A prospective, randomized, multicenter, open-label study of the safety and feasibility of bone marrow aspirate concentrate (BMAC) infused retrograde into the coronary sinus was performed. All patients receiving BMAC experienced improvements in left ventricular ejection fraction without complications, but only those with nonischemic heart failure (HF) showed improvements in left ventricular end-systolic diameter and B-type natriuretic peptide. These results provide the basis for a larger clinical trial in HF patients.
Keywords: Heart failure, Cell transplantation, Coronary sinus, Retrograde approach
Abstract
Cell therapy is an evolving option for patients with end-stage heart failure and ongoing symptoms despite optimal medical therapy. Our goal was to evaluate retrograde bone marrow cell delivery in patients with either ischemic heart failure (IHF) or nonischemic heart failure (NIHF). This was a prospective randomized, multicenter, open-label study of the safety and feasibility of bone marrow aspirate concentrate (BMAC) infused retrograde into the coronary sinus. Sixty patients were stratified by IHF and NIHF and randomized to receive either BMAC infusion or control (standard heart failure care) in a 4:1 ratio. Accordingly, 24 subjects were randomized to the ischemic BMAC group and 6 to the ischemic control group. Similarly, 24 subjects were randomized to the nonischemic BMAC group and 6 to the nonischemic control group. All 60 patients were successfully enrolled in the study. The treatment groups received BMAC infusion without complications. The left ventricular ejection fraction in the patients receiving BMAC demonstrated significant improvement compared with baseline, from 25.1% at screening to 31.1% at 12 months (p = .007) in the NIHF group and from 26.3% to 31.1% in the IHF group (p = .035). The end-systolic diameter decreased significantly in the nonischemic BMAC group from 55.6 to 50.9 mm (p = .020). Retrograde BMAC delivery is safe. All patients receiving BMAC experienced improvements in left ventricular ejection fraction, but only those with NIHF showed improvements in left ventricular end-systolic diameter and B-type natriuretic peptide. These results provide the basis for a larger clinical trial in HF patients.
Significance
This work is the first prospective randomized clinical trial using high-dose cell therapy delivered via a retrograde coronary sinus infusion in patients with heart failure. This was a multinational, multicenter study, and it is novel, translatable, and scalable. On the basis of this trial and the safety of retrograde coronary sinus infusion, there are three other trials under way using this route of delivery.
Introduction
Cardiovascular disease is the leading cause of death in the world [1, 2]. The overall mortality from coronary artery disease (CAD) has declined because of several factors, including improvements in pharmacologic therapy and revascularization techniques; in particular, reperfusion therapy with primary percutaneous coronary intervention [3]. These life-saving advances in the management of acute and chronic CAD have been associated with an increased prevalence of patients with heart failure (HF), with an estimated 250,000–350,000 patients who might be candidates for more advanced therapies [4]. HF, like all cardiovascular diseases, is highly age-dependent, and the prevalence will potentially double by 2030 with the advancing age of the U.S. and world populations [5].
The therapeutic options for patients who develop advanced HF are limited to heart transplantation or the use of ventricular assist devices [6]. This results in a large HF patient population with progressive symptoms and limited treatment options [7]. Biologically based cell and gene therapies for advanced HF have shown promise [8–11]. The goal of biological therapy, which uses the body’s native repair mechanisms, is to reverse or restore the function of organs, tissues, and blood vessels. As such, it is ideal for HF patients. Many sources of stem cells are available for biological therapies, including abdominal fat [12], genetically engineered fibroblasts [13, 14], resident cardiac stem cells [15], human umbilical cords [16], and human endometrium [17]. However, the most widely used biological therapy has been autologous bone marrow for the treatment of cardiovascular disease [8, 11].
The route of delivery is an important variable in biologically based therapy. Direct epicardial injection at open heart surgery [18] and intracoronary infusion for acute myocardial infarction (MI) were the first methods described. Intracoronary delivery depends on inflammatory signals to recruit the cells to areas of injury and might be suboptimal in patients with advanced CAD or previous coronary artery bypass grafting. Therefore, catheters were developed to deliver biologic agents into the myocardium percutaneously using electrical mapping, fluoroscopy, or echocardiography to obtain precise localizations [19, 20]. Percutaneous, subendocardial, intramyocardial delivery is effective but carries risk, including perforation. In addition, patients with aortic valve disease, a thin myocardium, or left ventricular (LV) thrombus are not candidates. Both intracoronary and intramyocardial delivery are also limited in the number of cells that can be safely delivered. This has made translation from preclinical models to human dosing more challenging.
Retrograde venous delivery via the coronary sinus has been shown to be successful for cardioplegic solution delivery in open heart surgical procedures [21]. Preclinical models have demonstrated delivery efficacy equal to intramyocardial delivery. Additionally, a large number of cells can be delivered with this technique, and few patient contraindications exist. Tuma et al. demonstrated the safety of retrograde delivery in a study in which a large number of bone marrow cells were delivered retrograde to patients with chronic myocardial ischemia [22]. Based on their study results, we conducted a clinical trial to evaluate the safety and efficacy using the retrograde delivery of bone marrow-derived stem cells in patients with advanced HF.
Materials and Methods
Study Design
An institutional review board-approved prospective, randomized, multicenter, open-label study (ClinicalTrials.gov identifier NCT01299324) was conducted to assess the safety and feasibility of retrograde infusion of a concentrate of nucleated bone marrow cells via the coronary sinus in patients with congestive HF. All patients were deemed to have received optimal medical treatment by a HF specialist. The primary endpoints of the study were the safety and feasibility of using bone marrow aspirate concentrate (BMAC) delivered retrograde into the coronary sinus of patients with heart failure. The secondary endpoints were the safety of retrograde delivery, quality of life (QOL) measured by the Minnesota Living with Heart Failure questionnaire (MLWHFQ), cardiovascular symptoms measured by the New York Heart Association (NYHA) HF classification and the Canadian Cardiovascular Society (CCS) angina classification, B-type natriuretic peptide (BNP) levels, LV ejection fraction (LVEF) by echocardiography, and ischemia and LVEF measured by single photon emission computed tomography (SPECT) in the ischemic group. Additionally, the efficacy of retrograde BMAC delivery was compared with optimized standard of medical care directed by a heart failure cardiologist. All clinical adverse events were evaluated by the adjudication committee at the time of the incident, and a determination was made of whether the event was related or unrelated to the experimental treatment. The enrolled patients were stratified to ischemic and nonischemic groups. Within these groups, the patients were randomized electronically at enrollment to receive either BMAC via retrograde delivery or the medical standard of care in a 4:1 ratio. Only the patients randomized to receive BMAC underwent bone marrow aspiration and infusion. The patients randomized to the control group continued to receive the standard of care medical therapy as prescribed by a heart failure specialist. All imaging and follow-up information was blinded for the reviewers (i.e., echocardiography).
Patient Selection
The eligible patients were aged 18 years or older with congestive HF, an LVEF <40% by contrast echocardiography, NYHA class III or IV, stable with standard medical therapy for at least 1 month before screening, and had a life expectancy of 6 months or longer. The exclusion criteria included previous radiation treatment, bone marrow disorders, cirrhosis, bleeding/clotting disorders, acute MI less than 7 days from the treatment date, uncontrolled diabetes (hemoglobin A1c >9%), known active malignancy or risk of active malignancy (defined as abnormal PAP, chest radiograph, or mammogram findings or positive fecal hemoccult result), antibiotics within 7 days, high-dose steroids within 1 month before the procedure, dialysis, serum creatinine >3.0 mg/dl, and pregnancy.
Procedure
The patients in the BMAC group were taken to the catheterization laboratory, where both the harvest of bone marrow and the infusion of cells occurred during the same visit. Patients taking warfarin were corrected to an international normalized ratio of <1.6 at the time of the procedure. The patient’s heart rate, blood pressure, and electrocardiographic (ECG) tracing were monitored throughout the procedure. Using a standard technique, a 240-ml sample of bone marrow was obtained from the posterior iliac crest. The sample was then processed and concentrated to a volume of 60 ml over 15 minutes using the Harvest Bone Marrow Aspirate Concentrate System (Harvest Technologies, Terumo BCT, Plymouth, MA, http://www.harvesttech.com). The Harvest BMAC system concentrates the entire nucleated cell population from the marrow aspirate and the platelets while reducing the number of red blood cells in the final product. It is a point-of-care system and concentrates the bone marrow at the patient’s bedside. This concentrate was then drawn up into a series of sterile syringes for infusion into the coronary sinus.
The coronary sinus was accessed via the right femoral vein using a 7F 8-mm × 40-mm balloon catheter (Cook Medical Inc., Lafayette, IN, http://www.cookmedical.com) under fluoroscopic guidance. The proper catheter position and balloon occlusion were confirmed with fluoroscopy. The prepared cell concentrate of 60 ml was infused continuously over a 5-minute period. The balloon remained inflated for 10 minutes after infusion to permit the migration of the cells into the cardiac tissue. After this dwell time, the balloon was deflated and the catheter removed. Local hemostasis was obtained, and the patient was transferred to a telemetry monitored unit for 24 hours of observation. The average time to perform the cell delivery from venous access to catheter removal was 29 ± 14 minutes.
Follow-Up
The patients returned for follow-up visits at 1, 3, 6, and 12 months. Cardiac biomarkers and chemistry and hematological laboratory tests were collected at their initial screening and subsequent 3-, 6-, and 12-month follow-up visits. For the BMAC patients, laboratory tests were obtained both before and after the procedure, including markers of cardiac injury, troponin I and creatine phosphokinase, and the complete blood count and renal function tests. Adverse events were recorded at each visit. SPECT scans were obtained at baseline and at 6 months in the ischemic group to assess LV ischemia and function. Echocardiography, ECG, and the MLWHFQ were performed at the 3-, 6-, and 12-month follow-up visits.
Statistical Analysis
Study data were collected using a dedicated electronic data capture system prospectively with real-time queries. Patient demographics, clinical characteristics, and safety data are summarized by ischemic stratification (ischemic vs. nonischemic) and treatment group (BMAC vs. control). Descriptive statistics are provided in the analysis of all safety, cardiac function, laboratory, health status, and quality-of-life endpoints in the present study. Data analysis was performed as intent to treat. The descriptive statistics for continuous variables included the mean, median, SD, median, minimum, maximum, and sample size. Categorical variables are described with numbers and percentages. The incidence rates of adverse events (AEs) are tabulated by treatment group and ischemic stratification for the various types of adverse events (e.g., all AEs, serious AEs [SAEs], deaths, and nonfatal AEs possibly related to the procedure), and body system. The severity, duration, and relationship of AEs to BMAC were recorded on the electronic case report forms. The incidence of AEs by severity and relationship to BMAC was compared by treatment group and ischemic stratification. Cardiac function and QOL parameters are tabulated and summarized by treatment group, ischemic stratification, and time point with descriptive statistics. Statistical analysis was performed using SAS, version 9.2 or higher (SAS Institute, Cary, NC, http://www.sas.com). The p values, when presented, are two-sided and considered statistically significant at the two-sided .05 level of significance, unless otherwise specified. No imputations for missing data were performed, and no adjustment for multiple comparisons was made, given the exploratory nature of this safety study.
Results
Disposition
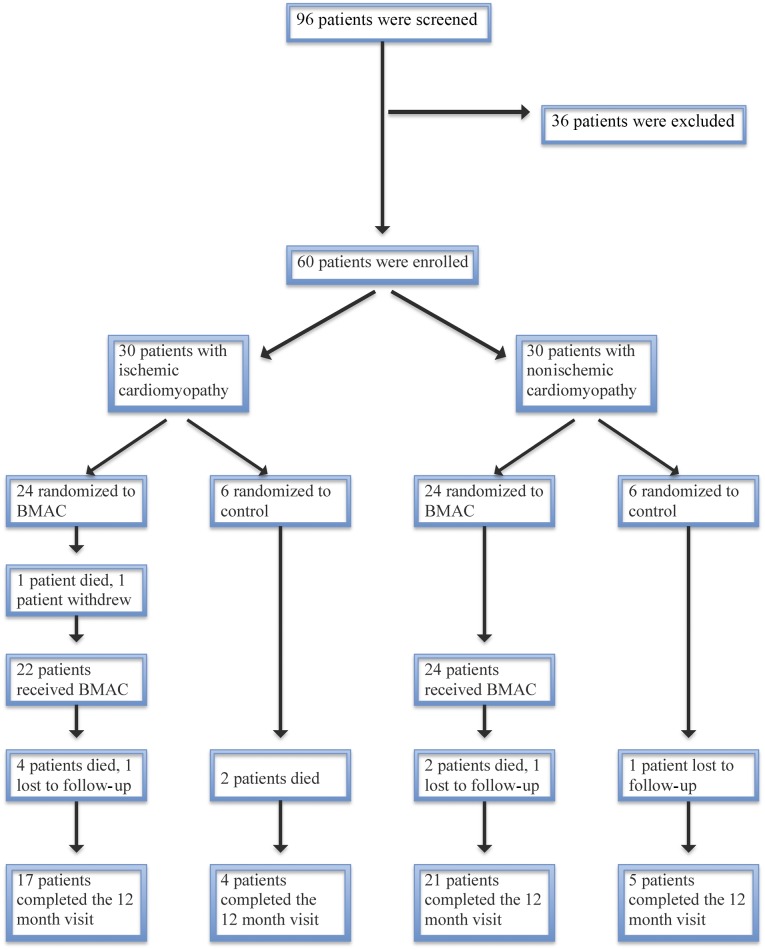
Between March 2011 and February 2012, 96 patients underwent the consent process and were screened, leading to 60 who met inclusion criteria and were randomized. Of the 60 patients, 30 were in each group (ischemic and nonischemic). Within each group, 24 patients were randomized to receive BMAC and 6 to serve as the controls.
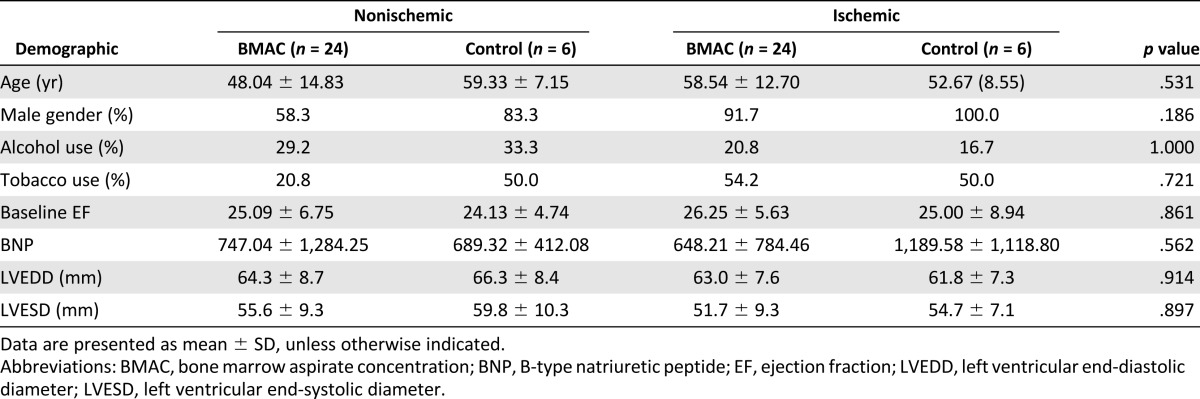
Of the patients in the ischemic and nonischemic groups, 93% and 63% were male, respectively. The mean age was 57 years in the ischemic group and 53 years in the nonischemic group. No significant differences were present in age, race, gender, or alcohol or tobacco use between the control and treatment arms within the ischemic and nonischemic groups (Table 1). For patients in the ischemic group, the baseline mean LVEF was 25.0% (SD, 8.94%) and 26.25% (SD, 5.63%) for the control and BMAC groups, respectively. In the nonischemic group, the baseline mean LVEF was 24.13% (SD, 4.74%) and 25.09% (SD, 6.75%) in the control and BMAC groups, respectively, as determined by echocardiography. All patients were in NYHA class III or IV at the time of screening. The demographics of all patients by randomization assignment are listed in Table 1.
Table 1.
Patient demographics
All enrolled subjects were randomized with no crossover between the groups. Two patients in the ischemic treatment group did not receive BMAC; 1 died after randomization, and 1 withdrew because of an unrelated adverse event. No technical difficulties occurred with the cell delivery procedure, and the mean number of cells infused in the treatment group was 3.7 ± 0.9 × 109 nucleated cells. Flow cytometry of the concentrate showed a mean CD34+ count of 39.4 × 106 cells (range, 22.1–51.6 × 106), with 84% cellular viability (range, 81%–90%). Total occlusion of the proximal coronary sinus was obtained in every case, with no adverse events or hemodynamic changes related to BMAC delivery.
Of the original 60 patients enrolled, 47 completed the study; 9 patients died, 1 before receiving the treatment, and 3 were lost to follow-up. Figure 1 shows patient disposition.
Figure 1.
Patient disposition. Patients with heart failure were stratified by the presence of ischemic or nonischemic disease. Within each stratification, the patients were randomized to BMAC or control in a 4:1 ratio. The numbers of patients randomized, treated, and followed for 12 months are shown for each study arm. Abbreviation: BMAC, bone marrow aspirate concentrate.
Safety
Of the ischemic control group subjects and ischemic BMAC group patients, 83% (5 of 6) and 100% (22 of 22) experienced at least one AE, respectively. Similarly, 83% (5 of 6) and 88% (21 of 24) of the nonischemic control and BMAC patients, respectively, experienced an AE (p = .253).
The incidence of nonserious adverse events possibly related to the procedure among patients receiving BMAC was 22.7% (5 of 22) among the ischemic patients and 16.7% (4 of 24) among the nonischemic patients. These nonserious events included elevated troponin levels in 2 patients, catheterization site hematomas in 4 patients, bleeding at the marrow aspiration site in 1 patient, elevated serum creatinine in 1 patient, and pain at the aspiration site in 1 patient.
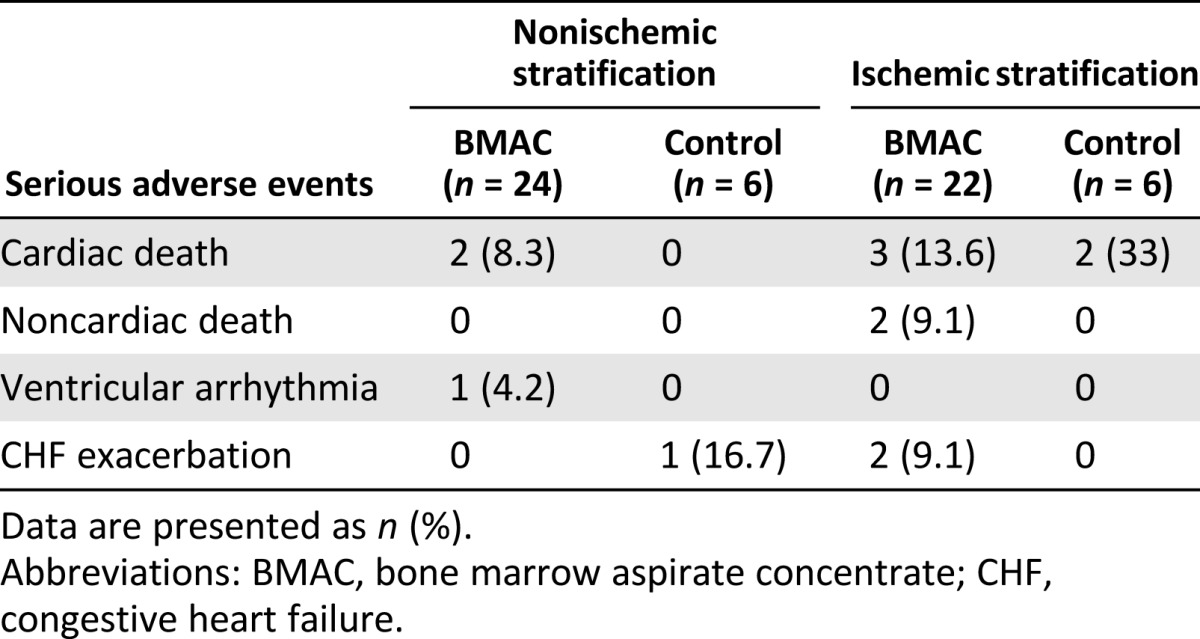
The incidence of SAEs was 33% and 31.8% in the ischemic control and BMAC groups and 16.7% and 16.7% in the nonischemic control and BMAC groups, respectively. Nine patients died, two in the ischemic control group, five in the ischemic BMAC group, and two in the nonischemic BMAC group (p = .928). Five patients experienced congestive heart failure exacerbation requiring hospital admission, and one patient developed a ventricular arrhythmia; all resolved with treatment. All the SAEs, including the 9 deaths, were classified by the site primary investigators as “unrelated” or “unlikely” to be related to the procedure. SAEs deemed to be related to the procedure included hematomas at the catheterization site and elevated serum creatinine. Serious adverse events are listed in Table 2.
Table 2.
Serious adverse events by stratification and treatment group
Quality of Life
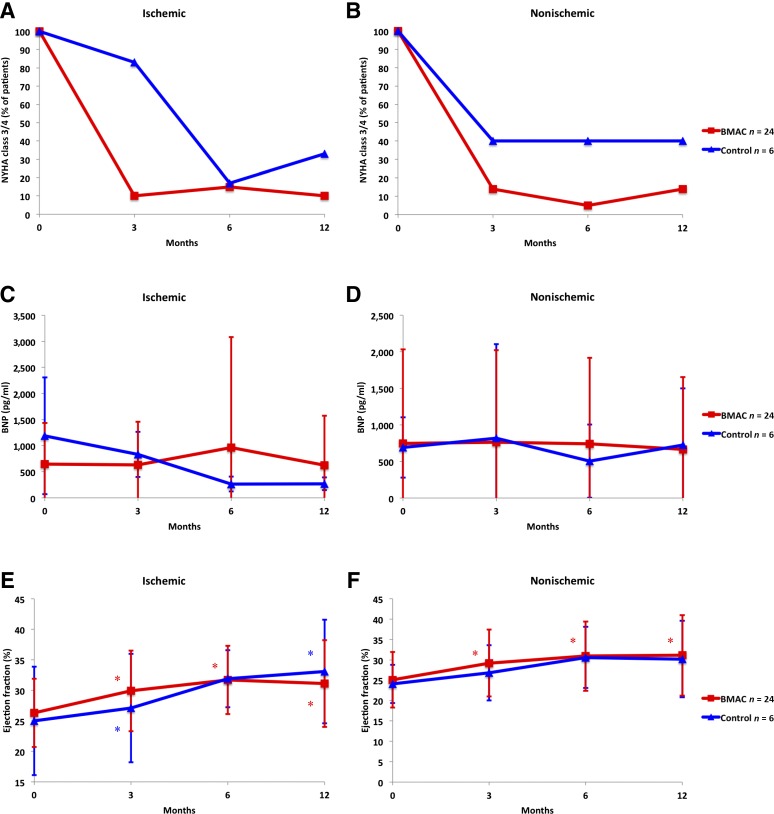
In general, the BMAC-treated patients did not progress to worsening NYHA classifications from baseline to the 12-month follow-up visit. All patients were in NYHA class 3 or 4 at the time of screening. At the 12-month follow-up visit, 8, 10, and 3 patients were in NYHA class 1, 2, and 3 in the nonischemic BMAC group and 3, 1, and 1 patient in the ischemic control group were in NYHA class 2, 3, and 4, respectively. In the ischemic BMAC group, 6, 9, 1, and 1 patient were in NYHA class 1, 2, 3, and 4 and 1, 1, and 2 patients in the ischemic control group were NYHA class 1, 2, and 3, respectively. The progression in health status as measured by the NYHA classification among the nonischemic BMAC patients was better than that in the nonischemic control group at all time points. A similar, although less pronounced, trend was seen in the ischemic stratification. The progression of NYHA status is shown in Figure 2A, 2B. The changes seen in NYHA class were not significant. The MLWHFQ scores improved from screening to 6 months among the BMAC and control patients in the ischemic and nonischemic stratifications both, with no significant different between groups. The CCS scores improved minimally in the nonischemic stratification, with 24 of 27 patients in class 1 at screening, and 27 of 27 in class 1 at 12 months. In the ischemic stratification, the CCS class worsened in the BMAC group, with an initial median CCS of 1 and a median of 2 at 12 months. In the ischemic control group, the distribution of CCS scores did not change.
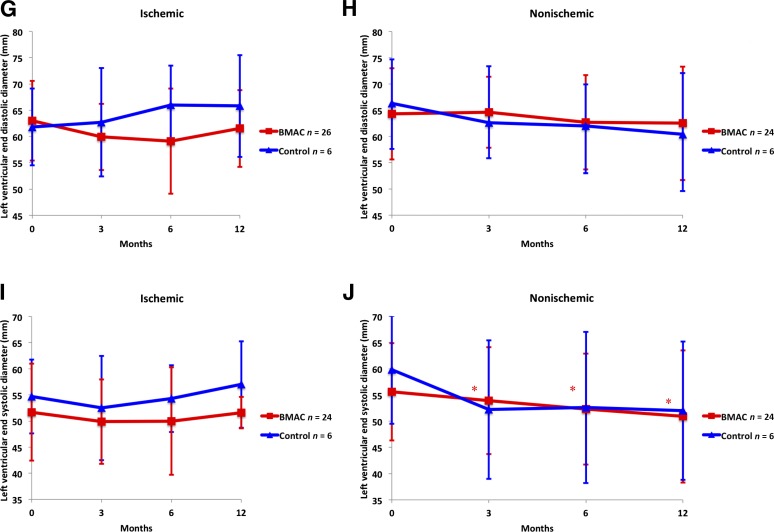
Figure 2.
Cardiovascular parameters. The cardiovascular parameters for both ischemic and nonischemic cardiomyopathy patients in the BMAC and control groups. The percentage of BMAC (red squares) or control (blue triangles) patients with a NYHA class score of III or IV is shown for ischemic (A) and nonischemic (B) patients. (C, D): BNP in ischemic (C) and nonischemic (D) patients. (E, F): Ejection fraction in ischemic (E) and nonischemic (F) patients. (G, H): Left ventricular end-diastolic diameter in ischemic (G) and nonischemic (H) patients. (I, J): Left ventricular end-systolic diameter in ischemic (I) and nonischemic (J) patients. Results are presented as mean and standard deviation. ∗, p < .05, statistically significant compared with baseline. Abbreviations: BMAC, bone marrow aspirate concentrate; BNP, B-type natriuretic peptide; NYHA, New York Heart Association.
Objective Measures
In the ischemic stratification, 22 of the 24 BMC group patients had an elevated BNP level at screening. In the control group, all 6 patients had elevated BNP. In the nonischemic stratification, 20 of 24 patients in the BMAC group had elevated BNP, and 5 control group patients had elevated BNP. BNP decreased in the nonischemic BMAC group. For this group, the average change in BNP from before infusion was −21.9, −26.2, −47.7, and −100.7 pg/ml at discharge and 3, 6, and 12 months respectively, compared with the nonischemic control group with BNP changes of +118.6, −162.6, and +24.0 pg/ml at 3, 6, and 12 months, respectively. In the ischemic stratification, the BNP changes in the BMAC group were −34.1, −12.11, +417.6, and +182.5 pg/ml at discharge and 3, 6, and 12 months compared with −355.7, −240.0, and −234.4 at 3, 6, and 12 months in the control group, respectively. The ischemic control group had very high BNP levels at baseline, nearly double that of the other groups. Changes in BNP between the BMAC and control groups did not reach statistical significance in either the ischemic or nonischemic stratification (Fig. 2C, 2D).
The mean ejection fraction in the BMAC groups in both the ischemic and the nonischemic stratifications demonstrated significant improvement compared with baseline screening by contrast echocardiography. The EF improved from 25.1% at screening to 31.1% at 12 months (p = .007) in the nonischemic group. The mean EF in the ischemic control group also demonstrated significant improvement compared with baseline, from 25.0% at screening to 33.1% at 12 months (p = .006; Fig. 2E, 2F). In both the ischemic and the nonischemic stratifications, no significant differences were seen between the mean EF in the BMAC and control groups at 12 months (p = .814 and p = .954, respectively).
A significant decrease was seen in the LV end-systolic diameter (LVESD) as measured by echocardiography in the BMAC group of the nonischemic stratification, which during the 12-month period changed from 55.6 mm to 50.9 mm (p = .020). A similar result was not seen in the ischemic BMAC group, with the LVESD changing from 51.7 mm to 51.6 mm at 12 months (p = .0692). No significant changes were seen in the end-diastolic diameter in either stratification. (Fig. 2G–2J).
SPECT was performed at the screening visit and at the 6-month follow-up visit only in the patients in the ischemic stratification. No significant change was seen in LVEF or perfusion in either the control or BMAC groups at the 6-month follow-up visit. The LVEF in the ischemic BMAC group changed from a mean of 27.09% ± 7.46% to 29.29% ± 6.74% at 6 months and 23.33% ± 10.39% to 31.25% ± 14.24% in the ischemic control group.
Discussion
The present study is the first randomized trial using retrograde coronary sinus delivery of bone marrow mononuclear cells in HF patients. The trial has demonstrated both the safety and the feasibility in patients with NYHA class 3 or 4 HF. Preclinical studies have demonstrated that total occlusion of the coronary sinus for up to 60 minutes is safe owing to the presence of the Thebesian veins, which also drain cardiac venous blood. This allows biologic agents to be infused retrograde under low pressure into the great and middle cardiac veins. Zakharova et al. also recently demonstrated the preclinical feasibility of retrograde cell delivery with distribution throughout the left ventricle [23]. Retrograde delivery of cells was developed to minimize the risks associated with cardiac cell delivery. Most endocardial, epicardial, and intracoronary delivery techniques have limitations such as myocardial wall thickness, invasiveness, or coronary blockage that would cause perforation or poor cell distribution. The number of cells delivered by these techniques is limited by microclumping, leading to infarcts. Thus far, cell doses of no greater than 300 million cells have been safely used owing to these issues. Retrograde delivery enables a larger cell dose, which is translatable based on most preclinical models [24]. The optimal retention of cells remains an ongoing investigation. Thus, retrograde coronary sinus delivery was chosen for the cell delivery route in the present trial.
Although adverse events occurred in all treatment arms, most were classified as unrelated to the procedure and should not raise safety concerns for this procedure. The rate of death in these patients should be considered a function of the poor health of this patient population that we aim to improve with this procedure, rather than a function of the procedure or cell infusion itself.
Although the present study was not adequately powered to show statistical significance between the control and BMAC groups, the significant change in ejection fraction in all patients receiving BMAC with both etiologies of heart failure and the reduction in end-systolic diameter in the nonischemic group is encouraging compared with the patients’ baseline parameters. Although the control group for the ischemic stratification demonstrated significant improvement at some time points, the BMAC groups demonstrated significance at all time points.
The decrease in end-systolic diameter indicates a signal for positive left ventricular remodeling, although this was significant only in the nonischemic patients. Although not statistically significant, a trend was also seen toward decreasing BNP levels in patients with nonischemic cardiomyopathy receiving BMAC compared with the ischemic BMAC group, in which the BNP level fluctuated to a greater degree. This decrease in BNP, coupled with the improved ejection fraction, potentially demonstrates an increased ability of the cardiac myocytes to tolerate stretch and volume overload. The presence of this finding mostly in the nonischemic patients might indicate that the ability for cardiac myocytes to remodel is dependent on cellular functions that are lost in ischemia. This is an important distinction, because data from the International Society for Heart and Lung Transplant [25] have shown that the percentage of patients with an ischemic etiology for their heart failure has declined from 50% 10 years ago to now only 37% of patients undergoing heart transplant.
The exact mechanism for improvement with stem cell therapy remains controversial. Recent research by Mirotsou et al. has indicated that transplanted cells do not remain in the heart for more than a few days; therefore, the benefit is likely related to the release of paracrine factors by stem cells into the injured myocardium that mediate neovascularization and remodeling [26]. Specifically, BMAC has been shown to significantly increase the levels of angiogenic ligands and interleukin-10 (IL-10). In a comparison of BMAC and IL-10-deficient BMAC, the latter failed to decrease the infarct size compared with wild-type BMAC.
A number of different cell types have been used in heart failure. Heldman et al. compared patients with ischemic cardiomyopathy receiving either intramyocardially delivered mesenchymal stem cells or mononuclear bone marrow stem cells to a placebo group and demonstrated an improvement in the MLWHFQ score in both treatment groups, but not in the placebo group [27]. Henry et al. used a cultured multicellular product delivered intramyocardially in patients with NYHA class III/IV, with a LVEF <30%. The patients with ischemic dilated cardiomyopathy appeared to benefit more than the nonischemic patients, with fewer cardiac adverse events, improved wall motion index scores, and improved MLWHFQ scores [28]. A recent study by Vrtovec et al., using selected bone marrow cells in patients with nonischemic dilated cardiomyopathy, showed significant improvement in ventricular function, without significant changes in LV end-diastolic diameter or LVESD [29].
Conclusion
The present study is the first prospective randomized clinical trial using bone marrow mononuclear cells infused into the coronary sinus of HF patients. Our study met its primary end point, demonstrating that a large number of bone marrow cells can be safely delivered via retrograde coronary sinus delivery with no direct procedure-related adverse events. Although the present study was not powered to demonstrate statistical significance, the results show early clinical signals that warrant further investigation. The present study was limited in its sample size and was open labeled. However, the findings should lead to larger studies to further evaluate the potential benefits of BMAC in patients with ischemic and nonischemic cardiomyopathy.
Acknowledgment
This study was funded by Harvest Technologies, Plymouth, MA.
Author Contributions
A.N.P.: conception/design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.M., G.T., H.I., and N.T.: provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; A.A.W. and T.D.H.: data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.N.P. has compensated honoraria from Cook Medical Inc. The other authors indicated no potential conflicts of interest.
References
- 1.World Health Organization. Report of the global survey on the progress in national chronic diseases prevention and control. 2007. Available at: http://www.who.int/chp/ncd_capacity/CCS_2005.pdf?ua=1. Accessed April 1, 2015.
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: Heart disease and stroke statistics—2013 Update: A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Commerce Economics and Statistics Administration U.S. Census Bureau. Age projections. http://www.censusscope.org/us/chart_age.htm. Accessed April 1, 2015.
- 5.National Center for Health Care Statistics. http://www.cdc.gov/nchs. Accessed April 1, 2015.
- 6.Miller LW, Guglin M. Patient selection for ventricular assist devices: A moving target. J Am Coll Cardiol. 2013;61:1209–1221. doi: 10.1016/j.jacc.2012.08.1029. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 8.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsa E, Sallam K, Wu JC. Cardiac stem cell biology: Glimpse of the past, present, and future. Circ Res. 2014;114:21–27. doi: 10.1161/CIRCRESAHA.113.302895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajjar RJ. Potential of gene therapy as a treatment for heart failure. J Clin Invest. 2013;123:53–61. doi: 10.1172/JCI62837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeevanantham V, Butler M, Saad A, et al. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: A systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meliga E, Strem BM, Duckers HJ, et al. Adipose-derived cells. Cell Transplant. 2007;16:963–970. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 13.Murry CE, Pu WT. Reprogramming fibroblasts into cardiomyocytes. N Engl J Med. 2011;364:177–178. doi: 10.1056/NEJMcibr1013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song K, Nam YJ, Luo X, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: A paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Patel AN, Vargas V, Revello P, et al. Mesenchymal stem cell population isolated from the subepithelial layer of umbilical cord tissue. Cell Transplant. 2013;22:513–519. doi: 10.3727/096368912X655064. [DOI] [PubMed] [Google Scholar]
- 17.Bockeria L, Bogin V, Bockeria O, et al. Endometrial regenerative cells for treatment of heart failure: A new stem cell enters the clinic. J Transl Med. 2013;11:56. doi: 10.1186/1479-5876-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menasché P, Hagège AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 19.Dib N, Menasche P, Bartunek JJ, et al. Recommendations for successful training on methods of delivery of biologics for cardiac regeneration: A report of the International Society for Cardiovascular Translational Research. JACC Cardiovasc Interv. 2010;3:265–275. doi: 10.1016/j.jcin.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Perin EC, Silva GV. Stem cell therapy in end-stage ischaemic heart failure: A catheter-based therapeutic strategy targeting myocardial viability. Eur Heart J Suppl. 2006;8(suppl H):H46–H51. [Google Scholar]
- 21.Talwalkar NG, Lawrie GM, Earle N, et al. Can retrograde cardioplegia alone provide adequate protection for cardiac valve surgery? Chest. 1999;115:135–139. doi: 10.1378/chest.115.1.135. [DOI] [PubMed] [Google Scholar]
- 22.Tuma J, Fernández-Viña R, Carrasco A, et al. Safety and feasibility of percutaneous retrograde coronary sinus delivery of autologous bone marrow mononuclear cell transplantation in patients with chronic refractory angina. J Transl Med. 2011;9:183. doi: 10.1186/1479-5876-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakharova L, Nural-Guvener H, Feehery L, et al. Retrograde coronary vein infusion of cardiac explant-derived c-Kit+ cells improves function in ischemic heart failure. J Heart Lung Transplant. 2014;33:644–653. doi: 10.1016/j.healun.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Patel AN, Silva F, Winters AA. Stem cell therapy for heart failure. Heart Fail Clin. 2015;11:275–286. doi: 10.1016/j.hfc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: Thirtieth official adult heart transplant report—2013; focus theme: Age. J Heart Lung Transplan. 2013;32:951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Mirotsou M, Jayawardena TM, Schmeckpeper J, et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry TD, Traverse JH, Hammon BL, et al. Safety and efficacy of ixmyelocel-T: An expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circ Res. 2014;115:730–737. doi: 10.1161/CIRCRESAHA.115.304554. [DOI] [PubMed] [Google Scholar]
- 29.Vrtovec B, Poglajen G, Lezaic L, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128(suppl 1):S42–S49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]