Testing of well-characterized human cancer cell lines was applied to elaborate on whether drug transporters are involved in protection from the cytotoxic effects of the ionophore antibiotics salinomycin and nigericin. The results showed that ionophore antibiotics are less suited to cancer stem cell-targeted treatment than previously thought.
Keywords: Ionophore antibiotic, Salinomycin, Nigericin, Cancer stem cell, Drug transporter
Abstract
Ionophore antibiotics were reported to selectively kill cancer stem cells and to overcome multidrug resistance, but mechanistic studies of the significance of drug transporters for treatment with these compounds are lacking. We applied chemosensitivity testing of well-characterized human cancer cell lines to elaborate on whether drug transporters are involved in protection from the cytotoxic effects of the ionophore antibiotics salinomycin and nigericin. Our experiments demonstrated that ionophore antibiotics were ineffective against both stem-like ovarian cancer side population cells (expressing either ABCB1 or ABCG2) and K562/Dox-H1 cells, which constitute a genetically defined model system for ABCB1 expression. Considering that cancer stem cells often express high levels of drug transporters, we deduced from our results that ionophore antibiotics are less suited to cancer stem cell-targeted treatment than previously thought.
Significance
Ionophore antibiotics such as salinomycin have repeatedly been shown to target cancer stem and progenitor cells from various tumor entities. Meanwhile, cancer stem cell (CSC)-selective toxicity of ionophore antibiotics seems to be a commonly accepted concept that is about to encourage their clinical testing. This study provides data that challenge the concept of targeted elimination of CSC by ionophore antibiotics. Stem-like ovarian cancer side population (SP) cells expressing high levels of ABC drug transporters are shown to largely resist the cytotoxic effects of salinomycin and nigericin. Furthermore, using a small interfering RNA-based knockdown model specific for ABCB1, this study demonstrates that ABC drug transporters are indeed causally involved in mediating protection from ionophore antibiotics. Considering that it is a hallmark of CSCs to exhibit drug resistance conferred by ABC drug transporters, it must be deduced from these results that CSCs may also be protected from ionophore antibiotics by means of drug-transporter mediated efflux.
Introduction
Ionophore antibiotics (IAs) are a compound class targeting lipid bilayers for insertion, thereby establishing membrane pores supporting ionic conductivity [1]. Because of their antibacterial and antiparasitic activity, these drugs are widely used as a fodder additive in industrial livestock farming to prevent microbial-associated disease [2]; however, IAs have also been shown to be active against cancer cells.
In 2009, Gupta et al. reported on the identification of selective inhibitors of cancer stem cells (CSCs) using high-throughput screening [3]. Strikingly, their screen revealed significant CSC-selective toxicity for two IAs (i.e., salinomycin and nigericin), indicating strong susceptibility of CSCs to this substance class. Specific anti-CSC activity of IAs was subsequently observed in other CSC populations [4–8]. Moreover, it was reported that salinomycin is able to overcome apoptosis resistance in cancer cells [9] and to interfere with Wnt signaling [10].
In the study by Gupta et al., drug transporters were not differentially regulated in CSCs [3], thus a potential role of drug transporters in mediating protection from IAs could not be defined. CSCs, however, often display elevated expression levels of transporters such as ABCB1 and ABCG2 [11], conferring therapy resistance and side population (SP) appearance. These facts provide a rationale for elaborating on whether IAs are substrates of drug transporters.
Interestingly, it was reported that salinomycin eradicates leukemic stem cells overexpressing ABCB1, ABCG2, and ABCC11 [12]. Mechanistically, the authors speculated that rapid membrane insertion might prevent transporter-mediated drug efflux. Moreover, others showed that salinomycin directly inhibits ABCB1 via induction of a distinct conformational change [13]. Consequently, sufficiently high doses of salinomycin should target ABCB1-positive cells based on a chemosensitization effect.
In this report, we addressed the question whether IAs could target stem-like SP cells expressing high amounts of ABCB1 or ABCG2. Because these models could also exhibit drug transporter-unrelated mechanisms of protection, we applied a particular small interfering RNA (siRNA)-based knockdown model in which any observed effect can be attributed to ABCB1.
Materials and Methods
Cell Culture
A2780 ovarian cancer (OvCa) cells were purchased from Sigma-Aldrich (St. Louis, MO, https://www.sigmaaldrich.com), and the A2780 subline A2780V [14] was kindly obtained from Dr. R. Zeillinger (Medical University of Vienna, Vienna, Austria). B17/92 OvCa cells [15] were generously provided by Dr. C. Brumm (University of Mainz, Mainz, Germany), and IGROV1 OvCa cells [16] were a kind gift of Dr. R. Brown (Imperial College London, London, U.K.). K562/Dox-H1 and K562/Dox-MM represent genetically engineered leukemia cells and were established from parental K562 cells, as described previously [17]. A2780V and IGROV1 harbor an ABCB1-positive stem-like SP, whereas A2780 and B17/92 contain an ABCG2-expressing stem-like SP [18], and K562/Dox-H1 (empty vector control) and K562/Dox-MM (MDR1-targeting siRNA) are positive and negative for ABCB1, respectively [17]. Cells were cultured in appropriate medium (i.e., RPMI or Dulbecco’s modified Eagle’s medium; PAA Laboratories; GE Healthcare, Munich, Germany, http://gehealthcare.de) supplemented with 10% fetal bovine serum (Biochrom, Cambridge, U.K., http://www.biochrom.co.uk), 2 mM l-glutamine, and 1× penicillin/streptomycin (Gibco; Life Technologies, Thermo Fisher Scientific, Waltham, MA, http://www.lifetechnologies.com/us/en/home/brands/gibco.html).
Flow Cytometry
Flow cytometry and sorting were performed on a FACSCanto II and a FACSAria I, respectively (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com). SP detection was accomplished using DyeCycle Violet staining, as described previously [18, 19], and antibody stainings were performed according to standard protocols (30 minutes at 4°C). Antibodies against ABCB1 (clone UIC2) and ABCG2 (clone 5D3) were used. Cells were chilled and subjected to flow cytometry or sorting within 1 hour. To discriminate dead cells, 7-AAD staining was included, and debris and doublets were gated out based on forward scatter/side scatter characteristics. Viable single cells were finally analyzed using FlowJo (Tree Star, Ashland, OR, http://www.flowjo.com).
Chemosensitivity Testing
The MTT assay was used to determine the chemosensitivity of fluorescence-activated cell sorting-purified SP and non-SP (NSP) fractions (7.5 × 103 cells per 96 wells). Treatment with salinomycin or nigericin (Sigma-Aldrich) or paclitaxel (Ebewe; EBEWE Pharma, Sandoz, Holzkirchen, Germany, http://www.sandoz.de) was continued for 48 hours, and specific inhibition was calculated by normalization to untreated controls. In case of chemoselection, defined mixtures of ABCB1-positive K562/Dox-H1 cells (20%) and ABCB1-negative K562/Dox-MM cells (80%) were generated and treated in a competitive assay format for 7 days with salinomycin or nigericin (5 × 104 cells per 6 wells). In the ABCG2 setting, we used A2780 cells, which naturally harbor an ABCG2-positive SP of ∼1% [18]. The proportion of drug transporter-positive cells was determined using staining with the antibodies stated above and flow cytometry. Results refer to the viable cell fraction.
Statistical Analysis
Data represent the mean ± SEM of at least three independent experiments and were analyzed for significance using a paired Student´s t test. A p value <.05 was considered significant.
Results
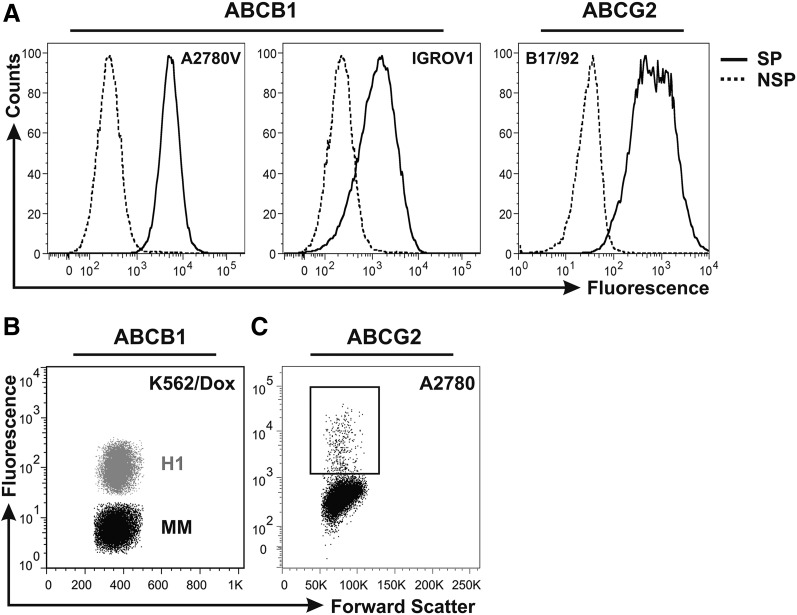
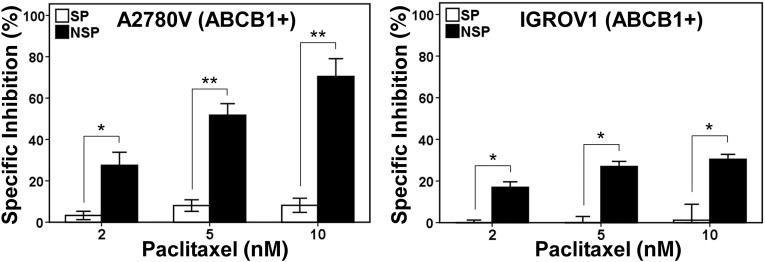
We [18] and others [20–23] recently identified the OvCa SP as a candidate CSC compartment driving tumor progression. Because these cells express high levels of ABCB1 and ABCG2 (Fig. 1A), they exhibit a characteristic resistance phenotype also covering clinically relevant agents. Indeed, we found that ABCB1-positive OvCa SP cells were resistant to taxane-based chemotherapy (Fig. 2), suggesting these cells as a potential source of recurrence. Further corroborating this notion, it was shown that SP cells were enriched in tumors of relapsing patients, indicating selection of these cells during in vivo chemotherapy [23]. These data highlight the need to identify novel therapeutics that are able to target this tumorigenic cell compartment.
Figure 1.
Drug transporter status of cell lines used within this study. (A): Histogram overlay of fluorescence-activated cell sorting-purified SP and NSP fractions of the ovarian cancer cell lines A2780V, IGROV1 (both ABCB1 positive), and B17/92 (ABCG2 positive). (B): Dot plot overlay of ABCB1-positive K562/Dox-H1 (empty vector control; gray) and ABCB1-negative K562/Dox-MM (MDR1-targeting small interfering RNA; black) chronic myelogenous leukemia cells. (C): Dot plot showing the ABCG2-expressing subpopulation of A2780 ovarian cancer cells (rectangular gate). Plots are representative examples of at least three independent stainings. Abbreviations: H1, ABCB1-positive K562/Dox-H1 leukemia cells; MM, ABCB1-negative K562/Dox-MM leukemia cells; NSP, non-side population; SP, side population.
Figure 2.
Resistance of ABCB1-positive SP cells to paclitaxel. Fluorescence-activated cell sorting-purified SP and NSP fractions of the ovarian cancer cell lines A2780V and IGROV1 were treated with increasing concentrations of paclitaxel and subsequently subjected to the metabolic colorimetric MTT assay. Nontreatment controls were included and used for calculation of the degree of specific inhibition of cell viability. ∗, p < .05; ∗∗, p < .01. Abbreviations: NSP, non-side population; SP, side population.
IAs Are Ineffective Against Stem-Like SP Cells
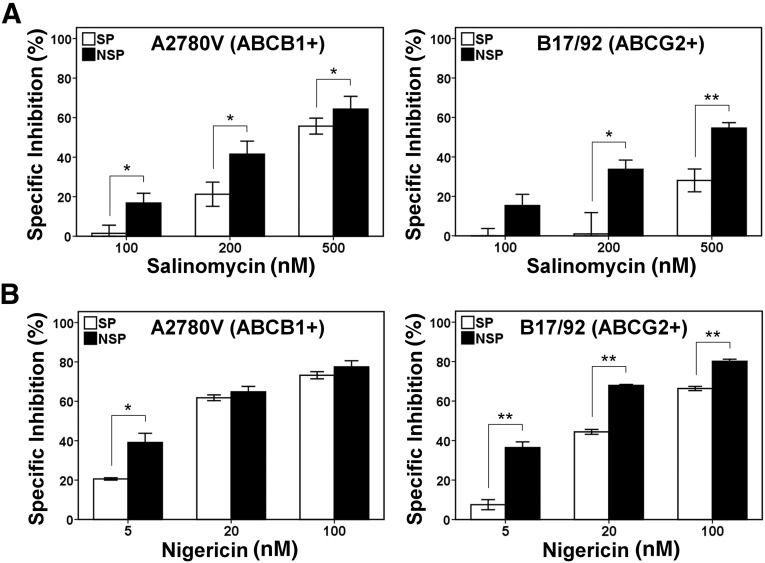
Based on the reported abilities of IAs to bear selectivity against epithelial and nonepithelial CSCs [3–8] and to overcome transporter-mediated drug resistance [12], we tested their effects on OvCa SP cells; however, using different cell lines expressing either ABCB1 or ABCG2, we found that—in contrast to non-SP cells—viability of SP cells was only marginally affected by salinomycin and nigericin (Fig. 3A, 3B) (data not shown). These results suggested that IAs do not selectively target the stem-like OvCa SP.
Figure 3.
Resistance of ABCB1- and ABCG2-expressing SP cells to ionophore antibiotics. Fluorescence-activated cell sorting-purified SP and NSP fractions of the ovarian cancer cell lines A2780V (ABCB1-positive SP) and B17/92 (ABCG2-positive SP) were treated with increasing concentrations of salinomycin (A) or nigericin (B) and subsequently subjected to the metabolic colorimetric MTT assay. Nontreatment controls were included and used for calculation of the degree of specific inhibition of cell viability. ∗, p < .05; ∗∗, p < .01. Abbreviations: NSP, non-side population; SP, side population.
ABCB1 Mediates Protection From IAs
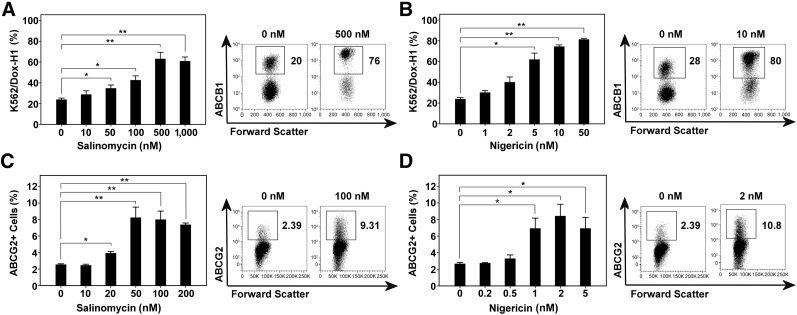
To elaborate on whether drug transporters can protect from IAs, we used a genetically defined model system for ABCB1 expression (Fig. 1B) [17]. Using this highly specific siRNA-based knockdown model, we showed in competitive assays that both salinomycin and nigericin dose-dependently selected ABCB1 high-expressing K562/Dox-H1 cells (empty vector) over ABCB1-negative K562/Dox-MM cells (MDR1-targeting siRNA) (Fig. 4A, 4B). Consequently, we show with genetic specificity that ABCB1 is causally involved in mediating protection from the cytotoxicity of IAs. Further corroborating the role of drug transporters in evasion of IA-induced cell death, both salinomycin and nigericin provided a selective advantage to the ABCG2-expressing subpopulation of A2780 OvCa cells (Fig. 1C), leading to an increase from 2% before treatment to 8% after treatment (Fig. 4C, 4D).
Figure 4.
Drug transporter expression protects from the cytotoxic effects of ionophore antibiotics. A mixture of 20% ABCB1-positive K562/Dox-H1 cells (empty vector control) and 80% ABCB1-negative K562/Dox-MM cells (MDR1-targeting small interfering RNA) was generated and exposed to increasing concentrations of salinomycin (A) or nigericin (B) in a competitive assay. The same assay was also applied to A2780 ovarian cancer cells, which naturally harbor an ABCG2-positive side population (C, D). After 7 days of treatment, the proportion of drug transporter-positive cells was determined using staining with respective monoclonal antibodies and flow cytometry. Representative fluorescence-activated cell sorting plots are shown. ∗, p < .05; ∗∗, p < .01.
Discussion and Conclusion
Beyond dispute, targeting of tumor-sustaining cancer cell subpopulations is an attractive therapeutic concept, the realization of which should prevent recurrence and improve patient outcomes [24]. Eradication of these CSCs, however, has proven difficult, partly because several protection mechanisms are operative in these cells [11, 25]. In the face of this challenge, the demonstration that IAs can target various CSC populations (including populations expressing ABCB1 and ABCG2) [3–8, 12] was particularly remarkable. Despite this, a definite role of drug transporters in the response to IA-based treatment has not been established.
In the current study, we addressed this question using well-characterized human cancer cell lines. We provided evidence that, unlike several published CSC compartments, stem-like OvCa SP cells expressing high levels of ABCB1 and ABCG2 were not selectively targeted by the IAs salinomycin and nigericin. Mechanistic studies finally revealed that drug transporter expression, exemplified by ABCB1, can mediate protection from the cytotoxicity of IAs, and we speculate that this effect is achieved through drug efflux, although our data cannot exclude indirect protective effects, for instance via gene regulation. Overall, our results establish a role, so far unrecognized, of drug transporters in protection from IAs. Considering the high conservation of drug transporters among stem cells [11], the use of IAs as CSC-selective drugs must be re-evaluated, although they might still be able to sensitize to other drugs in combination treatment approaches.
Acknowledgments
This work was supported by a research grant to D.W. and A.G.Z. from Oncotyrol. The Competence Center Oncotyrol is funded within the scope of the COMET-Competence Centers for Excellent Technologies through Bundesministerium für Verkehr, Innovation und Technologie, Bundesministerium für Wissenschaft, Forschung und Wirtschaft, the province of Salzburg, and the Tiroler Zukunftsstiftung/Standortagentur Tirol. The COMET program is conducted by the Austrian Research Promotion Agency. M.B. is supported by the Oesterreichische Krebshilfe Tirol. D.W. is supported by the Deutsche Forschungsgemeinschaft, the Austrian Society of Hemato-Oncology, the Forschungsfonds of the Austrian National Bank, and the Tiroler Landeskrankenanstalten. The excellent technical assistance of Elisabeth Hoflehner and Julia Roessler is gratefully acknowledged. We thank Dr. Walther Parson for cell line authentication using short tandem repeat profiling.
Author Contributions
M.B.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.G.Z.: conception and design, collection and/or assembly of data, final approval of manuscript; H.R. and G.G.: data analysis and interpretation, final approval of manuscript; S.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; D.W.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.G.Z. and D.W. have compensated research funding from Oncotyrol. The other authors indicated no potential conflicts of interest.
References
- 1.Mitani M, Yamanishi T, Miyazaki Y. Salinomycin: A new monovalent cation ionophore. Biochem Biophys Res Commun. 1975;66:1231–1236. doi: 10.1016/0006-291x(75)90490-8. [DOI] [PubMed] [Google Scholar]
- 2.Bolder NM, Wagenaar JA, Putirulan FF, et al. The effect of flavophospholipol (Flavomycin) and salinomycin sodium (Sacox) on the excretion of Clostridium perfringens, Salmonella enteritidis, and Campylobacter jejuni in broilers after experimental infection. Poult Sci. 1999;78:1681–1689. doi: 10.1093/ps/78.12.1681. [DOI] [PubMed] [Google Scholar]
- 3.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang QL, Zhao ZQ, Li JC, et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett. 2011;311:113–121. doi: 10.1016/j.canlet.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Ketola K, Hilvo M, Hyötyläinen T, et al. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br J Cancer. 2012;106:99–106. doi: 10.1038/bjc.2011.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo SZ, Blair KJ, Rahimy E, et al. Salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells despite activation of epithelial-mesenchymal transition and Akt. BMC Cancer. 2012;12:556. doi: 10.1186/1471-2407-12-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue W, Hamaï A, Tonelli G, et al. Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy. 2013;9:714–729. doi: 10.4161/auto.23997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusunoki S, Kato K, Tabu K, et al. The inhibitory effect of salinomycin on the proliferation, migration and invasion of human endometrial cancer stem-like cells. Gynecol Oncol. 2013;129:598–605. doi: 10.1016/j.ygyno.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs D, Heinold A, Opelz G, et al. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390:743–749. doi: 10.1016/j.bbrc.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Lu D, Choi MY, Yu J, et al. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci USA. 2011;108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs D, Daniel V, Sadeghi M, et al. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem Biophys Res Commun. 2010;394:1098–1104. doi: 10.1016/j.bbrc.2010.03.138. [DOI] [PubMed] [Google Scholar]
- 13.Riccioni R, Dupuis ML, Bernabei M, et al. The cancer stem cell selective inhibitor salinomycin is a p-glycoprotein inhibitor. Blood Cells Mol Dis. 2010;45:86–92. doi: 10.1016/j.bcmd.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Zeimet AG, Reimer D, Sopper S, et al. Ovarian cancer stem cells. Neoplasma. 2012;59:747–755. doi: 10.4149/neo_2012_094. [DOI] [PubMed] [Google Scholar]
- 15.Marth C, Zeimet AG, Herold M, et al. Different effects of interferons, interleukin-1beta and tumor necrosis factor-alpha in normal (OSE) and malignant human ovarian epithelial cells. Int J Cancer. 1996;67:826–830. doi: 10.1002/(SICI)1097-0215(19960917)67:6<826::AID-IJC12>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Bénard J, Da Silva J, De Blois MC, et al. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985;45:4970–4979. [PubMed] [Google Scholar]
- 17.Rumpold H, Wolf AM, Gruenewald K, et al. RNAi-mediated knockdown of P-glycoprotein using a transposon-based vector system durably restores imatinib sensitivity in imatinib-resistant CML cell lines. Exp Hematol. 2005;33:767–775. doi: 10.1016/j.exphem.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Boesch M, Zeimet AG, Reimer D, et al. The side population of ovarian cancer cells defines a heterogeneous compartment exhibiting stem cell characteristics. Oncotarget. 2014;5:7027–7039. doi: 10.18632/oncotarget.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boesch M, Reimer D, Rumpold H, et al. DyeCycle Violet used for side population detection is a substrate of P-glycoprotein. Cytometry A. 2012;81:517–522. doi: 10.1002/cyto.a.22038. [DOI] [PubMed] [Google Scholar]
- 20.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moserle L, Indraccolo S, Ghisi M, et al. The side population of ovarian cancer cells is a primary target of IFN-α antitumor effects. Cancer Res. 2008;68:5658–5668. doi: 10.1158/0008-5472.CAN-07-6341. [DOI] [PubMed] [Google Scholar]
- 22.Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102:1276–1283. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzo S, Hersey JM, Mellor P, et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 2011;10:325–335. doi: 10.1158/1535-7163.MCT-10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maugeri-Saccà M, Bartucci M, De Maria R. DNA damage repair pathways in cancer stem cells. Mol Cancer Ther. 2012;11:1627–1636. doi: 10.1158/1535-7163.MCT-11-1040. [DOI] [PubMed] [Google Scholar]