A novel method of odontoblast differentiation from induced pluripotent stem (iPS) cells using gene transfection is described. Mouse iPS cell-derived neural crest-like cells (iNCLCs) were generated and iNCLCs were differentiated into odontoblast-like cells by transfection of Pax9 and Bmp4 expression plasmids. The findings suggest an interaction between exogenous Pax9- and Bmp4-induced signaling modulated Dmp1 and Dspp expression. iPS cell-derived odontoblast-like cells could be a useful cell source for tooth regeneration.
Keywords: Induced pluripotent stem cells, Differentiation, Transcriptional factors, Cytokines
Abstract
The field of tooth regeneration has progressed in recent years, and human tooth regeneration could become viable in the future. Because induced pluripotent stem (iPS) cells can differentiate into odontogenic cells given appropriate conditions, iPS cells are a potential cell source for tooth regeneration. However, a definitive method to induce iPS cell-derived odontogenic cells has not been established. We describe a novel method of odontoblast differentiation from iPS cells using gene transfection. We generated mouse iPS cell-derived neural crest-like cells (iNCLCs), which exhibited neural crest markers. Next, we differentiated iNCLCs into odontoblast-like cells by transfection of Pax9 and Bmp4 expression plasmids. Exogenous Pax9 upregulated expression of Msx1 and dentin matrix protein 1 (Dmp1) in iNCLCs but not bone morphogenetic protein 4 (Bmp4) or dentin sialophosphoprotein (Dspp). Exogenous Bmp4 upregulated expression of Msx1, Dmp1, and Dspp in iNCLCs, but not Pax9. Moreover, cotransfection of Pax9 and Bmp4 plasmids in iNCLCs revealed a higher expression of Pax9 than when Pax9 plasmid was used alone. In contrast, exogenous Pax9 downregulated Bmp4 overexpression. Cotransfection of Pax9 and Bmp4 synergistically upregulated Dmp1 expression; however, Pax9 overexpression downregulated exogenous Bmp4-induced Dspp expression. Together, these findings suggest that an interaction between exogenous Pax9- and Bmp4-induced signaling modulated Dmp1 and Dspp expression. In conclusion, transfection of Pax9 and Bmp4 expression plasmids in iNCLCs induced gene expression associated with odontoblast differentiation, suggesting that iNCLCs differentiated into odontoblast-like cells. The iPS cell-derived odontoblast-like cells could be a useful cell source for tooth regeneration.
Significance
It has been reported that induced pluripotent stem (iPS) cells differentiate into odontogenic cells by administration of recombinant growth factors and coculture with odontogenic cells. Therefore, they can be potential cell sources for tooth regeneration. However, these previous methods still have problems, such as usage of other cell types, heterogeneity of differentiated cells, and tumorigenicity. In the present study, a novel method to differentiate iPS cells into odontoblast-like cells without tumorigenicity using gene transfection was established. It is an important advance in the establishment of efficient methods to generate homogeneous functional odontogenic cells derived from iPS cells.
Introduction
The treatment of congenital teeth absence or tooth loss from periodontitis and caries typically involves implantation of prosthetics made from artificial biomaterials. Recently, significant progress has been made in the study of rodent tooth regeneration [1–5], with regeneration of teeth in humans now potentially viable. However, several barriers remain, including establishing a suitable cell source that requires minimal donor invasion and overcomes ethical issues.
Induced pluripotent stem (iPS) cells are established by introducing Oct4, Sox2, c-Myc, and Klf4 into somatic cells [6]. Because iPS cells can differentiate into odontogenic cells by administration of recombinant growth factors and direct or indirect coculture with odontogenic cells [7–9], they represent a potential cell source for tooth regeneration. However, these methods still have problems, such as usage of other cell types, the heterogeneity of differentiated cells, and tumorigenicity. Takayama et al. [10] succeeded in inducing differentiation of human iPS and embryonic stem cells into nearly homogenous functional hepatocytes by transfection of critical transcription factors for hepatocyte differentiation. Their report indicated that transfection of differentiation-associated genes is a powerful tool to induce differentiation of iPS cells. However, application of transfection to differentiate iPS cells into odontogenic cells has not been accomplished.
Tooth morphogenesis is regulated by sequential and reciprocal interactions between many molecules, including cytokines and transcription factors from the epithelium and mesenchyme [11, 12]. Pax9 induces tooth development via upregulation of bone morphogenetic protein 4 (Bmp4) and Msx1 expression [13, 14]. Furthermore, a deficiency of Pax9 in mice arrests tooth development at the bud stage [15]. In turn, Bmp4 regulates expression of both Pax9 and Msx1 in dental mesenchyme [16, 17], with deficiency of Bmp4 leading to arrested molar development at the bud stage [18]. These findings indicate that Pax9 and Bmp4 are essential factors for odontogenic differentiation and led us to hypothesize that induction of these molecules could increase the odontogenic differentiation potential of iPS cells.
We describe a novel method of differentiating odontoblast-like cells from iPS cells. We first generated mouse iPS cell-derived neural crest-like cells (iNCLCs) as precursors of odontoblasts. We further examined whether transfection of Pax9 and Bmp4 into iNCLCs will promote differentiation into odontoblast-like cells.
Materials and Methods
Materials and methods are described in the supplemental online data.
Results and Discussion
Generation and Characterization of iNCLCs From Mouse iPS Cells
The establishment of differentiated cells from iPS cells is often achieved by mimicking natural developmental procedures. Odontoblasts differentiate from dental mesenchymal cells, which are derived from neural crest cells [11]. Otsu et al. [7] demonstrated the differentiation potential of iNCLCs into dental mesenchymal cells by means of combination cultures with dental epithelium. Therefore, it appears that iNCLCs can be a good model for odontoblast differentiation from iPS cells. We generated iNCLCs from mouse iPS cells, which showed a stellate morphology similar to that of neural crest cells [19] (supplemental online Fig. 1A). Furthermore, iNCLCs expressed the neural crest markers Pax3, Snai1, Snai2, p75NTR, and HNK-1 (supplemental online Fig. 1B–1E). These results indicate that our iNCLCs possessed features of neural crest cells.
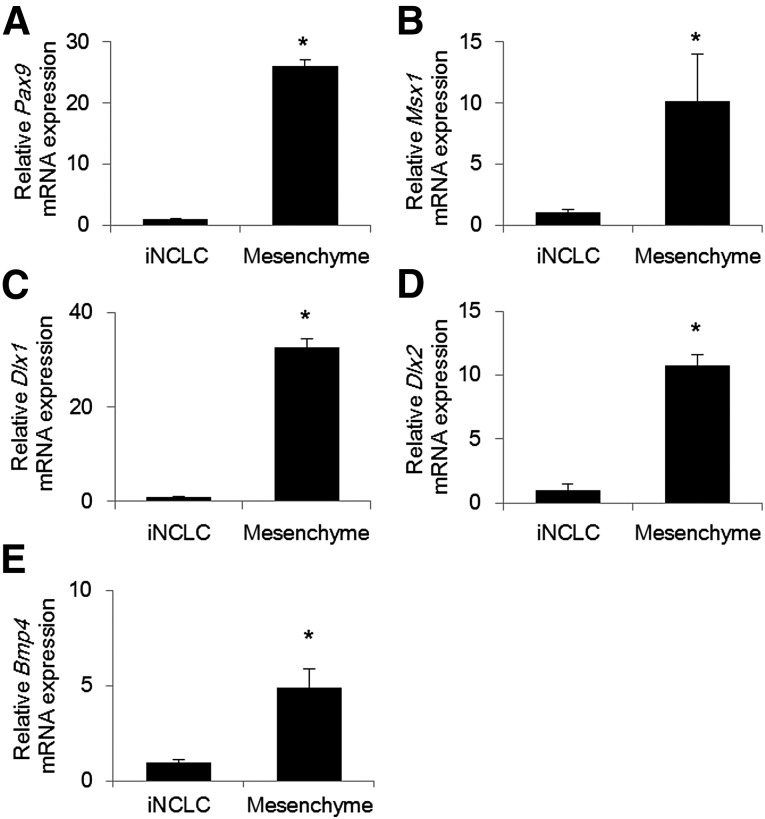
Dental mesenchymal cells express Pax9, Msx1, Dlx1, Dlx2, and Bmp4. These molecules regulate odontoblast differentiation during tooth development [12, 20]. Therefore, we evaluated the gene expression of these molecules to assess the odontogenic properties of iNCLCs. The expression levels of Pax9, Msx1, Dlx1, Dlx2, and Bmp4 were all significantly lower than the levels of dental mesenchymal cells (Fig. 1A–1E; supplemental online Fig. 2A–2F). These results suggest that the induction of the molecules associated with odontoblast differentiation is required for iNCLCs to acquire odontogenic properties.
Figure 1.
Expression of molecules associated with odontoblast differentiation in iNCLCs. Second passage iNCLCs were cultured for 2 days, and the expression of Pax9 (A), Msx1 (B), Dlx1 (C), Dlx2 (D), and Bmp4 (E) was assessed by real-time polymerase chain reaction (∗, p < .01; n = 3). Mesenchymal cells isolated from E14.5 murine tooth germs were used as control. Abbreviation: iNCLC, induced pluripotent stem cell-derived neural crest-like cell.
Overexpression of Pax9 Upregulates Gene Expression Associated With Odontoblast Differentiation in iNCLCs
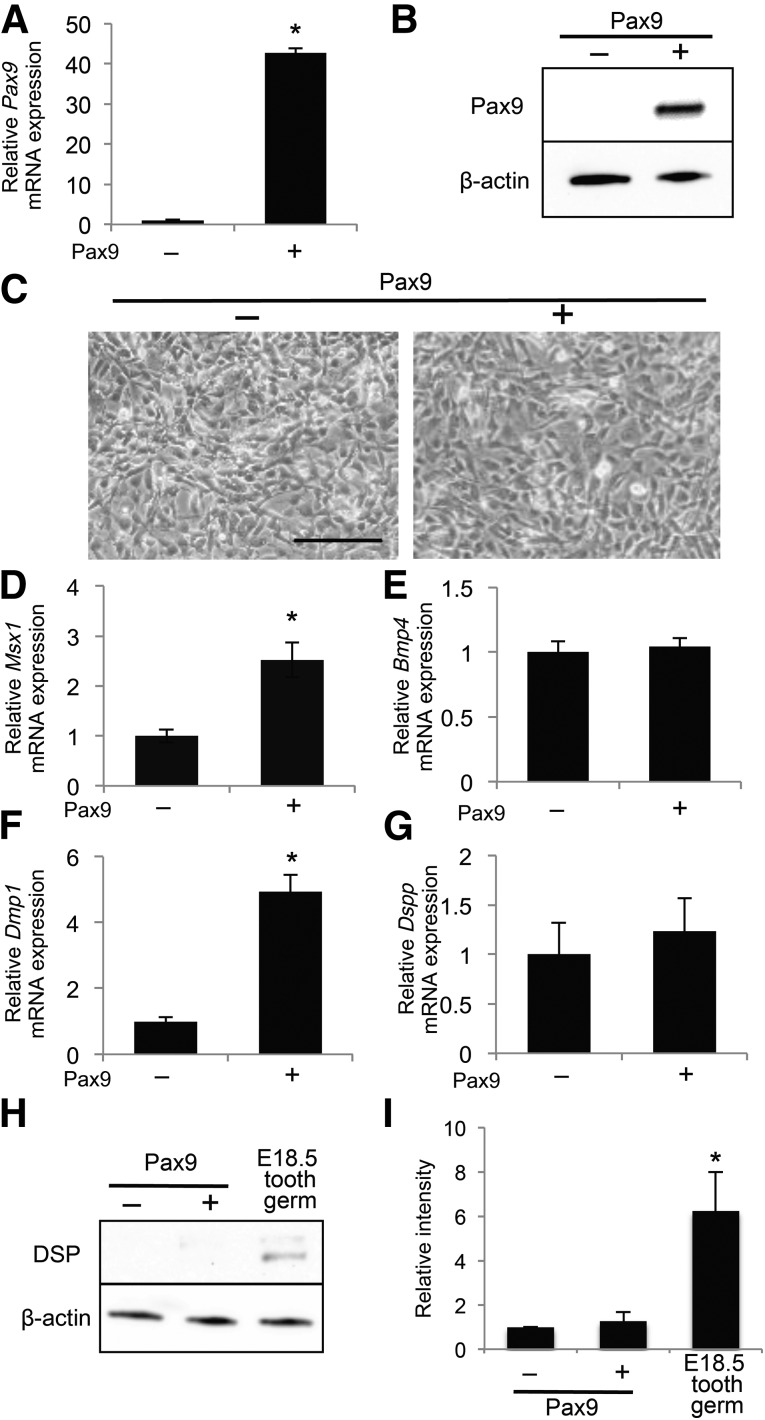
Pax9 is a critical modulator of tooth development expressed by dental mesenchymal cells [15]. Therefore, we examined the effects of exogenous Pax9 expression on the odontoblast differentiation of iNCLCs. Transfection of a Pax9 expression plasmid into iNCLCs increased Pax9 mRNA and protein levels (Fig. 2A, 2B). Pax9-overexpressing iNCLCs did not display any obvious morphological changes compared with the control (Fig. 2C). Pax9-overexpressing iNCLCs did not show significant changes in cell number or expression of proliferation markers (supplemental online Fig. 3A–3E). Pax9 upregulates Msx1 and Bmp4 promoter activity, suggesting it directly induces expression of both factors [14]. However, in the present study, exogenous Pax9 upregulated expression of Msx1 but not Bmp4 (Fig. 2D, 2E). These results suggest that exogenous Pax9 activates Msx1 signaling, with downstream targets possibly regulating differentiation of iNCLCs into odontoblast-like cells. Because nuclear factor interleukin-3 (Nfil3) inhibits Bmp4 promoter activity in osteoblast-like MC3T3-E1 cells [21], it could also negatively regulate Bmp4 transcription in iNCLCs. Supplemental online Figure 4A shows that Nfil3 was increased by exogenous Pax9, suggesting that Nfil3 might diminish Bmp4 transcription in Pax9-overexpressing iNCLCs. Exogenous Pax9 did not affect the expression of other molecules that regulate Bmp4 transcription (supplemental online Fig. 4B–4F). Dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (Dspp) are extracellular matrix proteins produced by odontoblasts [22]. Expression of Dmp1 and Dspp is detectable in murine tooth germs from embryonic days 13 and 14, respectively [22]. These findings suggest that Dmp1 is expressed in more immature odontoblasts compared with Dspp. Exogenous Pax9 upregulated Dmp1 expression but did not significantly affect expression of Dspp and dentin sialoprotein (DSP), which is the product of Dspp (Fig. 2F–2I). Therefore, our data suggest that Pax9-overexpressing iNCLCs differentiate into more immature odontoblasts.
Figure 2.
Overexpression of Pax9 upregulates gene expression associated with odontoblast differentiation in induced pluripotent stem cell-derived neural crest-like cells (iNCLCs). A Pax9 expression plasmid was electroporated into second passage iNCLCs followed by 2 days of culture. Pax9 expression was assessed by real-time polymerase chain reaction (PCR) (A) and Western blot (B); ∗, p < .01; n = 3. (C): Representative phase-contrast micrographs of control and Pax9-transfected iNCLCs. Scale bar = 100 μm. Expression levels of Msx1 (D), Bmp4 (E), Dmp1 (F), and Dspp (G) in Pax9-overexpressing iNCLCs were determined using real-time PCR; ∗, p < .01; n = 3. (H): Expression of DSP was analyzed by Western blot. Total protein from dissected tooth germs of embryonic day 18.5 mice was used as a positive control. (I): Quantification of the DSP protein level relative to β-actin; ∗, p < .01; n = 3. Abbreviation: DSP, dentin sialoprotein.
Exogenous Bmp4 Induces Gene Expression Associated With Odontoblast Differentiation in iNCLCs
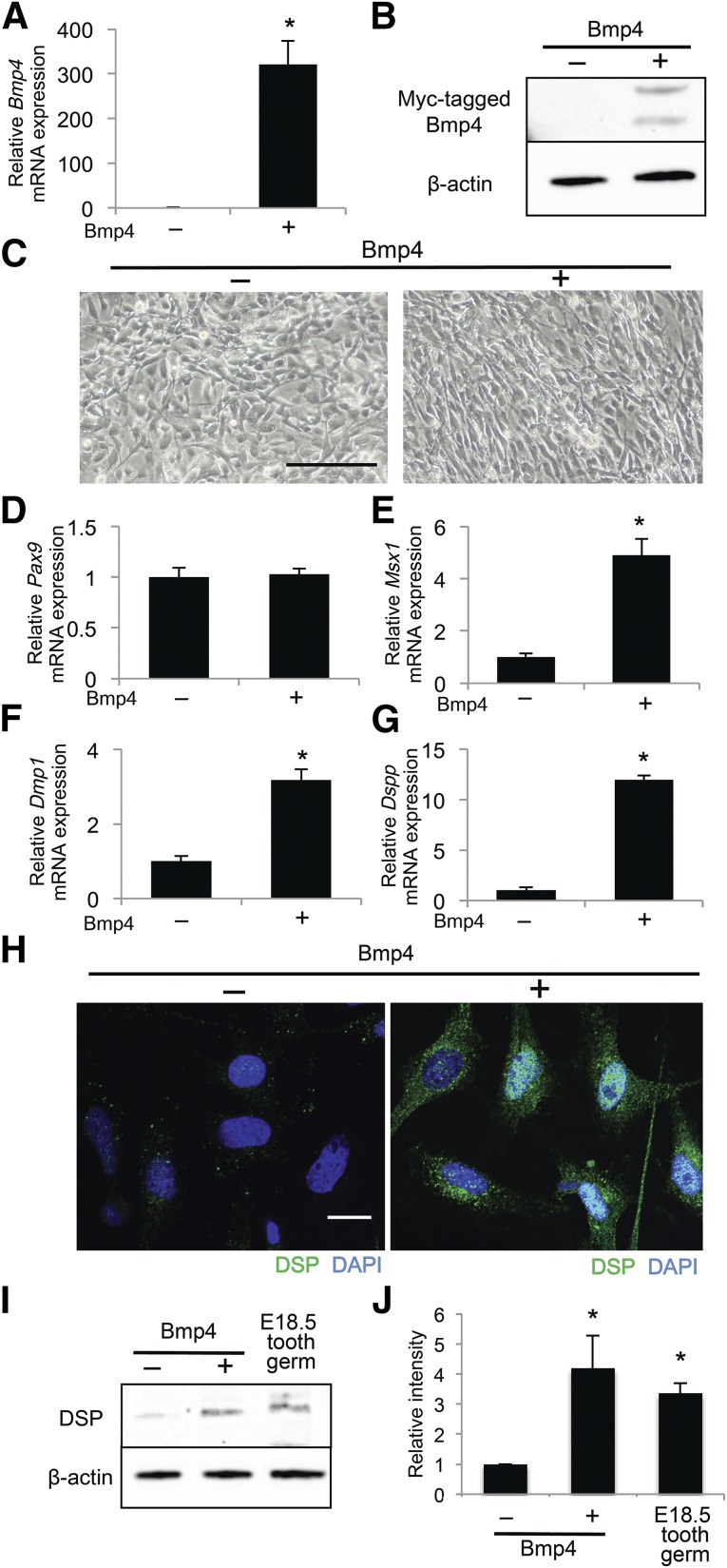
We next examined the effects of exogenous Bmp4 on the odontoblast differentiation of iNCLCs. Transfection of a Bmp4 expression plasmid into iNCLCs increased Bmp4 mRNA and protein expression (Fig. 3A, 3B). Both iNCLCs overexpressing Bmp4 and those administered recombinant Bmp4 demonstrated a fibroblast-like spindle shape similar to that of dental mesenchymal cells [23] (Fig. 3C; supplemental online Fig. 5A). Bmp4-overexpressing iNCLCs did not show significant changes in cell number or the expression of proliferation markers (supplemental online Fig. 3A–3E). Exogenous Bmp4 did not alter Pax9 expression but did induce Msx1, Dlx1, and Runx2 expression in iNCLCs (Fig. 3D, 3E; supplemental online Fig. 5B–5E). Additionally, exogenous Bmp4 significantly upregulated Dmp1, Dspp, and DSP expression (Fig. 3F–3J; supplemental online Fig. 5F, 5G). Maurin et al. analyzed microtubule-associated protein 1B (MAP1B) expression before and after odontoblast differentiation of dental pulp cells, resulting in an increase of MAP1B expression in the differentiated cells [24]. In the present study, MAP1B expression was significantly increased in Bmp4-overexpressing iNCLCs compared with iNCLCs and Pax9-overexpressing iNCLCs (supplemental online Fig. 6A, 6B). Taken together, these findings suggest that exogenous Bmp4 induces more matured odontoblast differentiation of iNCLCs.
Figure 3.
Exogenous Bmp4 induces gene expression associated with odontoblast differentiation in induced pluripotent stem cell-derived neural crest-like cells (iNCLCs). A Bmp4 expression plasmid was electroporated into second passage iNCLCs followed by 2 days of culture. Bmp4 expression was assessed by real-time polymerase chain reaction (PCR) (A) and Western blot (B); ∗, p < .01; n = 3. (C): Representative phase-contrast micrographs of control and Bmp4-transfected iNCLCs. Scale bar = 100 μm. Expression levels of Pax9 (D), Msx1 (E), Dmp1 (F), and Dspp (G) in Bmp4-overexpressing iNCLCs were determined by real-time PCR; ∗, p < .01; n = 3. (H): The expression of DSP (green) in control and Bmp4-transfected iNCLCs was analyzed by immunofluorescence. Nuclei are stained with DAPI (blue). Scale bar = 20 μm. (I): Expression of DSP was analyzed by Western blot. Total protein from dissected tooth germs of embryonic day 18.5 mice was used as a positive control. (J): Quantification of the DSP protein level relative to β-actin; ∗, p < .01; n = 3. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; DSP, dentin sialoprotein.
Pax9- and Bmp4-Overexpressing iNCLCs Do not Have Tumorigenicity
Because iPS cells possess the risk of tumorigenicity [6], we examined the tumorigenicity of iNCLCs and Pax9- and Bmp4-overexpressing iNCLCs. Transplantation of iPS cells induced teratoma formation (supplemental online Fig. 7A, 7E). However, iNCLCs and the transfected iNCLCs did not form teratomas (supplemental online Fig. 7B–7D, 7F–7H). Pluripotency markers, Oct3/4, Nanog, and Lin28, were rarely expressed in iNCLCs or the transfected iNCLCs (supplemental online Fig. 7L–7N). These results suggest that odontoblast-like cells derived from iNCLCs are safe cell sources for tooth regeneration.
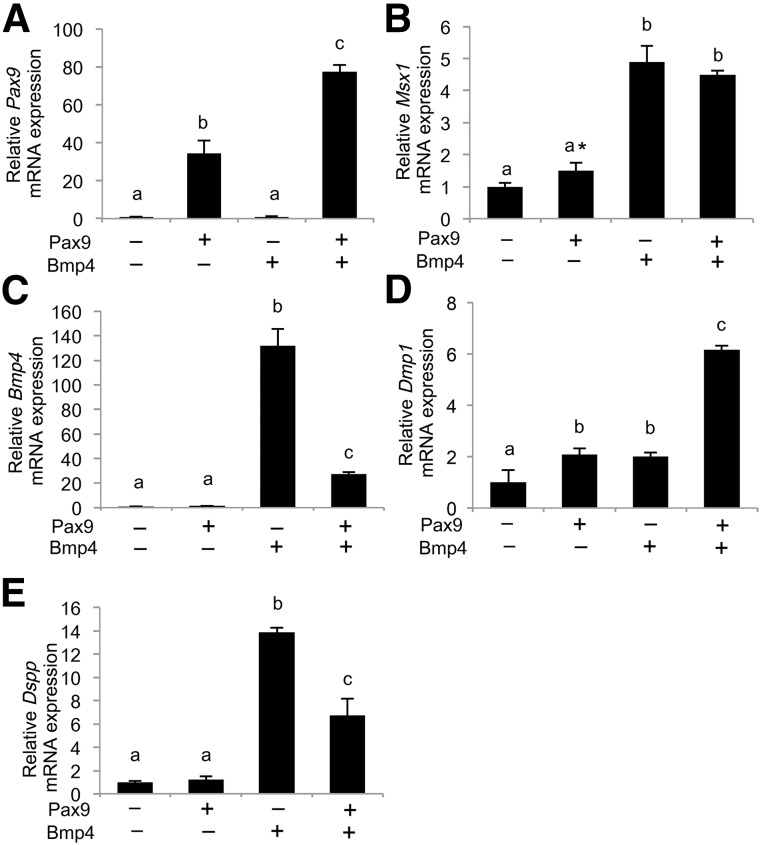
Combined Overexpression of Pax9 and Bmp4 Alters Gene Expression Associated With Odontoblast Differentiation in iNCLCs
We cotransfected Pax9 and Bmp4 expression plasmids into iNCLCs to analyze the combined effect of these factors. Cotransfection of Pax9 and Bmp4 plasmids resulted in higher expression of Pax9 than in iNCLCs overexpressing Pax9 alone (Fig. 4A). Pax9 overexpression did not affect exogenous Bmp4-induced Msx1 expression (Fig. 4B). Conversely, exogenous Pax9 downregulated Bmp4 overexpression, although Bmp4 expression was still higher than in that in the control (Fig. 4C). These data suggest that Pax9 and Bmp4 interact in iNCLCs. Combined overexpression of Pax9 and Bmp4 synergistically upregulated Dmp1 expression (Fig. 4D). However, Pax9 overexpression downregulated exogenous Bmp4-induced Dspp expression, although the expression was still higher than that in the control (Fig. 4E). These results suggest that the interaction of exogenous Pax9 and Bmp4-induced signaling in iNCLCs positively and negatively regulated Dmp1 and Dspp expression, respectively. Deshpande et al. [25] have demonstrated distinct differences in the effects of Dmp1 and dentin phosphoprotein, a proteolytic cleavage product of Dspp, on collagenous mineralization. Therefore, our results suggest that the cotransfection of Pax9 and Bmp4 regulates Dmp1 and Dspp expression, resulting in modulation of the mineralization function of odontoblast-like cells derived from iPS cells.
Figure 4.
Combined overexpression of Pax9 and Bmp4 alters gene expression associated with odontoblast differentiation in induced pluripotent stem cell-derived neural crest-like cells (iNCLCs). Pax9 and Bmp4 expression plasmids were cotransfected into second passage iNCLCs by electroporation followed by 2 days of culture. Expression levels of Pax9 (A), Msx1 (B), Bmp4 (C), Dmp1 (D), and Dspp (E) were determined by real-time polymerase chain reaction. The different letters (a, b, and c) indicate significant differences between the groups. Only in (B), asterisk represents significant difference between the control and Pax9 groups evaluated by Student’s t test; p < .01; n = 3.
Conclusion
We have demonstrated for the first time that transfection of Pax9 and Bmp4 each and cotransfection of them in iNCLCs induced gene expression associated with odontoblast differentiation, suggesting that iNCLCs differentiated into odontoblast-like cells. Our results have revealed that induction of iPS cell-derived odontoblasts by gene transfection is possible, and these cells could be a useful cell source for tooth regeneration. However, the molecular mechanisms underlying the induction of these odontoblastic cells from iNCLCs remain unknown. Therefore, the establishment of efficient methods to generate odontogenic cells derived from iPS cells requires further molecular analyses.
Supplementary Material
Acknowledgments
This study was supported by grants from the Japan Society for the Promotion of Science (to T.T.-Y.) and in part from the Japan Science and Technology Agency (to T.T.-Y.).
Author Contributions
D.S.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; N.T.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; T.O., S.S., I.T., and M.H.: collection and/or assembly of data, final approval of manuscript; T.T.-Y.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Duailibi MT, Duailibi SE, Young CS, et al. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 2.Hu B, Nadiri A, Kuchler-Bopp S, et al. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12:2069–2075. doi: 10.1089/ten.2006.12.2069. [DOI] [PubMed] [Google Scholar]
- 3.Nakao K, Morita R, Saji Y, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda E, Morita R, Nakao K, et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci USA. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oshima M, Mizuno M, Imamura A, et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE. 2011;6:e21531. doi: 10.1371/journal.pone.0021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Otsu K, Kishigami R, Oikawa-Sasaki A, et al. Differentiation of induced pluripotent stem cells into dental mesenchymal cells. Stem Cells Dev. 2012;21:1156–1164. doi: 10.1089/scd.2011.0210. [DOI] [PubMed] [Google Scholar]
- 8.Ozeki N, Mogi M, Kawai R, et al. Mouse-induced pluripotent stem cells differentiate into odontoblast-like cells with induction of altered adhesive and migratory phenotype of integrin. PLoS ONE. 2013;8:e80026. doi: 10.1371/journal.pone.0080026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Arakaki M, Ishikawa M, Nakamura T, et al. Role of epithelial-stem cell interactions during dental cell differentiation. J Biol Chem. 2012;287:10590–10601. doi: 10.1074/jbc.M111.285874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayama K, Inamura M, Kawabata K, et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Mol Ther. 2012;20:127–137. doi: 10.1038/mt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trainor PA. Neural Crest Cells: Evolution, Development and Disease. San Diego, CA: Academic Press; 2013. [Google Scholar]
- 12.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Gao Y, Lan Y, et al. Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development. 2013;140:4709–4718. doi: 10.1242/dev.099028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa T, Kapadia H, Feng JQ, et al. Functional consequences of interactions between Pax9 and Msx1 genes in normal and abnormal tooth development. J Biol Chem. 2006;281:18363–18369. doi: 10.1074/jbc.M601543200. [DOI] [PubMed] [Google Scholar]
- 15.Peters H, Neubüser A, Kratochwil K, et al. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vainio S, Karavanova I, Jowett A, et al. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 17.Neubüser A, Peters H, Balling R, et al. Antagonistic interactions between FGF and BMP signaling pathways: A mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 18.Jia S, Zhou J, Gao Y, et al. Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development. 2013;140:423–432. doi: 10.1242/dev.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loring J, Glimelius B, Erickson C, et al. Analysis of developmentally homogeneous neural crest cell populations in vitro. I. Formation, morphology and differentiative behavior. Dev Biol. 1981;82:86–94. doi: 10.1016/0012-1606(81)90430-9. [DOI] [PubMed] [Google Scholar]
- 20.Miletich I, Sharpe PT. Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today. 2004;72:200–212. doi: 10.1002/bdrc.20012. [DOI] [PubMed] [Google Scholar]
- 21.Hirai T, Tanaka K, Togari A. α1-Adrenergic receptor signaling in osteoblasts regulates clock genes and bone morphogenetic protein 4 expression through up-regulation of the transcriptional factor nuclear factor IL-3 (Nfil3)/E4 promoter-binding protein 4 (E4BP4) J Biol Chem. 2014;289:17174–17183. doi: 10.1074/jbc.M113.546135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Souza RN, Cavender A, Sunavala G, et al. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- 23.Feng XY, Zhao YM, Wang WJ, et al. Msx1 regulates proliferation and differentiation of mouse dental mesenchymal cells in culture. Eur J Oral Sci. 2013;121:412–420. doi: 10.1111/eos.12078. [DOI] [PubMed] [Google Scholar]
- 24.Maurin JC, Couble ML, Staquet MJ, et al. Microtubule-associated protein 1b, a neuronal marker involved in odontoblast differentiation. J Endod. 2009;35:992–996. doi: 10.1016/j.joen.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Deshpande AS, Fang PA, Zhang X, et al. Primary structure and phosphorylation of dentin matrix protein 1 (DMP1) and dentin phosphophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules. 2011;12:2933–2945. doi: 10.1021/bm2005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.