This study summarizes the current findings concerning the critical role of CD44/CD44v in regulation of cancer stemness and the research status of CD44/CD44v as biomarkers and therapeutic targets in cancer. This study also discusses the current challenges and future directions that may lead to best use of CD44/CD44v for clinical applications.
Keywords: CD44, Cancer stem cell, Biomarker, Therapeutic target, Splicing variants, Tumor microenvironment
Abstract
The reception and integration of the plethora of signals a cell receives from its microenvironment determines the cell’s fate. CD44 functions as a receptor for hyaluronan and many other extracellular matrix components, as well as a cofactor for growth factors and cytokines, and thus, CD44 is a signaling platform that integrates cellular microenvironmental cues with growth factor and cytokine signals and transduces signals to membrane-associated cytoskeletal proteins or to the nucleus to regulate a variety of gene expression levels related to cell-matrix adhesion, cell migration, proliferation, differentiation, and survival. Accumulating evidence indicates that CD44, especially CD44v isoforms, are cancer stem cell (CSC) markers and critical players in regulating the properties of CSCs, including self-renewal, tumor initiation, metastasis, and chemoradioresistance. Furthermore, there is ample evidence that CD44, especially CD44v isoforms, are valuable prognostic markers in various types of tumors. Therefore, therapies that target CD44 may destroy the CSC population, and this holds great promise for the cure of life-threatening cancers. However, many challenges remain to determining how best to use CD44 as a biomarker and therapeutic target. Here we summarize the current findings concerning the critical role of CD44/CD44v in the regulation of cancer stemness and the research status of CD44/CD44v as biomarkers and therapeutic targets in cancer. We also discuss the current challenges and future directions that may lead to the best use of CD44/CD44v for clinical applications.
Significance
Mounting evidence indicates that cancer stem cells (CSCs) are mainly responsible for cancer aggressiveness, drug resistance, and tumor relapse. CD44, especially CD44v isoforms, have been identified as CSC surface markers for isolating and enriching CSCs in different types of cancers. The current findings concerning the critical role of CD44/CD44v in regulation of cancer stemness and the research status of CD44/CD44v as biomarkers and therapeutic targets in cancer are summarized. The current challenges and future directions that may lead to best use of CD44/CD44v for clinical applications are also discussed.
Introduction
Despite recent progress in cancer therapy and increased knowledge of tumor biology, cancer remains a very common and lethal disease worldwide. Cancer-associated mortality is primarily caused by cancer recurrence and metastasis. Recent advances in cancer stem cell (CSC) research have indicated that CSCs, a type of cancer cell that can self-renew and differentiate into multiple cell types, are responsible for tumor initiation, recurrence, and metastasis [1, 2]. Therapies that specifically target CSCs hold great promise for improving survival and quality of life for cancer patients.
Solid tumors are regarded as “organs” that are made up of cancer cells and the tumor microenvironment. The tumor microenvironment is the cellular environment in which the tumor exists, including the extracellular matrix (ECM), mesenchymal stem cells, endothelial cells, and signaling molecules such as growth factors and cytokines. The tumor and the surrounding microenvironment are interacting constantly. The special microenvironment surrounding CSCs is called the CSC niche, and this microenvironment largely governs the cellular fate of CSCs [3–5]. (CD44, a multistructural and multifunctional transmembrane glycoprotein, is a receptor for hyaluronan (also called hyaluronic acid [HA]), a major component of the ECM, and a coreceptor for many growth factors and cytokines. CD44 has attracted considerable attention because of its important functions in mediating cell-cell and cell-matrix interactions and association with malignant process, particularly in cancer dissemination [6, 7]. Recent work has revealed that CD44 is the most common CSC surface marker and plays a pivotal role in CSCs in communicating with the microenvironment and regulating CSC stemness properties. Increasing evidence suggests that CD44, or more specifically, CD44v is a promising prognostic biomarker and therapeutic target for many cancers. Although much progress has been made in our understanding of the molecular structures and functions of CD44 and its various isoforms, most of them are provided by normal stem cells and cancer cells in general rather than by CSCs, and thus, many challenges are still ahead.
Molecular Structure and Functional Basis of CD44 and Its Splicing Variants
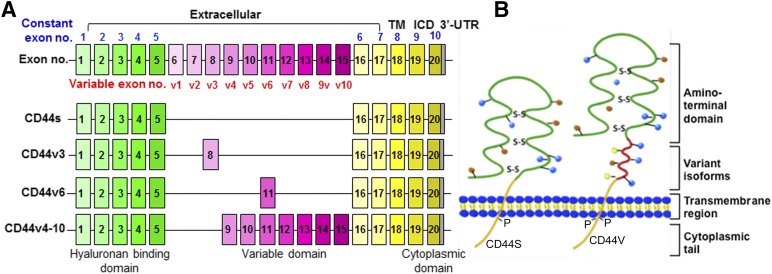
CD44 is encoded by the highly conserved CD44 gene on chromosome 11 in humans [8]. The full-length CD44 gene consists of 20 exons and 19 introns. Ten of these exons are expressed in all isoforms (known as “constant” exons). The ten central exons (known as “variable” exons) undergo extensive alternative splicing via excision or inclusion in various combinations in the membrane-proximal stem region to generate splicing variants (CD44v isoforms), which accounts for the heterogeneity of this protein family (Fig. 1A). The smallest or standard isoform (CD44s) lacking all variant exons in the extracellular domains, is expressed on most vertebrate cells, whereas CD44v isoforms are expressed only on some cells under specific conditions. Most strikingly, CD44v isoforms are expressed in a variety of cancers, particularly those in advanced stages [9].
Figure 1.
Diagrammatic structure of CD44 gene and protein. (A): Schematic structures of CD44 gene and pre-mRNAs. CD44 consists of several exons (top panel); some are constant region exons that are used in every CD44 mRNA and protein (yellow boxes), and others are variant exons (pink boxes) that are used in the CD44 splicing variant (CD44v) mRNAs and proteins (lower three panels), whereas the standard CD44 (CD44s) does not contain any variant exon. (B): CD44 protein structural domains. The CD44 protein is composed of an extracellular link domain; a stalk-like region in the extracellular domain close to the transmembrane region, where the variant exon products (red) are inserted; the TM; and the ICD. Abbreviations: ICD, intracellular cytoplasmic domain; TM, transmembrane region; UTR, untranslated region.
CD44 protein consists of three functional domains, including an extracellular domain (or ectodomain), a transmembrane (TM) domain, and an intracellular domain (ICD) (Fig. 1B) [8]. The first five exons of CD44 encode an amino-terminal globular protein domain containing motifs that function as ligand-binding receptors primarily for HA, an anionic, nonsulfated glycosaminoglycan that is one of the major components of the ECM [10]. Other CD44 ligands include the ECM components collagen, osteopontin, integrin, fibronectin, laminin, matrix metalloproteinases (MMPs), and others [11, 12]. The N-terminal globular domain of the CD44s isoform is separated from the plasma membrane by a short stem structure, which contains putative proteolytic cleavage sites [13].
The ectodomain is the most diverse region of the CD44 molecule. Sequences encoded by exons v1–v10 in different combinations through alternative splicing are inserted at a single site in the stem region [8]. The introduction of new exons into this region has been found to modulate HA-binding affinity by inducing conformational changes or allowing CD44 isoforms to have a new function as a coreceptor by generating new binding sites for many growth factors, receptors, and nonreceptor protein-tyrosine kinases; thus, the CD44v isoforms are involved in specific signaling pathways (see Table 3 in [12]). For example, the sequence encoded by exon v3 contains a heparin-sulfate site, which allows CD44v3 to bind several heparan sulfate-binding growth factors such as fibroblast growth factors (FGFs) and heparin-binding epidermal growth factor (EGF), whereas the sequence encoded by exon v6 contains a binding site for hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) [12, 14, 15]. It has been shown that the binding of HGF with CD44v6 induces CD44v6/HGF/cMet complex formation, leading to c-Met or HGF-induced Ras signaling activation [16, 17]. The inclusion of variant exons has been found to be dependent, at least in part, on mitogenic or oncogenic signals that regulate alternative splicing [18–20, 21]. Therefore, cancer cells often express a large variety of CD44 variants, particularly when the cancer is in an advanced stage.
The CD44 TM domain is a single-pass TM domain that provides a platform for CD44/CD44v oligomer formation or coupling to cofactors, adaptor proteins, receptor tyrosine kinases, or nonreceptor protein-tyrosine kinases to mediate CD44 activation signaling [22]. CD44-ICD is also crucial for the function of CD44 in signaling transduction. CD44-ICD contains a nuclear localization signal that is located immediately following the TM domain. Similar to the generation of Notch-ICD, CD44-ICD can be cleaved from the membrane by the presenilin-γ-secretase and then translocated into the nucleus, where it can transcriptionally regulate the expression of a number of genes through the formation of a complex with CBP/p300 or stat3 [23]. The target genes that CD44-ICD regulates include CD44, cyclin D1, MMP-9, HIF-2α, c-myc, and Twist1 [23–26]. Binding of the cytoskeletal proteins ankyrin and ERM (ezrin, radixin, and moesin) to the cytoplasmic tail of CD44 links CD44 to the actin-cytoskeleton and remodels the actin cytoskeleton with functions involved in the regulation of epithelial-mesenchymal transition (EMT), HA-dependent cell adhesion and motility, and cell migration and invasion [27]. The recruitment of ERM proteins to the cytoplasmic tail of CD44v6 is required to mediate cMet/HGF-dependent Ras signaling activation [16, 17, 28]. In contrast, the tumor suppressor protein Merlin (also called neurofibromin 2), which is related to the ERM family, also binds to the ERM-binding motif in the cytoplasmic tail of CD44 [27], but this binding seems to inhibit CD44 interaction with HA, leading to a tumor-suppressor function rather than an actin cross-linking function [29].
The complexity of the CD44 protein family and functions is further increased by post-translational modifications, including N- and O-linked glycosylation, phosphorylation, sulfation, and domain cleavage. For example, the proinflammatory cytokine tumor necrosis factor-α (TNF-α) was found to convert CD44 from its inactive, nonbinding form to its active form by inducing the sulfation of CD44 [30]. Membrane-bound CD44 can be cleaved by membrane-associated metalloproteases such as MT1-MMP, ADAM10, or MMP-9 at the membrane-proximal region of the ectodomain. This cleavage is required for CD44-ECM interactions during cell migration [31, 32], and the cleaved CD44 ectodomain may prevent attachment of cells to ECM or inhibit tumor growth by interfering with the membrane-bound CD44.
Cell Surface Marker and Multifunctional Roles of CD44 in CSCs
CD44 Acts as a Cell Surface Marker of CSCs
Recently, it was found that CD44 expression is associated with increased potential for tumor initiation and progression. For example, in a gastric cancer mouse model of K19-Wnt1/C2Me (Gan mouse, in which both Wnt and prostaglandin E2 signaling pathways are activated in the gastric mucosa), CD44+/+ animals developed large gastric tumors and became moribund around 30–50 weeks of age [33], and increased CD44 gene expression (mostly of variant isoforms CD44v8–10) was associated with gastric tumorigenesis. In contrast, CD44−/− Gan mice developed much smaller tumors with histologically confirmed hyperplastic lesions [33], suggesting that CD44 expression is required for gastric cancer initiation and progression. Similarly, in a mouse model of glioma, CD44+/+ animals developed significantly more high-grade gliomas than CD44+/− and CD44−/− mice and had much shorter survival durations [25]. All of these results clearly link CD44 to cancer stemness features.
Significantly, CD44 has been identified as a typical CSC surface marker, individually or in combination with other marker(s) such as CD24, CD133, and CD34, for enriching CSCs in various types of cancer (Table 1). For example, initially, Al-Hajj et al. [34] identified and isolated the tumorigenic cells as CD44+CD24−/lowLineage− in breast cancer tissues from eight of nine patients. As few as 100 cells with this phenotype were able to form tumors in immunocompromised mice, whereas tens of thousands of cells carrying alternate phenotypes failed to form tumors. The tumorigenic subpopulation could be serially passaged, and the new tumors generated in each round of passage contain additional CD44+CD24−/lowLineage− tumorigenic cells, as well as the phenotypically diverse mixed populations of nontumorigenic cells. This was the first demonstration that a subset of CD44+/CD24−/low cells is prospective breast CSCs. Later on, gastric cancer-initiating cells were identified from a panel of human gastric cancer cell lines using cell surface marker CD44. CD44+ cells showed spheroid colony formation in serum-free medium in vitro, as well as tumorigenic ability when injected into stomach and skin of severe combined immunodeficient (SCID) mice in vivo. The CD44+ gastric cancer cells showed the stem cell properties of self-renewal and the ability to form differentiated progeny and gave rise to CD44− cells. Also, the CD44+ gastric cancer cells showed increased resistance for chemotherapy- or radiation-induced cell death. On the contrary, CD44 knockdown resulted in much reduced spheroid colony formation and smaller tumor production in SCID mice, and the CD44− populations had significantly reduced tumorigenic ability in vitro and in vivo, whereas other potential CSC markers, such as CD24, CD133, CD166, stage-specific embryonic antigen-1 (SSEA-1), and SSEA-4, or sorting for side population did not show any correlation with tumorigenicity in vitro or in vivo. These results support the existence of gastric CSCs and identified CD44 as a reliable cell surface marker for gastric CSCs [35]. Recently, stem-like glioma cells have also been enriched experimentally based on the expression of CD44 [25]. More convincingly, the functional role of CD44 as a CSC marker was demonstrated by CD44 direct transcriptional reprogramming of CD44− colon cancer cells to CD44+ stem-like cancer cells [23, 36], whereas CD44 downregulation had the opposite effect in head and neck cancer cells [37].
Table 1.
CD44 as a cancer stem cell marker in various cancer types

Given that many CD44v isoforms are preferentially expressed on cancer cells and required for tumorigenesis and progression, CD44v isoforms presumably might be better CSC markers than the CD44s isoform. Experiments using knockin mice expressing either CD44v4–10 or CD44s isoform have demonstrated that CD44v isoform, but not the CD44s isoform, promote adenoma initiation in Apc(Min/+) mice [38]. Supporting this conclusion are findings that CD44v6 predicts poor prognosis and is a marker of constitutive and reprogrammed CSCs driving colon cancer metastasis [39], as well as findings that CD44v8–10 is a specific marker for gastric CSCs [40] and CD44v3 is a specific CSC marker of head and neck cancers [41, 42].
CD44 Integrates Environmental and Cellular Signaling Critical for Regulation of Cancer Stemness
The stem cell niche surrounding CSCs provides a regulatory microenvironment for CSCs, allowing them to regenerate the most tumor cells while maintaining self-renewal potential [3, 5, 12]. The molecular structure of CD44 functions as a receptor for HA and other ECM components, enabling CSCs to sense environmental changes and mediate signaling transduction to regulate CSC stemness properties. For example, binding of CD44 to HA and other ECM molecules such as osteopontin, the main component of metastatic niche, has been shown to activate the Nanog-Stat3, Oct4-Sox2-Nanog, or c-Src kinase signaling pathways, leading to upregulation of miR-21 or downregulation of miR-203 [43, 44]. Consequently, CD44 binding regulates CSC survival, self-renewal, maintenance, and chemoresistance [26, 44–46], which at least in part explains why CD44 is critical for disseminated cancer cells to adapt to new environments and why CD44 is required for metastatic colonization [3, 12, 47, 48].
CSCs are regulated by complex interactions with components of the tumor microenvironment, in which growth factors and cytokines play pivotal roles in intercellular communication. Acting as a coreceptor for many growth factors and cytokines produced by cells in the tumor microenvironment, including EGF, FGF, HGF, VEGF, transforming growth factor β (TGF-β), and MMPs, CD44 mediates the growth factor or cytokine signaling preferentially transduced into CD44-positive tumor cells to stimulate CSC self-renewal and promote tumor cell invasion and metastasis. The essential role of growth factors in regulating CSCs has been demonstrated by the fact that EGF and FGF are required as essential supplements for in vitro spheroid culture and enrichment of various types of CSCs. It has been demonstrated that CD44v3 functions as a coreceptor for FGF and VEGF, and CD44v6 functions as a coreceptor for EGF and HGF by forming a complex with related receptors to potentiate receptor tyrosine kinase-mediated or CD44-mediated signaling critical for CSC stemness [11, 49].
Hypoxia is one of the fundamental biological phenomena associated with a variety of solid tumors. The hypoxic microenvironment has been shown to maintain glioblastoma stem cells and promote reprogramming toward a CSC phenotype, in which hypoxia-induced HIF-2α expression is essential only in CSCs, and multiple HIF-regulated genes such as such as OCT4, NANOG, and c-MYC are preferentially expressed in CSCs compared with differentiated tumor cells [50]. The importance of HIF-2α was further supported by findings that forced expression of nondegradable HIF-2α induced a CSC-like phenotype and augmented tumorigenic potential in a nonstem population and that HIF-2α colocalized with CSC markers in tumor specimens [51, 52]. Mechanistically, osteopontin-CD44 signaling was found to regulate HIF-2α expression via the γ-secretase-regulated CD44-ICD in a CBP/p300-dependent mechanism in glioma, which promoted aggressive glioma growth in vivo and stem cell-like phenotypes [25]. In contrast, hypoxia-induced HIF-1α expression, which is primarily responsible for upregulating glycolytic genes and promoting angiogenesis [50], was found to upregulate CD44 and variant CD44v6 and CD44v7/8 expression [53], suggesting that CD44 plays a central role in signaling regulation circuits for the maintenance of cancer stemness under hypoxic conditions.
CD44 Acts as a Critical Regulator of EMT
Recent evidence shows that tumor cells that undergo EMT acquire stem cell-like properties and metastatic potential [54, 55]. HA binding to CD44 induces EMT, whereas blockage of HA synthesis reduces EMT and metastasis formation [56], and the aggressiveness of breast cancer cells with an EMT phenotype can be inhibited by CD44-specific antibodies [57]. Moreover, a recent study elegantly demonstrated the critical role of CD44 and EMT in relation to stem-like properties, in which gastric epithelial cells were cocultured with a cagA-positive Helicobacter pylori strain. CagA oncoprotein has been demonstrated to be responsible for a particular cell phenotype in vitro, the “hummingbird” phenotype, which corresponds to an elongation of the cells, mimicking EMT. Cell-sorting experiments showed that only the cells with high expression of CD44 induced by H. pylori infection displayed the mesenchymal phenotype and CSC properties in vitro, and these cells had higher tumorigenic properties than cells with low CD44 expression in mouse xenografts [58].
TGF-β is a ubiquitous cytokine that is often elevated in the tumor microenvironment. TGF-β elicits tumor-promoting effects through its ability to induce EMT and increase the number of CSCs, and CSC phenotypes can be abrogated by the novel TGF-β-targeting peptides [59]. It has been found that TGF-β receptor type I (RI) contains a CD44-binding site. The binding of HA to CD44 induces a complex between CD44 and TGF-βRI and stimulates TGF-βRI serine/threonine kinase activity, which in turn increases Smad2/Smad3 phosphorylation and activates downstream signaling pathways. More interestingly, TGF-βRI kinase activated by HA phosphorylates CD44, which enhances the interaction of CD44 with the cytoskeletal protein ankyrin, thus potentiating HA-CD44 signaling [60]. Functionally, in the setting of CD44s overexpression, treatment with TGF-β1 induced the mesenchymal phenotype in hepatocellular carcinoma cells, which was characterized by low E-cadherin and high vimentin expression. Loss of CD44s inhibited TGF-β-mediated vimentin expression, mesenchymal spindle-like morphology, and tumor invasiveness [61]. TNF-α, a common cytokine in the tumor microenvironment, was found to upregulate CD44v3 and CD44v6 expression through the JNK or p38 pathway and resulted in increased migration ability of breast cancer cells in vitro [62]. Similarly, TNF-α was found to up-regulate CD44 and, more significantly, CD44v expression in and promote migration, invasion, and EMT phenotype of clear cell renal cell carcinomas [63].
CD44 Acts as a Critical Regulator of ROS Metabolism in CSCs
In adult stem cells and CSCs, low reactive oxygen species (ROS) levels have been associated with the formation of a proliferation-permissive intracellular environment and with perseverance of self-renewal capacities [64, 65]. CSCs possess enhanced mechanisms of protection from stress induced by ROS. One of the significant characteristics of cancer cells differentiating them from normal cells is that cancer cells use glycolysis to produce ATP regardless of local availability of molecular oxygen (the Warburg effect), and pyruvate kinase isoenzyme type M2 (PKM2) plays a critical role in this process [66, 67]. It has been found that the PKM2 expression and low PKM2 activity promote the conversion of pyruvate to lactate and the flow of glycolytic intermediates into biosynthesis for the generation of reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) [67]. NADPH provides the reducing equivalents for biosynthetic reactions and the oxidation reduction involved in protecting the cell against the toxicity of ROS, allowing the regeneration of reduced glutathione (GSH). Expression of CD44, especially CD44v isoforms, contributes to ROS defense through two different mechanisms. First, CD44 (CD44-ICD) interacts with PKM2 and suppresses PKM2 activity by increasing PKM2 phosphorylation, thereby promoting the glycolytic pathway, leading to antioxidant status (increased GSH, reduced ROS) in CSCs [68]. Second, CD44v isoforms interact with and stabilize xCT, a subunit of the cystine-glutamate transporter xc ([minus]), thereby promoting cystine uptake for GSH synthesis [33]. Thus, CD44 and CD44v isoforms protect CSCs often exposed to high levels of ROS in the tumor microenvironment.
Clinical Significance of CD44 in Cancer
CD44 as a Valuable Biomarker
Given that overexpression of CD44, particularly CD44v isoforms, is common in various cancers, tremendous efforts have been focused on studying the diagnostic and prognostic value of CD44 and CD44v isoforms in cancer. Ample evidence now suggests that both CD44s and CD44v isoforms can be valuable diagnostic or prognostic markers in many cancers (Table 2). For example, a recent study showed that among 290 patients with gastric cancer, the overall survival rate was significantly higher in those whose tumors did not express CD44 than in those with tumors expressing CD44, and CD44 expression was an independent prognostic factor in gastric cancer [69]. Similarly, in a meta-analysis of 16 published studies with a total of 2,403 patients with gastric cancer, CD44 expression was associated with TNM stage, lymph node, and distant metastasis and reduced overall survival rates [70]. CD44 has also been found to function as a prognostic marker in many other tumors, including lung, colorectal, breast, hepatocellular, head, neck, and hypopharyngeal squamous cell carcinoma. CD44 expression predicts local recurrence after radiotherapy in laryngeal cancer, and the presence of CD44-positive circulating tumor cells is an independent predictor of recurrence for gastric cancer (Table 1).
Table 2.
CD44 as a prognostic marker in various cancer types
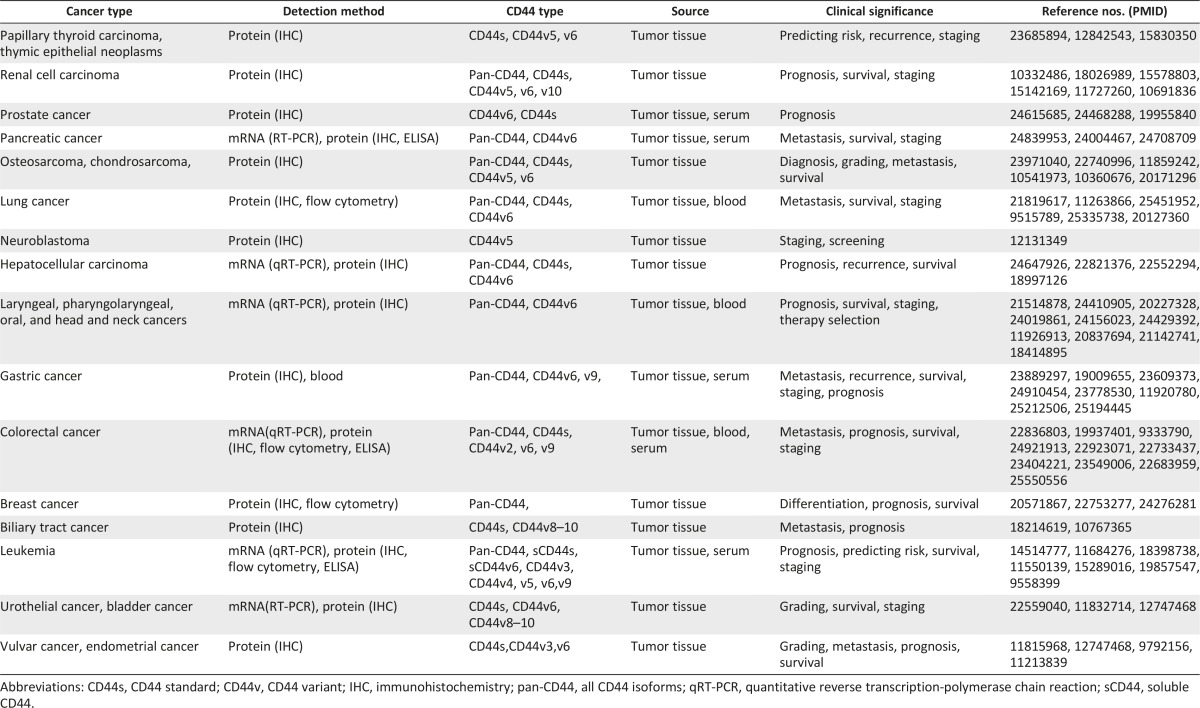
Because CD44v is more specifically expressed in CSCs than CD44, the prognostic value of CD44v isoforms has drawn much attention. A recent study showed that CD44v6 is a marker of constitutive and reprogrammed CSCs driving colon cancer metastasis, and low levels of CD44v6 predict increased probability of survival [39]. Consistently, CD44v6 is associated with poor prognosis in several cancers, including prostate cancer, pancreatic cancer, non-small cell lung cancer, uterine adenocarcinoma, gastric cancer, acute myeloid leukemia, esophageal squamous cell carcinoma, and laryngeal cancer (Table 2). Furthermore, CD44v2, CD44v3, CD44v5, CD44v9, CD44v10, and CD44v8–10 have also been reported to have prognostic value in different cancers (Table 2). For example, CD44v9 expression in primary early gastric cancer is a predictive marker for recurrence [71], and the presence of CD44v9-positive circulating cancer cells is strongly associated with refractory disease, recurrence, and poor survival rates in colorectal cancer [72].
However, the prognostic value of CD44s and CD44v isoforms seems to vary by cancer type, and expression of CD44s does not always indicate an increased likelihood of tumor promotion. One report showed that the loss of CD44s expression in cancer cells in the deepest invaded area was a good marker for predicting potential metastasis to the lymph nodes and liver in colorectal cancer with invasion into the subserosal layer. A meta-analysis including a total of 3,098 patients showed that CD44 is not a prognostic marker in colorectal cancer in terms of 5-year overall survival rates, histologic grade, and metastasis, whereas CD44v6 is associated with increased severity of histologic grade and poor overall survival [73]. Similar observations were made in non-small cell lung cancer [74]. However, another report showed that patients with colorectal cancer with high expression of CD44v2 had a poorer prognosis than patients with other CD44v isoforms [75]. The discrepancy in prognostic value between CD44s and CD44v isoforms among different reports and in different tumor types awaits further clarification.
CD44 as a Therapeutic Target
The multifunctional roles of CD44 in the cross-talk with the tumor microenvironment and in the regulation of cancer stemness, as well as the demonstrated prognostic value of CD44 in various cancers, indicate that targeting CD44 is a promising approach with the potential to eliminate CSCs [76]. However, therapeutic design by targeting CD44 should consider (a) the existence of the CD44s on many normal cells, (b) the expressing levels of CD44s/CD44v on tumor cells, and (c) the resemblance between CD44s and other molecules like lyve 1 [77]. Up to now, various CD44 targeting strategies have been developed (see Table 1 in [76] and Fig. 2 in [78]), among which targeting tumor specific CD44v strategies have been extensively studied.
Antibody-Based Strategies Against CD44
Over the past several years, various monoclonal antibodies and antibody constructs against CD44 or CD44v isoforms have been demonstrated to exhibit significant antitumor activity in vitro and in preclinical animal models of human xenograft tumors. H90 is a mouse monoclonal antibody directed against human CD44. In vivo administration of this antibody to immune-deficient mice transplanted with human acute myelogenous leukemia markedly reduced leukemic repopulation. The absence of leukemia in serially transplanted mice demonstrated that this monoclonal antibody directly targets acute myelogenous leukemic stem cells with mechanisms involved in inducing terminal differentiation and inhibiting engraftment, homing, and self-renewal [79]. This study was the first to demonstrate that CD44 is a key regulator of leukemic stem cell function that is essential for proper homing of the cells to microenvironmental niches and for maintaining the cells in a primitive state. Another exciting finding is that administration of the human CD44 monoclonal antibody p245 significantly reduced tumor growth in xenograft mice bearing human triple-negative basal-like breast cancer. Furthermore, tumor relapse, mediated by the residual CD44-positive breast CSCs, occurred 4–6 weeks after complete histologic regression resulting from combination chemotherapy, and this relapse could be effectively prevented when p245 was systemically administrated during the tumor regression period [80]. The results from this study further support the concept that CD44 plays a critical role in CSCs.
On the basis of promising preclinical antitumor studies, bivatuzumab, a humanized monoclonal antibody against CD44v6, labeled with 186Re or conjugated with the toxin mertansine, was first selected for clinical trials in patients with incurable squamous cell carcinoma of the head and neck or esophagus [81]. These trials showed that bivatuzumab had promising antitumor effects. However, the studies were abruptly ended after the death of a patient. Immunogenic accumulation of the antibody in nontumor areas (skin keratinocytes) limited the use of this antibody for cancer therapy [82]. Recently, RO5429083, a humanized CD44 antibody developed by Roche (Indianapolis, IN, http://www.roche.com) that targets a glycosylated, conformation-dependent epitope of CD44 to inhibit the binding of HA to CD44, was entered into clinical trials to treat patients with metastatic or locally advanced CD44-expressing malignant solid tumors or acute myelogenous leukemia (NCT01358903 and NCT01641250). Currently, a number of new humanized anti-CD44 or anti-CD44v antibodies are under preclinical investigation for anti-CSC therapy.
HA-CD44 Interaction-Based Strategies
HA binding to the CD44 ectodomain activates CD44-mediated oncogenic signaling, microRNA functions, and chemoradioresistance in cancer cells, leading to tumor progression. Therefore, interference with the binding of HA to CD44 expressed on tumor cells using soluble CD44 ectodomain or peptides as a competitor is expected to have antitumor effects. Indeed, local administration of CD44 ectodomain inhibited human melanoma growth in mice, whereas administration of a mutant, non-HA-binding CD44 ectodomain had no significant effect on tumor growth [83].
Interestingly, a peptide of A5G27 (RLVSYNGIIFFLK) identified in a synthetic peptide screening for cell attachment activity and confirmed to bind CD44 was demonstrated to have inhibitory effects on tumor growth and lung metastatic colonization of B16-F10 mouse melanoma cells [84]. Mutational analysis identified three amino acids in the exon v6 coding region that are absolutely required for CD44v6 coreceptor function for Met and VEGFR-2. Peptides comprising these three amino acids (the smallest containing only five amino acids) efficiently act as competitors to block ligand-dependent activation of Met or Ron and inhibit the migration and angiogenesis of cancer cells [15, 85].
Another peptide, A6, which is an 8-amino acid peptide (acetyl-KPSSPPEE-amino) derived from human urokinase plasminogen activator that acts in a urokinase plasminogen activator–independent pathway to inhibit migration, invasion, and metastasis of cancer cells [86], was found to bind specifically to CD44 [87]. On the basis of preclinical antitumor activities, A6 was advanced into clinical trials. In one study, conducted in ovarian cancer patients with early biochemical relapse, treatment with A6 was associated with prolongation of progression-free survival [88].
(pro)MMP-9 binds to chronic lymphocytic leukemia cells through the promatrix metalloproteinase-9 hemopexin (PEX9) domain and contributes to chronic lymphocytic leukemia progression. A novel peptide identified from the PEX9 domain impairs adhesion and migration of chronic lymphocytic leukemia cells by binding to CD44 [89], suggesting that this peptide has therapeutic potential.
By using phage display technology to screen the peptide library, researchers have identified several other CD44-binding peptides that exhibited similar binding affinity to that of the CD44 antibody to breast cancer stem cell-specific surface marker CD44 [90]. It is speculated that these peptides could have both diagnostic and therapeutic value.
HA has the unique features of biodegradability, biocompatibility, and nonimmunogenicity, and it contains multiple functional (hydroxyl and carboxylic acid) residues on its backbone. In addition, HA binding to CD44 can lead to internalization of the HA [91], which makes HA an ideal carrier for targeted delivery of therapeutic agents specifically to cancer cells because HA has high affinity toward CD44. The internalization of HA by cancer cells through highly expressed CD44 receptors enhances specific delivery of drugs, including chemical drugs, siRNA, shRNA, and microRNA [92, 93], to cancer cells, particularly CD44-positive and drug-resistant CSCs, via conjugation to HA or entrapment in HA-modified nanoparticles or micelles. This results in improved therapeutic efficacy with HA compared with conventional anticancer agents in different types of tumor model systems [94–96].
One of the significant advances in the study of CD44 is the development of a cell-specific delivery approach by targeting the HA/CD44v6-induced signaling pathway in colon cancer cells, in which two DNA plasmids of U6-promoter-loxP-CMV-GFP-STOP-loxP-CD44v6-shRNA and colon-specific (Fabpl) promoter-GFP-Cre are packaged in transferrin-coated PEG-PEI nanoparticles [97]. The successful delivery of CD44v6-shRNA was demonstrated by specific inhibition of CD44v6 expression in colon tumor cells and by perturbation of HA /CD44v6 signaling, as reflected in a reduced number of adenomas and reduced tumor growth in Apc Min/+ mice [97]. This approach has several advantages over other targeting strategies and may be easily designed to target other CD44v-expressing cancer cells. Other CD44-targeting strategies have also been investigated, such as targeting the CD44v-xCT system and using CD44v as a DNA vaccine [33, 98, 99]; these strategies deserve further attention.
Current Challenges and Future Directions
Identification of Novel CD44v Isoforms in CSCs
CD44 is one of the most complicated genes, possibly containing more than 1,000 isoforms owing to free inclusion or exclusion of 10 alternative exons in a central tandem array [100]. To date, only very few of these potential CD44v isoforms have been identified because of technical limitations [101], and few studies have used stem cells or CSCs for systemic analysis owing to the difficulty in obtaining sufficient cells [12]. The unique expression and function of CD44v isoforms in cancer cells makes it highly necessary to identify novel CD44v isoforms in cancers, particularly in CSCs. Recent advances in next-generation RNA sequencing and stem cell technology to enrich and isolate CSCs provide excellent opportunities to discover previously unidentified CD44v isoforms in CSCs [102]. Such research will not only further our understanding of the critical role of CD44v isoforms in cancers but also open up new avenues to use the identified CD44v isoforms as diagnostic and prognostic markers or therapeutic targets in cancer.
Understanding the Transcriptional Regulation of CD44 Expression
Increased transcription of CD44 at the RNA level is the fundamental basis for the high levels of CD44s or CD44v protein expression in various cancer cells. It is highly clinically significant to understand the molecular mechanisms by which CD44 transcription is regulated. Over the past few decades, tremendous efforts have been made toward defining CD44s and CD44v protein structure and function and the underlying signaling pathways [11, 12, 39, 71, 76, 103]. More recently, the effects of microRNAs on CD44 expression have also been studied [104–106], but few studies have examined transcriptional regulation of CD44 expression, though the magnitude of CD44s and CD44v isoform expression is critical for CD44 function in cancer. Fortunately, it was found that p53 transcriptionally suppresses CD44 expression and tumor growth [107], and a positive feedback loop couples Ras activation and CD44 expression [18]. These seminal findings may, at least in part, explain why CD44 is overexpressed in cancer cells.
However, we are still facing many challenges regarding the fundamental questions of CD44 expression in cancer and CSCs. For example, why do tumors contain both CD44-positive (or -high) and CD44-negative (or -low) subpopulations of cells? How and when do cancer cells gain CD44 expression when they change into stem-like cells? How do CSCs lose CD44 expression when differentiated? How do genetic and epigenetic factors cross-talk to regulate CD44 transcriptional expression in cancer and CSCs? The answers to these questions may help develop more effective strategies that simultaneously inhibit CD44 transcription and block CD44s or CD44v protein function to generate a synergistic effect.
Understanding the Molecular Regulation of CD44 Alternative Splicing
Compared with the ubiquitous expression of CD44s, the expression of CD44v isoforms seems to be restricted to cancer cells. Mounting clinical data clearly demonstrate that CD44v isoforms are associated with aggressive cancers and poor prognosis. Isoform switching from CD44s to CD44v is a critical event during EMT, and EMT is an important step in the metastatic process and acquisition of stemness in cancer cells [54, 55, 103, 108]. Although CD44 exons are not chosen at random [100], the reason cancer cells but not nontumor cells express a large variety of CD44v isoforms remains largely unknown [9]. One possible explanation is that cancer cells express a variety of functionally diverse CD44v isoforms, providing the cancer cells with a flexible and quick means to adapt to or cope with their constantly changing environment. Thus, it is a great challenge to investigate how environmental or epigenetic signals regulate CD44 alternative splicing or CD44v isoform switching, particularly during the reprograming of cancer cells to CSCs and CSC differentiation.
Unfortunately, little progress has been made in the past several years in our understanding the molecular mechanisms by which CD44 alternative splicing is regulated in CSCs [21], partially owing to the high complexity of CD44 splicing variants and technical difficulties in direct RNA transcript detection, analysis, and manipulation. Recently, the powerful clustered regulatory interspaced short palindromic repeat (CRISPR) and CRISPR-associated protein 9 (Cas9) system for genome engineering and gene regulation has been successfully programmed to target RNA [109] in which specially designed protospacer adjacent motif (PAM)-presenting oligonucleotides, or PAMmers, are used to direct the CRISPR/Cas9 complex (called RNA-targeting CRISPR/Cas9 complex, or RCas9) to bind or cut specific RNA targets while avoiding corresponding DNA sequences [110]. It is expected that RCsa9, when fused to select protein domains, could promote or exclude specific introns or exons, and RCas9 could cut or bind RNA in a sequence-specific manner. Thus, the application of the RCas9 technique will open up a new era for the mechanistic study of CD44 alternative splicing regulation. For example, in combination with the minigene report system [111], the RCas9 technique might enable us to know which transacting splicing factor is important for expression of a specific CD44v isoform, which cis-element in pre-CD44 mRNA is required for the binding of a specific transacting splicing factor, and which signaling pathway is important to activate specific transacting splicing factor(s) to regulate the expression of specific CD44v isoforms in CSCs. It is anticipated that identifying the mechanisms by which specific CD44v isoforms are preferentially expressed in CSCs or differentiated CSCs could allow these isoforms to be used to develop strategies to eliminate CSCs.
Developing Key CD44 Reagents and Standardizing Protocols for Experimental and Clinical Applications
Although hundreds of studies have been published on CD44 over the last two decades, CD44v isoforms have not been thoroughly studied. One of the main reasons is lack of the critical reagents for characterizing, detecting, and targeting CD44v isoforms. For example, only limited antibodies to the variable CD44v exon products are commercially available, and no primers and probe sets are commercially available to cover all possible CD44v isoforms for the detection of CD44v expression at the mRNA level. Many studies have examined the clinical significance of specific CD4v isoforms in various cancers; however, discrepancies or controversial results have been reported even within the same tumor type [112–115], and the discrepancies or controversies may be caused by using different sources of antibodies or assay protocols [7]. Therefore, commercially available CD44 assay kits containing well-characterized CD44s and various CD44v antibodies with standardized assay protocols are urgently needed for clinical studies and for comparison among different studies or different laboratories to reach general conclusions. It has been suggested that detection of CD44 expression in clinical cancer tissues using both immunohistochemistry and reverse transcription-polymerase chain reaction (or in situ RNA hybridization) methods would lead to more reliable results.
The heterogenetic nature of cancer and the highly complicated and context-dependent expression of CD44v isoforms substantiate the necessity for personalized screening of unique CD44v isoforms and the use of these isoforms as prognostic biomarkers or therapeutic targets. For example, pan-CD44 was previously identified as a gastric CSC marker (IM7 antibody) [35]. However, in a recent study using 28 paired primary tumor and adjacent nontumor gastric tissue samples to screen for cell surface protein expression, CD44v8–10 was identified as the predominant CD44v isoform expressed in gastric cancer cells and was found to function as a gastric CSC marker [40]. Consistent with previous observations in the Gan gastric cancer mouse model [33], this finding suggests that CD44v8–10 is both an ideal biomarker for early detection of gastric cancer and an ideal target for developing clinical therapeutics against gastric CSCs. Additionally, the limitations demonstrated in previous clinical trials of anti-CD44 therapies also highlight the need for developing better anti-CD44v reagents such as high-affinity anti-CD44v peptides to replace highly immunogenic antibodies [90], as well as the use of CSC-specific CD44v peptides for cancer vaccines on the basis of promising CD44 DNA vaccine studies in animal models [99, 116].
Conclusion
CD44, especially CD44v isoforms, have been identified as CSC markers and critical players in regulating the properties of CSCs in many types of tumors. With better understanding of the fundamental basis of how CD44 and CD44v expressions are regulated and identification of novel and unique CD44v isoforms in CSCs, effective therapeutic strategies that aim to eliminate CSCs by targeting CSC-specific CD44 isoform(s) may be developed that will bring new hope to patients with life-threatening cancer.
Acknowledgments
We thank Erica Goodoff for helpful comments. This work was supported in part by grants from NIH (CA124523), the American Institute for Cancer Research (10A073), an Institutional Research Grant, and the Texas Medical Center Digestive Diseases Center Pilot/Feasibility Fund (to D.W.).
Author Contributions
Y.Y., X.Z., and D.W.: manuscript writing; final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Merlos-Suárez A, Barriga FM, Jung P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sneddon JB, Werb Z. Location, location, location: The cancer stem cell niche. Cell Stem Cell. 2007;1:607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: Complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plaks V, Kong N, Werb Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naor D, Wallach-Dayan SB, Zahalka MA, et al. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Naor D, Nedvetzki S, Golan I, et al. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein LA, Zhou DF, Picker LJ, et al. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989;56:1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura Y, Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992;340:1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- 10.Aruffo A, Stamenkovic I, Melnick M, et al. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 11.Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 12.Williams K, Motiani K, Giridhar PV, et al. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med (Maywood) 2013;238:324–338. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto I, Kawano Y, Tsuiki H, et al. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene. 1999;18:1435–1446. doi: 10.1038/sj.onc.1202447. [DOI] [PubMed] [Google Scholar]
- 14.Bennett KL, Jackson DG, Simon JC, et al. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995;128:687–698. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremmel M, Matzke A, Albrecht I, et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009;114:5236–5244. doi: 10.1182/blood-2009-04-219204. [DOI] [PubMed] [Google Scholar]
- 16.Orian-Rousseau V, Chen L, Sleeman JP, et al. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orian-Rousseau V, Morrison H, Matzke A, et al. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell. 2007;18:76–83. doi: 10.1091/mbc.E06-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng C, Yaffe MB, Sharp PA. A positive feedback loop couples Ras activation and CD44 alternative splicing. Genes Dev. 2006;20:1715–1720. doi: 10.1101/gad.1430906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang W, Crossman DK, Mitchell EH, et al. WNT5A inhibits metastasis and alters splicing of Cd44 in breast cancer cells. PLoS One. 2013;8:e58329. doi: 10.1371/journal.pone.0058329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weg-Remers S, Ponta H, Herrlich P, et al. Regulation of alternative pre-mRNA splicing by the ERK MAP-kinase pathway. EMBO J. 2001;20:4194–4203. doi: 10.1093/emboj/20.15.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prochazka L, Tesarik R, Turanek J. Regulation of alternative splicing of CD44 in cancer. Cell Signal. 2014;26:2234–2239. doi: 10.1016/j.cellsig.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Ilangumaran S, Borisch B, Hoessli DC. Signal transduction via CD44: Role of plasma membrane microdomains. Leuk Lymphoma. 1999;35:455–469. doi: 10.1080/10428199909169610. [DOI] [PubMed] [Google Scholar]
- 23.Su YJ, Lai HM, Chang YW, et al. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto I, Kawano Y, Murakami D, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155:755–762. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietras A, Katz AM, Ekström EJ, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014;14:357–369. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourguignon LY, Earle C, Wong G, et al. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene. 2012;31:149–160. doi: 10.1038/onc.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukita S, Oishi K, Sato N, et al. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasenauer S, Malinger D, Koschut D, et al. Internalization of Met requires the co-receptor CD44v6 and its link to ERM proteins. PLoS One. 2013;8:e62357. doi: 10.1371/journal.pone.0062357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai Y, Liu YJ, Wang H, et al. Inhibition of the hyaluronan-CD44 interaction by merlin contributes to the tumor-suppressor activity of merlin. Oncogene. 2007;26:836–850. doi: 10.1038/sj.onc.1209849. [DOI] [PubMed] [Google Scholar]
- 30.Maiti A, Maki G, Johnson P. TNF-alpha induction of CD44-mediated leukocyte adhesion by sulfation. Science. 1998;282:941–943. doi: 10.1126/science.282.5390.941. [DOI] [PubMed] [Google Scholar]
- 31.Chetty C, Vanamala SK, Gondi CS, et al. MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell Signal. 2012;24:549–559. doi: 10.1016/j.cellsig.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Pan Y, Han C, Wang C, et al. ADAM10 promotes pituitary adenoma cell migration by regulating cleavage of CD44 and L1. J Mol Endocrinol. 2012;49:21–33. doi: 10.1530/JME-11-0174. [DOI] [PubMed] [Google Scholar]
- 33.Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JL, Wang MJ, Chen JY. Acetylation and activation of STAT3 mediated by nuclear translocation of CD44. J Cell Biol. 2009;185:949–957. doi: 10.1083/jcb.200812060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kidwai F, Costea DE, Hutchison I, et al. The effects of CD44 down-regulation on stem cell properties of head and neck cancer cell lines. J Oral Pathol Med. 2013;42:682–690. doi: 10.1111/jop.12076. [DOI] [PubMed] [Google Scholar]
- 38.Zeilstra J, Joosten SP, van Andel H, et al. Stem cell CD44v isoforms promote intestinal cancer formation in Apc(min) mice downstream of Wnt signaling. Oncogene. 2014;33:665–670. doi: 10.1038/onc.2012.611. [DOI] [PubMed] [Google Scholar]
- 39.Todaro M, Gaggianesi M, Catalano V, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Lau WM, Teng E, Chong HS, et al. CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 2014;74:2630–2641. doi: 10.1158/0008-5472.CAN-13-2309. [DOI] [PubMed] [Google Scholar]
- 41.Bourguignon LY, Wong G, Earle C, et al. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287:32800–32824. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SJ, Wreesmann VB, Bourguignon LY. Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck. 2007;29:550–558. doi: 10.1002/hed.20544. [DOI] [PubMed] [Google Scholar]
- 43.Bourguignon LY, Spevak CC, Wong G, et al. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284:26533–26546. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju SY, Chiou SH, Su Y. Maintenance of the stemness in CD44(+) HCT-15 and HCT-116 human colon cancer cells requires miR-203 suppression. Stem Cell Res (Amst) 2014;12:86–100. doi: 10.1016/j.scr.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Bourguignon LY, Shiina M, Li JJ. Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv Cancer Res. 2014;123:255–275. doi: 10.1016/B978-0-12-800092-2.00010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu Z, Goodyear SM, Rao S, et al. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2011;108:12740–12745. doi: 10.1073/pnas.1109987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Psaila B, Lyden D. The metastatic niche: Adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malanchi I, Santamaria-Martínez A, Susanto E, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 49.Zhao P, Damerow MS, Stern P, et al. CD44 promotes Kras-dependent lung adenocarcinoma. Oncogene. 2013;32:5186–5190. doi: 10.1038/onc.2012.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Heddleston JM, Li Z, McLendon RE, et al. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishnamachary B, Penet MF, Nimmagadda S, et al. Hypoxia regulates CD44 and its variant isoforms through HIF-1α in triple negative breast cancer. PLoS One. 2012;7:e44078. doi: 10.1371/journal.pone.0044078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nieto MA. Epithelial plasticity: A common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 55.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshihara S, Kon A, Kudo D, et al. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005;579:2722–2726. doi: 10.1016/j.febslet.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 57.Uchino M, Kojima H, Wada K, et al. Nuclear beta-catenin and CD44 upregulation characterize invasive cell populations in non-aggressive MCF-7 breast cancer cells. BMC Cancer. 2010;10:414. doi: 10.1186/1471-2407-10-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bessède E, Staedel C, Acuña Amador LA, et al. Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene. 2014;33:4123–4131. doi: 10.1038/onc.2013.380. [DOI] [PubMed] [Google Scholar]
- 59.Zubeldia IG, Bleau AM, Redrado M, et al. Epithelial to mesenchymal transition and cancer stem cell phenotypes leading to liver metastasis are abrogated by the novel TGFβ1-targeting peptides P17 and P144. Exp Cell Res. 2013;319:12–22. doi: 10.1016/j.yexcr.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Bourguignon LY, Singleton PA, Zhu H, et al. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 61.Mima K, Okabe H, Ishimoto T, et al. CD44s regulates the TGF-β-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012;72:3414–3423. doi: 10.1158/0008-5472.CAN-12-0299. [DOI] [PubMed] [Google Scholar]
- 62.Li J, Zha XM, Wang R, et al. Regulation of CD44 expression by tumor necrosis factor-α and its potential role in breast cancer cell migration. Biomed Pharmacother. 2012;66:144–150. doi: 10.1016/j.biopha.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 63.Mikami S, Mizuno R, Kosaka T, et al. Expression of TNF-alpha and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomas. Int J Cancer. 2015;136:1504–1514. doi: 10.1002/ijc.29137. [DOI] [PubMed] [Google Scholar]
- 64.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 67.Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: Multiple faces for conferring benefits on cancer cells. Clin Cancer Res. 2012;18:5554–5561. doi: 10.1158/1078-0432.CCR-12-0859. [DOI] [PubMed] [Google Scholar]
- 68.Tamada M, Nagano O, Tateyama S, et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438–1448. doi: 10.1158/0008-5472.CAN-11-3024. [DOI] [PubMed] [Google Scholar]
- 69.Cao X, Cao D, Jin M, et al. CD44 but not CD24 expression is related to poor prognosis in non-cardia adenocarcinoma of the stomach. BMC Gastroenterol. 2014;14:157. doi: 10.1186/1471-230X-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Fu Z, Xu S, et al. The prognostic value of CD44 expression in gastric cancer: A meta-analysis. Biomed Pharmacother. 2014;68:693–697. doi: 10.1016/j.biopha.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Hirata K, Suzuki H, Imaeda H, et al. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379–386. doi: 10.1038/bjc.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katoh S, Goi T, Naruse T, et al. Cancer stem cell marker in circulating tumor cells: Expression of CD44 variant exon 9 is strongly correlated to treatment refractoriness, recurrence and prognosis of human colorectal cancer. Anticancer Res. 2015;35:239–244. [PubMed] [Google Scholar]
- 73.Fan CW, Wen L, Qiang ZD, et al. Prognostic significance of relevant markers of cancer stem cells in colorectal cancer: A meta analysis. Hepatogastroenterology. 2012;59:1421–1427. doi: 10.5754/hge10727. [DOI] [PubMed] [Google Scholar]
- 74.Jiang H, Zhao W, Shao W. Prognostic value of CD44 and CD44v6 expression in patients with non-small cell lung cancer: Meta-analysis. Tumour Biol. 2014;35:7383–7389. doi: 10.1007/s13277-014-2150-3. [DOI] [PubMed] [Google Scholar]
- 75.Ozawa M, Ichikawa Y, Zheng YW, et al. Prognostic significance of CD44 variant 2 upregulation in colorectal cancer. Br J Cancer. 2014;111:365–374. doi: 10.1038/bjc.2014.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zöller M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 77.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orian-Rousseau V, Ponta H. Perspectives of CD44 targeting therapies. Arch Toxicol. 2015;89:3–14. doi: 10.1007/s00204-014-1424-2. [DOI] [PubMed] [Google Scholar]
- 79.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 80.Marangoni E, Lecomte N, Durand L, et al. CD44 targeting reduces tumour growth and prevents post-chemotherapy relapse of human breast cancers xenografts. Br J Cancer. 2009;100:918–922. doi: 10.1038/sj.bjc.6604953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tijink BM, Buter J, de Bree R, et al. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12:6064–6072. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- 82.Rupp U, Schoendorf-Holland E, Eichbaum M, et al. Safety and pharmacokinetics of bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: Final results of a phase I study. Anticancer Drugs. 2007;18:477–485. doi: 10.1097/CAD.0b013e32801403f4. [DOI] [PubMed] [Google Scholar]
- 83.Bartolazzi A, Peach R, Aruffo A, et al. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hibino S, Shibuya M, Engbring JA, et al. Identification of an active site on the laminin alpha5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004;64:4810–4816. doi: 10.1158/0008-5472.CAN-04-0129. [DOI] [PubMed] [Google Scholar]
- 85.Matzke A, Herrlich P, Ponta H, et al. A five-amino-acid peptide blocks Met- and Ron-dependent cell migration. Cancer Res. 2005;65:6105–6110. doi: 10.1158/0008-5472.CAN-05-0207. [DOI] [PubMed] [Google Scholar]
- 86.Boyd DD, Kim SJ, Wang H, et al. A urokinase-derived peptide (A6) increases survival of mice bearing orthotopically grown prostate cancer and reduces lymph node metastasis. Am J Pathol. 2003;162:619–626. doi: 10.1016/S0002-9440(10)63855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piotrowicz RS, Damaj BB, Hachicha M, et al. A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells. Mol Cancer Ther. 2011;10:2072–2082. doi: 10.1158/1535-7163.MCT-11-0351. [DOI] [PubMed] [Google Scholar]
- 88.Gold MA, Brady WE, Lankes HA, et al. A phase II study of a urokinase-derived peptide (A6) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:635–639. doi: 10.1016/j.ygyno.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ugarte-Berzal E, Bailón E, Amigo-Jiménez I, et al. A novel CD44-binding peptide from the pro-matrix metalloproteinase-9 hemopexin domain impairs adhesion and migration of chronic lymphocytic leukemia (CLL) cells. J Biol Chem. 2014;289:15340–15349. doi: 10.1074/jbc.M114.559187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park HY, Lee KJ, Lee SJ, et al. Screening of peptides bound to breast cancer stem cell specific surface marker CD44 by phage display. Mol Biotechnol. 2012;51:212–220. doi: 10.1007/s12033-011-9458-7. [DOI] [PubMed] [Google Scholar]
- 91.Culty M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992;116:1055–1062. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi S, Han L, Deng L, et al. Dual drugs (microRNA-34a and paclitaxel)-loaded functional solid lipid nanoparticles for synergistic cancer cell suppression. J Control Release. 2014;194:228–237. doi: 10.1016/j.jconrel.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Skandalis SS, Gialeli C, Theocharis AD, et al. Advances and advantages of nanomedicine in the pharmacological targeting of hyaluronan-CD44 interactions and signaling in cancer. Adv Cancer Res. 2014;123:277–317. doi: 10.1016/B978-0-12-800092-2.00011-3. [DOI] [PubMed] [Google Scholar]
- 94.Goodarzi N, Ghahremani MH, Amini M, et al. CD44-targeted docetaxel conjugate for cancer cells and cancer stem-like cells: A novel hyaluronic acid-based drug delivery system. Chem Biol Drug Des. 2014;83:741–752. doi: 10.1111/cbdd.12288. [DOI] [PubMed] [Google Scholar]
- 95.Shah V, Taratula O, Garbuzenko OB, et al. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: An optimal delivery of siRNA and anticancer drug. Clin Cancer Res. 2013;19:6193–6204. doi: 10.1158/1078-0432.CCR-13-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu M, Jambhrunkar S, Thorn P, et al. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale. 2013;5:178–183. doi: 10.1039/c2nr32145a. [DOI] [PubMed] [Google Scholar]
- 97.Misra S, Hascall VC, De Giovanni C, et al. Delivery of CD44 shRNA/nanoparticles within cancer cells: Perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ MICE. J Biol Chem. 2009;284:12432–12446. doi: 10.1074/jbc.M806772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshikawa M, Tsuchihashi K, Ishimoto T, et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855–1866. doi: 10.1158/0008-5472.CAN-12-3609-T. [DOI] [PubMed] [Google Scholar]
- 99.Wallach-Dayan SB, Rubinstein AM, Hand C, et al. DNA vaccination with CD44 variant isoform reduces mammary tumor local growth and lung metastasis. Mol Cancer Ther. 2008;7:1615–1623. doi: 10.1158/1535-7163.MCT-07-2383. [DOI] [PubMed] [Google Scholar]
- 100.Bell MV, Cowper AE, Lefranc MP, et al. Influence of intron length on alternative splicing of CD44. Mol Cell Biol. 1998;18:5930–5941. doi: 10.1128/mcb.18.10.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Screaton GR, Bell MV, Jackson DG, et al. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bánky B, Rásó-Barnett L, Barbai T, et al. Characteristics of CD44 alternative splice pattern in the course of human colorectal adenocarcinoma progression. Mol Cancer. 2012;11:83. doi: 10.1186/1476-4598-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yae T, Tsuchihashi K, Ishimoto T, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- 104.Cheng W, Liu T, Wan X, et al. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012;279:2047–2059. doi: 10.1111/j.1742-4658.2012.08589.x. [DOI] [PubMed] [Google Scholar]
- 105.Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saini S, Majid S, Shahryari V, et al. miRNA-708 control of CD44(+) prostate cancer-initiating cells. Cancer Res. 2012;72:3618–3630. doi: 10.1158/0008-5472.CAN-12-0540. [DOI] [PubMed] [Google Scholar]
- 107.Godar S, Ince TA, Bell GW, et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown RL, Reinke LM, Damerow MS, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Connell MR, Oakes BL, Sternberg SH, et al. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature. 2014;516:263–266. doi: 10.1038/nature13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cooper TA. Use of minigene systems to dissect alternative splicing elements. Methods. 2005;37:331–340. doi: 10.1016/j.ymeth.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 112.Jansen RH, Joosten-Achjanie SR, Arends JW, et al. CD44v6 is not a prognostic factor in primary breast cancer. Ann Oncol. 1998;9:109–111. doi: 10.1023/a:1008220917687. [DOI] [PubMed] [Google Scholar]
- 113.Ma W, Deng Y, Zhou L. The prognostic value of adhesion molecule CD44v6 in women with primary breast carcinoma: A clinicopathologic study. Clin Oncol (R Coll Radiol) 2005;17:258–263. doi: 10.1016/j.clon.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 114.Shah NG, Trivedi TI, Vora HH, et al. CD44v6 expression in primary breast carcinoma in western India: A pilot clinicopathologic study. Tumori. 2010;96:971–977. [PubMed] [Google Scholar]
- 115.Yu P, Zhou L, Ke W, et al. Clinical significance of pAKT and CD44v6 overexpression with breast cancer. J Cancer Res Clin Oncol. 2010;136:1283–1292. doi: 10.1007/s00432-010-0779-x. [DOI] [PubMed] [Google Scholar]
- 116.Parmiani G, Russo V, Maccalli C, et al. Peptide-based vaccines for cancer therapy. Hum Vaccin Immunother. 2014;10:3175–3178. doi: 10.4161/hv.29418. [DOI] [PMC free article] [PubMed] [Google Scholar]