Human adipose stem cells (hASCs) have the potential to treat patients with a variety of clinical conditions. The progress and challenges of bringing adipose stem cell therapy into mainstream clinical use are discussed.
Summary
Human adipose stem cells (hASCs) have the potential to treat patients with a variety of clinical conditions. Recent advancements in translational research, regulatory policy, and industry have positioned hASCs on the threshold of clinical translation. We discuss the progress and challenges of bringing adipose stem cell therapy into mainstream clinical use.
Significance
This article details the advances made in recent years that have helped move human adipose stem cell therapy toward mainstream clinical use from a translational research, regulatory policy, and industrial standpoint. Four recurrent themes in translational technology as they pertain to human adipose stem cells are discussed: automated closed-system operations, biosensors and real-time monitoring, biomimetics, and rapid manufacturing. In light of recent FDA guidance documents, regulatory concerns about adipose stem cell therapy are discussed. Finally, an update is provided on the current state of clinical trials and the emerging industry that uses human adipose stem cells. This article is expected to stimulate future studies in translational adipose stem cell research.
Introduction
Human mesenchymal stem cells are multipotent adult stem cells that have been isolated from almost every major tissue in the body. Within the last quarter of a century, human mesenchymal stem cells have garnered much attention for use in tissue engineering, regenerative medicine, and immunomodulatory applications. The acronym hMSC is used to refer to human mesenchymal stem cells or human multipotent stem cells and often is within the context of bone marrow MSCs (BM-hMSCs) [1]. BM-hMSCs were the first hMSCs to be isolated and have thus received much attention in basic and translational research. However, human adipose stromal/stem cells (hASCs), as defined by the International Society of Cellular Therapy (ISCT) and International Federation for Adipose Therapeutics and Science (IFATS) [2], have been demonstrated to possess similar therapeutic potential but to have several distinct translational advantages. These advantages result because hASCs are derived from a generally undesired and excess tissue source: fat tissue. This allows hASCs to be obtained in large quantities from a minimally invasive procedure. Recent advancements in hASC research have capitalized on these advantages to position hASCs on the threshold of clinical translation. In the past 5 years, the number of clinical trials using hASCs has rapidly risen—from 18 to 152 studies (January 1, 2010 to March 9, 2015; clinicaltrials.gov). These clinical trials have addressed a wide range of conditions, including fistula, musculoskeletal disorders, ischemia, soft tissue damage, host-versus-graft disease, and many more. In recent years, a number of reviews have discussed the clinical translation of hASCs [3–5]. We offer a new perspective to these data by discussing the technology, regulation, and industry that must be considered to effectively translate hASCs into widespread clinical use.
Trends in Translation
Although a wealth of innovative and important research in hASC biology has occurred within the past decade, it has become apparent that to effectively translate hASCs within the near future into clinical practice will require great reliance on technologies that can simplify and engineer around the gaps in our hASC understanding. We highlight four recurrent themes in translational technology as they pertain to hASCs: automated closed-system operations, biosensors and real-time monitoring, biomimetics, and rapid manufacturing.
Automated closed-system devices will become an essential component of translating hASCs. They greatly reduce the required resources for in vitro cell handling and effectively minimize human error. In addition, automated closed-system operations assist in the implementation of the Food and Drug Association (FDA) guidelines for “process analytical technology,” a framework for controlling and regulating the manufacturing process of pharmaceutical products. Currently, automated closed-system devices have two major functions: isolation and expansion. Automated closed-system isolation devices allow clinicians to isolate a patient’s cells and readminister the cells back to the patient within the same surgery. Several companies already manufacture such devices to isolate the stromal vascular fraction (SVF) from adipose tissue [6], and the SVF has been used in a number of clinical trials for soft tissue repair. In a clinical trial encompassing both breast augmentation and facial reconstruction, the Tissue Genesis Icellator Isolation System (Tissue Genesis, Honolulu, HI, http://www.tissuegenesis.com) was used to isolate the SVF. Forty-two patients were successfully treated by cell-assisted lipotransfer, a procedure that enriches traditional lipotransfer methods with the addition of the SVF [7]. Cytori Therapeutics (San Diego, CA, http://www.cytori.com) and collaborators have also conducted a number of clinical trials using their Celution System. In a breast reconstruction study, they reported that SVF-enriched fat grafts did not elicit any serious adverse effects and showed satisfactory aesthetic results in 57 of 67 patients [8]. The clinical trials conducted by both Tissue Genesis and Cytori Therapeutics have demonstrated that closed-system machines can isolate SVF reliably. Yet another form of automated closed-system devices is bioreactors used for cell expansion. A number of recent studies have focused on optimizing bioreactors for hASC expansion [9, 10]. In the future, automated closed systems for isolation and expansion will likely be combined into the same device.
A critical component to the design of closed system devices is the ability to monitor internal conditions through the use of biosensors and real-time monitoring technologies. Biosensors are already incorporated into commercial stirred tank bioreactors to ensure batch control for commercial fermentation or pharmaceutical applications. However, the use of cell lines from different donors presents a new layer of complexity to the biomanufacturing process. It is known that hASCs isolated from different donors have differing proliferation and differentiation potentials [11, 12]. Thus, interest has been increasing in quantifying and monitoring the variability between cell lines to generate reproducible results from variable input. A technology that has the potential to be integrated into hASC expansion to monitor donor cells is Raman spectroscopy, which can be used to noninvasively quantify biochemical changes within a cell line. It has been shown that cell source-dependent variations in bone formation capacities can be monitored using Raman spectroscopy [13]. We have also shown that Raman spectroscopy can be used to noninvasively measure lipid production during hASC adipogenesis within as little as 1 day after the onset of adipogenesis [14]. Mass spectroscopy also holds promise for these types of applications. Mass spectroscopy has previously been used to monitor the proteome [15] and secretome [16] of hASCs and has the potential to monitor the hASC metabolome in real time. The use of mass spectroscopy to track hASCs allows for the use of a minimal amount of conditioned medium to provide a rapid, comprehensive, and potentially quantitative method of assessing hASCs throughout expansion and differentiation. Finally, electrical impedance spectroscopy holds similar promise, having also been used to track adipogenic and osteogenic differentiation of hASCs in real time [17]. The likely integration of biosensors into closed-system devices will allow for real-time monitoring and, if needed, the correction of conditions within such devices for the desired hASC response.
Biomimetics is essential to effective in vitro hASC technologies, in particular when extended in vitro culture or manipulation is required. Soluble chemical signals have long been used to differentiate and manipulate stem cells. However, it has become increasingly clear that mechanical and other physical stimuli also play a key role in directing stem cell fate. A wealth of recent research has been performed on the use of biomimetic mechanical loading to direct hMSC and hASC fate. We, and others, have used biomimetic magnitudes of cyclic tensile strain [12, 18–20] or fluid shear stress to promote osteogenesis [21, 22], hydrostatic pressure to promote chondrogenesis [23], oscillatory shear stress to alter actin organization and differentiation potential [24], and unloading to promote adipogenesis [25, 26] or maintain the stemness of hASC spheroids [27]. Electrical stimulation has been shown to enhance hASC differentiation for cardiac [28], neuronal [29], and osteogenic [30] applications. The use of biomimetics to direct stem cell fate will likely be incorporated into automated closed-system devices through physiologic chemical, mechanical, and electrical stimuli to further optimize hASC performance for specific applications.
Another theme that can be observed throughout current translational tissue engineering and regenerative medicine research is the use of rapid manufacturing technologies such as three-dimensional (3D) printing. The advantages of these techniques include automation, ease of generating patient-specific designs, reduced manufacturing costs, tunability, and three-dimensional tissue architecture. Numerous options and considerations are available for generating bioprinted tissues [31], many of which have not yet been used in hASC applications. However, a few recent publications have incorporated hASCs into rapid manufacturing technologies. Patient-specific reconstruction of mandibular ameloblastoma resection defects using computer-aided additive manufacturing of β-tricalcium phosphate constructs seeded with a hASC biologic component was recently reported [32]. Rapid manufacturing has also been used for cartilage applications, and it has been demonstrated that hASCs seeded on 3D printed chitosan scaffolds could be induced to differentiate down the chondrogenic lineage [33]. In an effort to generate a more biologically relevant scaffold, Pati et al. recently demonstrated that hASCs and other cell lines could be printed in a cell-laden extracellular matrix bioink to generate adipose, cartilage, and heart tissue [34]. These rapid manufacturing technologies are expected to gain even more momentum as hASC translation moves forward into patient-specific applications in the coming years.
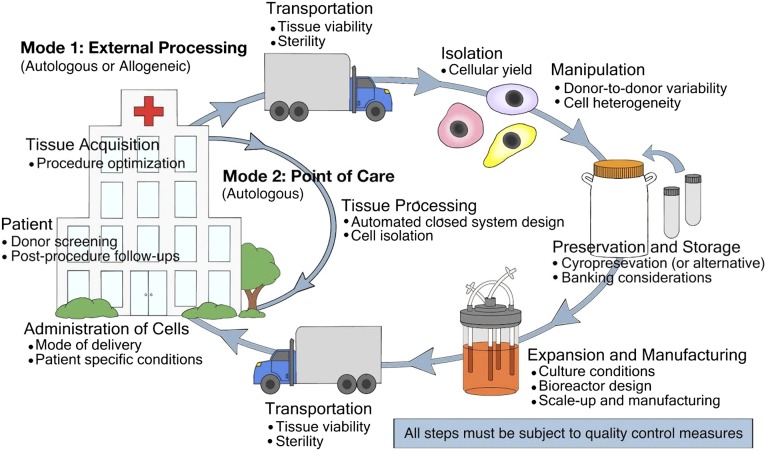
Although many advances have occurred in hASC research in the past few years, fully successful hASC translation still requires significantly more innovation. The entire process of autologous hASC therapy and the potential barriers to translation are illustrated in Figure 1. Research and the development of technologies that simplify, standardize, and enhance quality control within this process will be particularly instrumental in facilitating hASC translation.
Figure 1.
The steps of adipose stem cell therapy. Two modes of human adipose stem cell (hASC) therapy are highlighted, and examples of some critical issues at each step are shown (but by no means are all inclusive). In mode 1 of hASC therapy, standardized methods should be developed to prescreen each patient for hASC therapy candidacy and to determine the best method of adipose tissue acquisition (whether resection, liposuction, or an alternative). Because Current Good Manufacturing Practice facilities for hASCs could be located off-site, technologies for shipping hASCs should be optimized. Cell isolation technologies should maximize cellular yield. There will be many issues to consider when manipulating cells, including the high level of hASC variability between donors and the inherently heterogeneous cell population. The development of closed-system devices that continually monitor cells and adjust culture conditions to deliver a consistent hASC output might be especially useful in achieving this goal. In addition, if the patient would prefer to bank cells for future procedures, long-term storage methods must be validated for safety and efficacy. An ideal mode of hASC administration would be both condition-specific and patient-specific. After hASC treatment, standard methods are needed to monitor a patient for adverse side effects. In mode 2, hASCs are isolated, processed, and administered back to the patient at the point of care. This method will require the optimization of closed-system isolation devices and the determination of whether the stromal vascular fraction or hASCs will be the final cell therapy delivered back to the patient.
Into the Regulatory Unknown
Although impressive progress has been made in hASC research and translation, since hASCs were first isolated in the early 2000s, major challenges remain in the standardization, regulation, and quality control of hASC therapies.
In 2013, a major step toward standardization of hASC research was taken when the International Federation for Adipose Therapeutics and Science and the ISCT released a joint statement to define both adipose tissue-derived SVF and culture-expanded adipose tissue-derived stromal cells [2]. The three criteria that IFATS and ISCT used to define hASCs are plastic adherence, a specific surface antigen expression profile of greater than 80% expression of CD13, CD29, CD44, CD73, CD90, and CD105 and less than 2% expression of CD31, CD45, and CD235a, and a capacity for trilineage differentiation into osteoblasts, adipocytes, and chondroblasts. This definition has been adopted by many hASC investigators and could serve to standardize the diverse field of hASC research. Beyond the international standardization of hASC research, the field must also work toward international harmonization of translational aspects of hASC therapy. As materials and biologics from hASC therapies begin to cross international borders, national and international regulatory agencies will need to coordinate to define policies that can safely and effectively moderate the global effort toward hASC translation.
The regulatory guidelines for hASC products have been ambiguous as companies have begun to enter the hASC industry. To address this problem, in December 2014, the U.S. FDA released a draft guidance document for industry, outlining the criteria to determine whether a product derived from adipose tissue is regulated as a drug, device, and/or biological product [35]. This document, currently in draft form, outlines the four criteria that a product derived from adipose tissue must meet to avoid premarket review. The four criteria for such a product are as follows: (a) be minimally manipulated, (b) be intended for homologous use, (c) not be combined with most other agents, and (d) be derived from autologous or a first- or second-degree blood relative (unless it does not have a systemic effect or depend on the activity of living cells). Adipose tissue is defined by the FDA as a structural tissue; hence, if this document is approved, it will require adipose therapies that are intended to serve functions other than structural to undergo the entire FDA premarket approval process. This is of particular concern for breast augmentation and reconstruction therapies that currently transplant subcutaneous adipose tissue to the breast, which has other functions than structural (i.e., lactation). A wealth of evidence has shown that adipose tissue serves numerous functions other than structural, including participating in endocrine, hematopoietic, immunological, and regenerative functions within the human body [36, 37]. The FDA is currently revising this guidance document and is expected to include these topics within the final document.
Another important regulatory concern is the standardization of quality control practices in hASC therapies. In order to ensure that a safe and effective hASC product is administered to the patient, quality control must occur throughout the hASC processing shown in Figure 1. After an adipose tissue sample has been taken, it is desirable to perform an initial test to determine whether the tissue will be an acceptable donor source for the desired procedure. Karyotyping the sample to screen for any major chromosomal abnormalities has been suggested [38]. Other testing procedures that are often performed on initial isolation are colony-forming unit assays and flow cytometry cell marker analyses, based on recommendations from the IFATS and ISCT, as previously discussed [2]. During the expansion process, some have suggested performing phenotypic analysis, cytogenetics, sterility, trilineage differentiation potential testing, and colony-forming unit assays after each split [38]. This testing should also be performed after removing cells from cryopreservation. Before administering the cell product to a patient, testing often includes all or a subset of the following tests: cell number, viability, purity and identity based on cell markers, cytogenetics, bacterial and fungal sterility, endotoxin, and mycoplasma [38–40]. Quality control must also be considered for both manufacturing facilities and reagents [41]. Additional information on such Current Good Manufacturing Practice facilities and regulations for cell therapy has been previously reviewed [42]. As hASCs enter clinical translation, standard quality control measures must be developed to ensure that the hASC products are safe and effective for patient treatment.
The Big Fat Industry
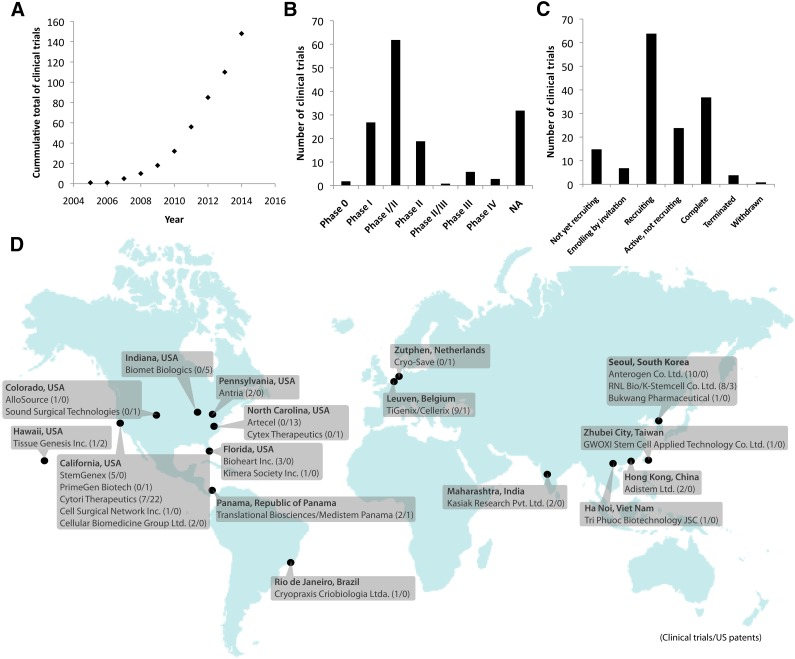
With the transition of hASCs from the laboratory bench to widespread clinical application, the emergence of an hASC-based industry will naturally occur. The beginnings of such an industry can already be identified from clinical trial and patent data. As of March 9, 2015, after steadily increasing for the past decade (Fig. 2A), 152 results could be found on clinicaltrials.gov with the search term “adipose stem cell.” Most of these trials are in phase I (18%), phase II (13%), or phase I and/or II (41%) (Fig. 2B). Most of the studies are still in the process of recruiting, although 37 trials have already been completed (Fig. 2C). The total enrollment capacity of these 152 trials includes 11,162 patients. Of the current clinical trials, 49% have been sponsored by the private sector and 51% by noncommercial entities. Clearly, significant interest exists from the private sector in hASC research. As of March 9, 2015, 25 companies were the primary sponsor of hASC clinical trials or patents specifically pertaining to hASCs registered with the U.S. government (Fig. 2D). These companies include a mixture of established companies expanding into hASC research and new companies specifically focused on hASC therapy. One of the advantages to industrial translation of hASC compared with other stem cell sources is that it is possible to perform simple, low-risk procedures such as isolating fat tissue from a patient and retransplanting it into a patient within the same procedure. This has provided a relatively low-risk entry point for firms seeking to enter the field. However, as regulatory agencies implement higher regulatory standards on nonhomologous and allogenic hASC products, entry into this field is becoming more complex. Nevertheless, if the wide range of clinical trials is any indication of the coming industry trends, the industry is likely to expand and diversify in the future. For now, it is clear that hASC companies have emerged across the globe and no sign exists of this trend slowing down.
Figure 2.
Human adipose stem cell (hASC) clinical trials and the emerging global industry. (A): The number of hASC clinical trials registered on clinicaltrials.gov has been gaining momentum for the past decade. As of March 9, 2015, most trials were still in phase I and II (B) and in the process of recruiting (C). (D): In addition to clinical trials, a global industry has emerged. The 25 mapped companies throughout the globe are current leaders within hASC commercialization as identified by hASC-related clinical trials and patents. These companies were identified from either the 152 previously mentioned clinical trials or a U.S. patent search (ABST/(adipose AND stem AND cells) or TTL/(adipose AND stem AND cells)), where ABST indicates “abstract” and TTL indicates “title,” on http://patft.uspto.gov on March 9, 2015. General mesenchymal stem cell patents and patents held by noncommercial institutions were not included within this industry map, although a number of universities and hospitals have also performed clinical trials and hold patents. Company locations and URLs can be found in the supplemental online data. Abbreviation: NA, not available.
Conclusion
Translational research pertaining to hASCs is advancing rapidly and could soon allow clinicians to treat patients with hASC therapies for a variety of conditions ranging from tissue engineering to immunomodulatory applications. As hASCs find their way into clinical practice, it is essential that researchers, industry, physicians, and regulatory agencies work together to bring promising hASC therapies to patients in a safe and effective manner.
Supplementary Material
Acknowledgments
This work was funded by a North Carolina Space Grant Fellowship (to R.C.N.), National Science Foundation (NSF) Chemical, Bioengineering, Environmental and Transport Systems (CBET) Grant 1133427 (to E.G.L.), NSF CBET Grant 1133427 (to E.G.L.), William R. Kenan Jr. Institute for Engineering, Technology and Science grant (to E.G.L.), NIH Clinical and Translational Science Award (CTSA) Grant 550KR71418 (to E.G.L.), and NIH CTSA Grant 550KR61325 (to E.G.L.). We thank Alison Nordberg for the diagram.
Author Contributions
R.C.N.: conception/design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; E.G.L.: conception/design, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gir P, Oni G, Brown SA, et al. Human adipose stem cells: Current clinical applications. Plast Reconstr Surg. 2012;129:1277–1290. doi: 10.1097/PRS.0b013e31824ecae6. [DOI] [PubMed] [Google Scholar]
- 4.Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: An update on the transition to clinical translation. Regen Med. 2012;7:225–235. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokai LE, Marra K, Rubin JP. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Doi K, Tanaka S, Iida H, et al. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: Bench and bed analysis. J Tissue Eng Regen Med. 2013;7:864–870. doi: 10.1002/term.1478. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: The RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382–389. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- 9.Schirmaier C, Jossen V, Kaiser S, et al. Scale-up of adipose tissue-derived mesenchymal stem cell production in stirred single-use bioreactors under low-serum conditions. Eng Life Sci. 2014;14:292–303. [Google Scholar]
- 10.Dos Santos F, Campbell A, Fernandes-Platzgummer A, et al. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol Bioeng. 2014;111:1116–1127. doi: 10.1002/bit.25187. [DOI] [PubMed] [Google Scholar]
- 11.Bodle JC, Teeter SD, Hluck BH, et al. Age-related effects on the potency of human adipose-derived stem cells: Creation and evaluation of superlots and implications for musculoskeletal tissue engineering applications. Tissue Eng Part C Methods. 2014;20:972–983. doi: 10.1089/ten.tec.2013.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson AD, Marvel SW, Bernacki SH, et al. Osteogenic effects of rest inserted and continuous cyclic tensile strain on hASC lines with disparate osteodifferentiation capabilities. Ann Biomed Eng. 2009;37:955–965. doi: 10.1007/s10439-009-9648-7. [DOI] [PubMed] [Google Scholar]
- 13.Gentleman E, Swain RJ, Evans ND, et al. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat Mater. 2009;8:763–770. doi: 10.1038/nmat2505. [DOI] [PubMed] [Google Scholar]
- 14.Moody B, Haslauer CM, Kirk E, et al. In situ monitoring of adipogenesis with human-adipose-derived stem cells using surface-enhanced Raman spectroscopy. Appl Spectrosc. 2010;64:1227–1233. doi: 10.1366/000370210793335106. [DOI] [PubMed] [Google Scholar]
- 15.DeLany JP, Floyd ZE, Zvonic S, et al. Proteomic analysis of primary cultures of human adipose-derived stem cells: Modulation by adipogenesis. Mol Cell Proteomics. 2005;4:731–740. doi: 10.1074/mcp.M400198-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Zvonic S, Lefevre M, Kilroy G, et al. Secretome of primary cultures of human adipose-derived stem cells: Modulation of serpins by adipogenesis. Mol Cell Proteomics. 2007;6:18–28. doi: 10.1074/mcp.M600217-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Bagnaninchi PO, Drummond N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc Natl Acad Sci USA. 2011;108:6462–6467. doi: 10.1073/pnas.1018260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall ME, Rachlin A, Otey CA, et al. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. Am J Physiol Cell Physiol. 2007;293:C1532–C1538. doi: 10.1152/ajpcell.00065.2007. [DOI] [PubMed] [Google Scholar]
- 19.Charoenpanich A, Wall ME, Tucker CJ, et al. Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: Osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors. Tissue Eng Part A. 2011;17:2615–2627. doi: 10.1089/ten.tea.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charoenpanich A, Wall ME, Tucker CJ, et al. Cyclic tensile strain enhances osteogenesis and angiogenesis in mesenchymal stem cells from osteoporotic donors. Tissue Eng Part A. 2014;20:67–78. doi: 10.1089/ten.tea.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knippenberg M, Helder MN, Doulabi BZ, et al. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780–1788. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech Model Mechanobiol. 2010;9:659–670. doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- 23.Puetzer J, Williams J, Gillies A, et al. The effects of cyclic hydrostatic pressure on chondrogenesis and viability of human adipose- and bone marrow-derived mesenchymal stem cells in three-dimensional agarose constructs. Tissue Eng Part A. 2013;19:299–306. doi: 10.1089/ten.tea.2012.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo YC, Chang TH, Hsu WT, et al. Oscillatory shear stress mediates directional re-organization of actin cytoskeleton and alters differentiation propensity of mesenchymal stem cells. Stem Cells. 2015;33:429–442. doi: 10.1002/stem.1860. [DOI] [PubMed] [Google Scholar]
- 25.Sheyn D, Pelled G, Netanely D, et al. The effect of simulated microgravity on human mesenchymal stem cells cultured in an osteogenic differentiation system: A bioinformatics study. Tissue Eng Part A. 2010;16:3403–3412. doi: 10.1089/ten.tea.2009.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Liu P, Chen L, et al. The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials. 2015;41:15–25. doi: 10.1016/j.biomaterials.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Pavesi A, Soncini M, Zamperone A, et al. Electrical conditioning of adipose-derived stem cells in a multi-chamber culture platform. Biotechnol Bioeng. 2014;111:1452–1463. doi: 10.1002/bit.25201. [DOI] [PubMed] [Google Scholar]
- 29.Jaatinen L, Salemi S, Miettinen S, et al. The combination of electric current and copper promotes neuronal differentiation of adipose-derived stem cells. Ann Biomed Eng. 2015;43:1014–1023. doi: 10.1007/s10439-014-1132-3. [DOI] [PubMed] [Google Scholar]
- 30.McCullen SD, McQuilling JP, Grossfeld RM, et al. Application of low-frequency alternating current electric fields via interdigitated electrodes: Effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells. Tissue Eng Part C Methods. 2010;16:1377–1386. doi: 10.1089/ten.tec.2009.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 32.Wolff J, Sándor GK, Miettinen A, et al. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: Experience with three cases. Ann Maxillofac Surg. 2013;3:114–125. doi: 10.4103/2231-0746.119216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye K, Felimban R, Traianedes K, et al. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS One. 2014;9:e99410. doi: 10.1371/journal.pone.0099410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pati F, Jang J, Ha DH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Food and Drug Association. Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) from Adipose Tissue: Regulatory Considerations; Draft Guidance (2014). Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/ucm427795.htm. Accessed March 9, 2015.
- 36.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;6:312–321. doi: 10.4252/wjsc.v6.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanley PJ, Mei Z, da Graca Cabreira-Hansen M, et al. Manufacturing mesenchymal stromal cells for phase I clinical trials. Cytotherapy. 2013;15:416–422. doi: 10.1016/j.jcyt.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 40.Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Gimble JM, Bunnell BA, Chiu ES, et al. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 42.Giancola R, Bonfini T, Iacone A. Cell therapy: cGMP facilities and manufacturing. Muscles Ligaments Tendons J. 2012;2:243–247. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.