide metronomic regimens induced marked decrease of Treg cells in the spleen and tumor mass, while high-dose temozolomide regimen did not significantly modify the percentage of Tregs [40]. Interestingly, although the treatment with metronomic temozolomide reduced tumor progression when compared to untreated animals, the effect did not reach statistical significance, indicating that Treg depletion alone is not sufficient to significantly impact tumor growth in the used model of fully established tumor.
A single administration of cyclophosphamide depletes CD4+CD25+ T cells in tumor-bearing animals could delay tumor growth and cure rats bearing established tumors when followed by an immunotherapy, which had no curative effect when administered alone [41]. It was also established that low-dose cyclophosphamide not only decreased cell number but led to decreased functionality of Treg cells: cyclophosphamide treatment enhanced apoptosis and decreased homeostatic proliferation of these cells [42]. Expression of GITR and FoxP3, which are involved in the suppressive activity of Tregs, was down-regulated after cyclophosphamide administration, though the level of expression varied depending on the time studied. Others reported that the cyclophosphamide-mediated inhibition of inducible nitric oxide synthase was directly linked to its immunostimulatory effects [43]. Kan et al., have demonstrated that cyclophosphamide and gemcitabine at low concentrations is able to preferentially reduce the induction and viability of human CD4+FoxP3+ Treg cells without affecting the viability of total CD4+ T cells in vitro [44]. This is consistent with an in vivo study by Di Paolo et al. that showed that low-dose cyclophosphamide in a murine model results in a decreased number of tumor-infiltrating Treg cells [45]. Similar effects of the preferential deletion of Treg cells have been observed in cancer patients receiving gemcitabine. Rettig et al. have reported that administration of gemcitabine results in a significant reduction of the proportion FoxP3+ Treg cells within the CD4+ T cell compartment, producing a transient hyperimmunoactive state [46]. While this particular study utilized gemcitabine at standard therapeutic concentrations, it does suggest that Treg cells are preferentially susceptible to chemotherapy and warrants further study into their susceptibility to the drug at low-dose concentrations.
In addition to CD4+FoxP3+ Treg cells, another means by which the tumor microenvironment acquires an immunosuppressive state is through the activity of a heterogenous group of cells of the myeloid lineage known as myeloid-derived suppressor cells (MDSCs). MDSCs represent a group of both granulocytic and monocytic cells whose physiological maturation is arrested by factors in the tumor microenvironment and acquire an immunosuppressive phenotype through mediators such as iNOS, arginase 1, cyclooxygenase-2, prostaglandin E2, TGF-β, IL-10 and the induction of Treg cells [47]. There has been great interest in MDSCs as a therapeutic target in order to allow for increased antitumor immunity, such as with attempts to promote their differentiation or inhibit their immunosuppressive function [48, 49]. Similar to efforts utilizing low-dose chemotherapy for the depletion of Treg cells, there have been several recent studies characterizing the effects of antineoplastic drugs on MDSCs with varying conclusions [50]. A recent study by Sevko et al. showed that utilization of low-dose cyclophosphamide therapy may actually be detrimental to the establishment of antitumor immunity in melanoma [51]. Although they observed that cyclophosphamide therapy resulted in the preferential deletion of Treg cells, similar to previous studies, this therapy also enhanced the production of chronic inflammatory mediators by melanoma cells associated with an accumulation of Gr1+CD11b+MDSCs. However, in a similar study of murine colon cancer, the ability of low-dose cyclophosphamide to promote MDSC expansion was reversed when low-dose gemcitabine was concurrently administered, resulting in reduced levels of MDSCs and potent antitumor immune responses [52]. This is consistent with a study by Suzuki et al. demonstrating that gemcitabine (a single dose of 120 mg/kg) can selectively eliminate Gr1+CD11b+MDSCs in tumor-bearing mice [53]. Similar success in selectively targeting MDSCs has been achieved with a single administration of the pyrimidine analog 5-fluorouracil [54].
Importantly, the antitumor efficacy of ultra-low dose paclitaxel was recently revealed in the murine model of spontaneous melanoma, which mimics human cutaneous melanoma [55]. Administration of paclitaxel (three weekly injections) significantly decreased accumulation and immunosuppressive activities of tumor-infiltrating MDSCs associated with the inhibition of p38 MAPK activity, TNF-α production and S100A9 expression in MDSCs. Importantly, reduced tumor burden and increased animal survival upon paclitaxel injection was mediated by the restoration of CD8+ T cell effector functions [55]. This suggests that the ability of paclitaxel in ultra-low noncytotoxic dose to block the immunosuppressive potential of MDSCs in vivo represents a new therapeutic strategy to down-regulate immunosuppression in the tumor microenvironment for enhancing the efficacy of concomitant anticancer therapies. Using an in vitro model system, several potential mechanisms of the direct effect of paclitaxel on MDSCs were tested, which might be responsible for the antitumor potential of low-dose paclitaxel therapy in mice [56]. It was hypothesized that a decreased level of MDSC in vivo after paclitaxel administration might be due to (i) the blockage of MDSC generation, (ii) an induction of MDSC apoptosis, or (iii) the stimulation of MDSC differentiation. The results revealed that paclitaxel in ultra-low concentrations neither increased MDSC apoptosis nor blocked MDSC generation, but stimulated MDSC differentiation towards DCs.
It is also important to mention that some chemotherapeutic agents, e.g., paclitaxel, have been reported to prevent polarization of conventional DCs into immunosuppressive regulatory DCs (regDCs). Based on the phenotypic and functional analysis of DCs, it was shown that paclitaxel blocked cDCs→regDCs polarization in mice when used in extra low doses in vivo or extra low concentrations in vitro [57–59]. These new data not only revealed an important tumor supporting function of immunosuppressive/tolerogenic DCs in cancer, but demonstrated that regDCs could be successfully targeted by noncytotoxic noncytostatic doses of chemotherapeutic drugs.
Together, these results support a new concept that certain chemotherapeutic agents in ultra-low noncytotoxic doses may suppress tumor progression by targeting several immune regulatory cell populations in the tumor microenvironment, including MDSCs, regDCs and Treg cells. Thus, low-dose chemotherapy/chemomodulation is a promising strategy to rescue the tumor immunoenvironment from the tumorigenic conditions imposed by various immunosuppressive cell types and restore immunity.
Elevation of Cellular Cytotoxicity by Chemotherapeutic Drugs
There is considerable evidence that effective antitumor immunity can be established with a T helper type 1 (Th1) and CD8+ cytotoxic T cells and that these effector cells are strongly associated with increased patient survival in several human tumors [60–62]. As such, many of the efforts at designing tumor immunotherapies have focused on the expansion or activation of these cell types, but unfortunately, with a very few exceptions (e.g., CAR-modification T cells), these have thus far been met with limited success. However, it has been demonstrated that there may also be utility in using low doses of various chemotherapeutic drugs to enhance the effects of the various immunotherapy strategies that are currently being considered.
In a study using recombinant lentiviruses (rLVs) to induce antigen-specific T cells to tumor antigens, Sierro et al. showed that despite being able to prime larger numbers of tumor-specific T cells by utilizing rLV, they were unable to mount an immunogenic response in a murine model. However, low-dose cyclophosphamide given to the mice was able to enhance rLV vaccine efficacy and antitumor immunity [63]. Similarly, the combination of low-dose 5-fluorouracil with an adenoviral based tumor vaccine was shown to provide a synergistic improvement in survival in a murine model [64]. In using a different approach to harness T cell based immunity, Li et al. have also demonstrated in a study using bispecific antibodies to recruit T cells to antibody-target-specific tumor cells that T cell cytotoxicity is enhanced both in vitro and in vivo with the administration of low concentrations of cytarabine [65]. These T cells were found to express higher levels of CD25 and CD69 and released an increased level of IL-2.
There may also be a role for low doses of common chemotherapeutic drugs in enhancing the cytotoxic activity of other effector immune cells, such as NK cells or γδT cells. It has been shown that human myeloma cells treated with low concentrations of doxorubicin or melphalan increase their expression of NKG2D and DNAM-1 ligands, leading to increased NK cell degranulation [66]. In a recent study aimed at targeting colon “cancer stem cells” (CSCs), low concentrations of 5-fluorouracil and doxorubicin were able to sensitize cancer stem cells to cytotoxic killing by Vγ9Vδ2 T cells in vitro, an effect mediated by the TRAIL apoptotic pathway [67]. These results may be especially significant due to the well-described resistance of CSCs to both radio- and chemotherapy, suggesting that low-dose chemotherapy combined with γδT cell-based therapy may hold potential as a clinically-effective means to overcome the limitations of targeting CSCs with conventional treatment modalities.
Conclusions
Both the direct and indirect effects of traditional chemotherapeutic drugs given in standard, MTD concentrations on the immune system and tumor environment have been well described. However, recent research efforts present compelling evidence that suggests that these same drugs given in low or ultra-low doses may also hold clinical utility through their ability to promote antitumor immunity [2]. If successful, employing these drugs in such a manner would allow for prevention of the significant morbidity associated with current chemotherapy regimens as well as potentially abrogate the potential for the development of tumor resistance. Moreover, the availability and established safety profiles of these drugs would allow for rapid translation into clinical practice. However, although anticancer efficacy of conventional chemotherapies seems to be due, in part, to augmentation of the host immune reactivity, a comprehensive analysis of immunomodulating activities of chemotherapeutic agents is still required: a new study recently revealed that two common chemotherapeutic agents, gemcitabine and 5-fluorouracil, could also activate immune regulatory cells, which stimulated the emergence of protumorigenic cytokines via inflammasome pathways, limiting the antitumor efficacy of the drugs [68]. The results of these studies have revealed that gemcitabine and 5-fluorouracil, in addition to depleting immunosuppressive MDSCs, also induce the release of cathepsin B from lysosomes and the activation of the NLRP3 inflammasome and caspase-1, which causes IL-1β secretion from MDSCs, resulting in IL-17 production by T cells and promotion of tumor growth. The importance of these new data is in providing, probably for the first time, a mechanism-based, rather than empirical, rationale for combination of specific chemotherapeutic agents with specific immunotherapeutic approaches for cancer treatment: IL-1 receptor antagonist was shown to enhance the antitumor effect of 5-fluorouracil [69]. Finally, while the concept of chemoimmunomodulation using ultra-low doses of various antineoplastic drugs remains in its infancy, and many of the mechanisms of their action remain to be described, there is compelling experimental evidence that several classes of chemotherapeutic drugs administered in extra low concentrations have effects on several aspects of the immune system relevant to tumor immune surveillance and therapy.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Shurin MR, Naiditch H, Gutkin DW, Umansky V, Shurin GV. ChemoImmunoModulation: immune regulation by the antineoplastic chemotherapeutic agents. Curr Med Chem. 2012;19:1792–1803. doi: 10.2174/092986712800099785. [DOI] [PubMed] [Google Scholar]
- 3.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 4.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 6.Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U (2013) Low-dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer 49:3387–3395 [DOI] [PubMed]
- 7.Ademuyiwa FO, Miller KD. Incorporation of antiangiogenic therapies in the treatment of metastatic breast cancer. Clin Breast Cancer. 2008;8(Suppl 4):S151–S156. doi: 10.3816/CBC.2008.s.011. [DOI] [PubMed] [Google Scholar]
- 8.Barber EL, Zsiros E, Lurain JR, Rademaker A, Schink JC, Neubauer NL. The combination of intravenous bevacizumab and metronomic oral cyclophosphamide is an effective regimen for platinum-resistant recurrent ovarian cancer. J Gynecol Oncol. 2013;24:258–264. doi: 10.3802/jgo.2013.24.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hida K, Ohga N, Akiyama K, Maishi N, Hida Y (2013) Heterogeneity of tumor endothelial cells. Cancer Sci 104:1391–1395 [DOI] [PMC free article] [PubMed]
- 10.Hida K, Akiyama K, Ohga N, Maishi N, Hida Y. Tumour endothelial cells acquire drug resistance in a tumour microenvironment. J Biochem. 2013;153:243–249. doi: 10.1093/jb/mvs152. [DOI] [PubMed] [Google Scholar]
- 11.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 12.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–2572. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eralp Y, Wang X, Wang JP, Maughan MF, Polo JM, Lachman LB. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004;6:R275–R283. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells. 2000;18:10–18. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ustinova EE, Shurin GV, Gutkin DW, Shurin MR. The role of TLR4 in the paclitaxel effects on neuronal growth in vitro. PLoS ONE. 2013;8:e56886. doi: 10.1371/journal.pone.0056886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 18.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 19.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 20.Inoue H, Tani K (2013) Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. doi:10.1038/cdd.2013.84 [DOI] [PMC free article] [PubMed]
- 21.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C, Ullrich E, de Botton S, Zitvogel L, Kroemer G. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 23.Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spisek R, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle (Georgetown Tex) 2007;6:1962–1965. doi: 10.4161/cc.6.16.4601. [DOI] [PubMed] [Google Scholar]
- 25.Garg AD, Martin S, Golab J, Agostinis P (2013) Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ. doi:10.1038/cdd.2013.48 [DOI] [PMC free article] [PubMed]
- 26.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Matsushima H, Mizumoto N, Takashima A. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res. 2009;69:6978–6986. doi: 10.1158/0008-5472.CAN-09-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J Immunother. 2008;31:491–499. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneno R, Shurin GV, Kaneno FM, Naiditch H, Luo J, Shurin MR. Chemotherapeutic agents in low noncytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol (Dordr) 2011;34:97–106. doi: 10.1007/s13402-010-0005-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, Shurin GV. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13:5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sevko A, Kremer V, Falk C, Umansky L, Shurin MR, Shurin GV, Umansky V. Application of paclitaxel in low non-cytotoxic doses supports vaccination with melanoma antigens in normal mice. J Immunotoxicol. 2012;9:275–281. doi: 10.3109/1547691X.2012.655343. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 35.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol. 2013;4:190. doi: 10.3389/fimmu.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 39.Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70:7800–7809. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 40.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 42.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 43.Loeffler M, Kruger JA, Reisfeld RA. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 2005;65:5027–5030. doi: 10.1158/0008-5472.CAN-05-0646. [DOI] [PubMed] [Google Scholar]
- 44.Kan S, Hazama S, Maeda K, Inoue Y, Homma S, Koido S, Okamoto M, Oka M. Suppressive effects of cyclophosphamide and gemcitabine on regulatory T-cell induction in vitro. Anticancer Res. 2012;32:5363–5369. [PubMed] [Google Scholar]
- 45.Di Paolo NC, Tuve S, Ni S, Hellstrom KE, Hellstrom I, Lieber A. Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rettig L, Seidenberg S, Parvanova I, Samaras P, Curioni A, Knuth A, Pascolo S. Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int J Cancer. 2011;129:832–838. doi: 10.1002/ijc.25756. [DOI] [PubMed] [Google Scholar]
- 47.Khaled YS, Ammori BJ, Elkord E (2013) Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol 91:493–502 [DOI] [PubMed]
- 48.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, Liu A, Wang TC, Yang CS. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 2012;5:205–215. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naiditch H, Shurin MR, Shurin GV. Targeting myeloid regulatory cells in cancer by chemotherapeutic agents. Immunol Res. 2011;50:276–285. doi: 10.1007/s12026-011-8213-2. [DOI] [PubMed] [Google Scholar]
- 51.Sevko A, Sade-Feldman M, Kanterman J, Michels T, Falk CS, Umansky L, Ramacher M, Kato M, Schadendorf D, Baniyash M, Umansky V. Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J Invest Dermatol. 2013;133:1610–1619. doi: 10.1038/jid.2012.444. [DOI] [PubMed] [Google Scholar]
- 52.Tongu M, Harashima N, Monma H, Inao T, Yamada T, Kawauchi H, Harada M. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother. 2013;62:383–391. doi: 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 54.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 55.Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, Shurin GV, Shurin MR, Umansky V. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190:2464–2471. doi: 10.4049/jimmunol.1202781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol. 2012;9:292–300. doi: 10.3109/1547691X.2011.642418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Y, Shurin GV, Gutkin DW, Shurin MR. Tumor associated regulatory dendritic cells. Semin Cancer Biol. 2012;22:298–306. doi: 10.1016/j.semcancer.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shurin GV, Ma Y, Shurin MR. Immunosuppressive mechanisms of regulatory dendritic cells in cancer. Cancer Microenviron. 2013;6:159–167. doi: 10.1007/s12307-013-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shurin GV, Ouellette CE, Shurin MR. Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother. 2012;61:223–230. doi: 10.1007/s00262-011-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 61.Fridman WH, Galon J, Dieu-Nosjean MC, Cremer I, Fisson S, Damotte D, Pages F, Tartour E, Sautes-Fridman C. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344:1–24. doi: 10.1007/82_2010_46. [DOI] [PubMed] [Google Scholar]
- 62.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 63.Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, Romero P. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217–2228. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- 64.Geary SM, Lemke CD, Lubaroff DM, Salem AK. The combination of a low-dose chemotherapeutic agent, 5-Fluorouracil, and an adenoviral tumor vaccine has a synergistic benefit on survival in a tumor model system. PLoS ONE. 2013;8:e67904. doi: 10.1371/journal.pone.0067904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Yang M, Fan D, Yan Y, Shi R, Cheng J, Li Z, Zhang M, Wang J, Xiong D (2013) Cytosine arabinoside promotes cytotoxic effect of T cells on leukemia cell mediated by bispecific antibody. Hum Gene Ther 24:751–760 [DOI] [PMC free article] [PubMed]
- 66.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 67.Todaro M, Orlando V, Cicero G, Caccamo N, Meraviglia S, Stassi G, Dieli F. Chemotherapy sensitizes colon cancer initiating cells to Vgamma9Vdelta2 T cell-mediated cytotoxicity. PLoS ONE. 2013;8:e65145. doi: 10.1371/journal.pone.0065145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 69.Shurin MR. Dual role of immunomodulation by anticancer chemotherapy. Nat Med. 2013;19:20–22. doi: 10.1038/nm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]


