In a multicenter, randomized controlled clinical trial, administration of valacyclovir for 90 days following standard intravenous acyclovir therapy for herpes simplex encephalitis did not improve outcomes, as measured by neuropsychological testing at 12 months.
Keywords: herpes simplex virus, encephalitis, antiviral therapy, valacyclovir, acyclovir
Abstract
Background. Despite the proven efficacy of acyclovir (ACV) therapy, herpes simplex encephalitis (HSE) continues to cause substantial morbidity and mortality. Among patients with HSE treated with ACV, the mortality rate is approximately 14%–19%. Among survivors, 45%–60% have neuropsychological sequelae at 1 year. Thus, improving therapeutic approaches to HSE remains a high priority.
Methods. Following completion of a standard course of intravenous ACV, 87 adult patients with HSE (confirmed by positive polymerase chain reaction [PCR] for herpes simplex virus DNA in cerebrospinal fluid) were randomized to receive either valacyclovir (VACV) 2 g thrice daily (n = 40) or placebo tablets (n = 47) for 90 days (12 tablets of study medication daily). The primary endpoint was survival with no or mild neuropsychological impairment at 12 months, as measured by the Mattis Dementia Rating Scale (MDRS). Logistic regression was utilized to assess factors related to the primary endpoint.
Results. The demographic characteristics of the 2 randomization groups were statistically similar with no significant differences in age, sex, or race. At 12 months, there was no significant difference in the MDRS scoring for VACV-treated vs placebo recipients, with 85.7% and 90.2%, respectively, of patients demonstrating no or mild neuropsychological impairment (P = .72). No significant study-related adverse events were encountered in either treatment group.
Conclusions. Following standard treatment with intravenous ACV for PCR-confirmed HSE, an additional 3-month course of oral VACV therapy did not provide added benefit as measured by neuropsychological testing 12 months later in a population of relatively high-functioning survivors.
Clinical Trials Registration. NCT00031486.
(See the Editorial Commentary by Tyler on pages 692–4.)
Herpes simplex encephalitis (HSE) is a rare disease with an incidence of 1 case per 250 000–500 000 individuals per year [1–3]. Nonetheless, HSE remains the most common cause of sporadic fatal encephalitis [4–9]. More than 90% of cases are caused by herpes simplex virus (HSV) type 1 [10, 11]. In adults, HSE classically presents as acute necrotizing encephalitis with initial lesions localizing to the temporal region unilaterally. Without effective antiviral therapy, HSE mortality exceeds 70%, and <10% of patients return to normal neurologic function [12]. Common sequelae include memory impairment, personality and behavioral abnormalities, and seizures.
Studies in the 1980s demonstrated benefit from therapy with vidarabine and subsequently with acyclovir (ACV), with reduction in mortality to approximately 19%–28% [1, 2, 12]. With the advent of reliable molecular diagnostic tests [13, 14] and longer durations of intravenous ACV therapy (14–21 days), mortality has been reduced to approximately 14%–19% [3, 15–19]. Overall, the proportion of survivors who have no or mild neurological impairment and are able to resume activities of daily living is approximately 40%–55%. Thus, even with optimal medical management, long-term morbidity among HSE survivors remains unacceptably high [16, 20–22].
We hypothesized that persistent low-level HSV replication in the central nervous system (CNS) following intravenous ACV therapy could result in a chronic inflammatory response, through either direct viral cytotoxicity or immune-mediated mechanisms [23–25]. This study of high-dose oral valacyclovir (VACV) compared with a placebo administered for 90 days was designed to test the hypothesis that extended antiviral therapy would result in reduced neuropsychological morbidity and improved outcomes.
METHODS
From 2000 to 2009, we conducted a prospective, randomized, double-blind, placebo-controlled trial of adjunctive VACV therapy for HSE. This multinational study was performed by the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group (CASG; study number 204). Active study sites were located in the United States, Canada, England, and Sweden. Immunocompetent subjects ≥12 years of age with signs and symptoms of encephalitis were screened at the time of initial clinical presentation. Those who were confirmed to have HSE by detection of HSV DNA in the cerebrospinal fluid (CSF) by polymerase chain reaction (PCR) were reassessed for study eligibility after completing 14–21 days of intravenous ACV. Written informed consent was obtained from each participating patient or legal guardian. Patients were randomized to either 2 g of VACV daily (administered as four 500-mg tablets 3 times daily) or identical placebo tablets for 90 days.
The primary endpoint was survival with no or mild neuropsychological impairment at 12 months after the initiation of study medication, as measured by the Mattis Dementia Rating Scale (MDRS). Secondary endpoints included survival with no or mild neuropsychological impairment at 90 days and 6, 12, and 24 months as measured by the MDRS, the Mini-Mental State Examination (MMSE), and the Glasgow Coma Scale (GCS). The impact on quality of life (QOL) was assessed using the 36-Item Short-Form Health Survey (SF-36).
Study Population
Individuals ≥12 years of age with PCR-confirmed HSE were eligible for enrollment and randomization if the subject or legal guardian provided informed consent. Initial PCR analysis of CSF was performed at the participating sites and results were confirmed at the CASG Central Laboratory at the University of Alabama at Birmingham. Patients were required to complete 14–21 days of intravenous ACV therapy at a minimum dose of 30 mg/kg/day (or as adjusted for creatinine clearance). Other inclusion criteria included expectation for successful follow-up for 24 months, patient weight >45.5 kg, and a negative pregnancy test for female patients. Exclusion criteria included HSV meningitis only; anticipated life expectancy of <90 days; inability to swallow pills at the time of study randomization; creatinine clearance of ≤50 mL/minute/1.73 m2 (as calculated using the Jelliffe equation); receipt of an alternative antiherpesvirus medication (eg, ganciclovir, foscarnet) for acute therapy; expectation for long-term (>30 days/year) antiherpesvirus therapy for other indications; and/or individuals >3 days beyond completion of the treatment course with intravenous ACV.
Study Procedures
Samples were collected at each clinic visit during the drug treatment phase for safety laboratory assessments (complete blood count with differential, platelet count, blood urea nitrogen, creatinine, glucose, aspartate aminotransferase, alanine aminotransferase, total bilirubin, alkaline phosphatase, and urinalysis). Adverse events were assessed throughout the first 6 months of study participation. Serious adverse clinical events were reported directly to the Central Unit at the University of Alabama at Birmingham within 48 hours of knowledge of the occurrence. During the treatment period, the National Institutes of Health (NIH) Stroke Scale was performed at baseline; on days 7, 14, 28, 42, 56, 70, and 90; and at 6, 12, and 24 months. Neuropsychological evaluations (MDRS, MMSE, GCS, and SF-36) were performed at baseline (day 0, first day of study drug administration), 90 days, 6 months, 12 months, and 24 months.
The MDRS, a brief psychometric instrument designed to assess the nature and severity of dementia, was employed to measure cognitive changes. Based on guidance from the literature, cut-points for severity of impairment as measured by the MDRS were established prior to the study: very severe, <86; severe, 87–113; moderate, 114–120; mild, 121–138; none/normal, 139–144. Similarly, severity cut-points for the MMSE were established: very severe, <10; severe, 11–15; moderate, 16–22; mild, 23–26; none/normal, 27–30. The SF-36 QOL survey is based on domains of subject-reported physical health and mental health; the QOL score is the sum of the physical and mental health scores.
Statistical Analyses
This study was designed to test the null hypothesis that the proportion of randomized study subjects who survived at 1 year with no or mild impairment (as defined by MDRS) would be the same for the treated and placebo groups vs the alternative hypothesis that they would differ. Based on previous reports, the proportion of HSE patients receiving standard-of-care treatment who survived with no or mild impairment was estimated to be approximately 60%; we projected that this proportion would increase to 85% among study subjects receiving VACV. With an 80% power and a 2-sided type I error of 5%, 50 evaluable subjects per group was planned. A 20% dropout rate was estimated, thus increasing the sample size to 60 per group. The randomization allotments were computer-generated, blocked, and stratified for each enrolling country. The randomization scheme was maintained by the CASG Biostatistics Unit and was kept confidential until after the database closed. An independent data and safety monitoring board (DSMB) monitored this trial utilizing the Lan-DeMets stopping boundaries to assess increased efficacy between the 2 arms of the study. In addition, a futility analysis was provided at each interim analysis.
An intent-to-treat analysis of all subjects receiving at least 1 dose of study drug was performed. Demographic and baseline characteristics of the 2 treatment groups were compared using Fisher exact test for categorical factors and the Kruskal–Wallis test for continuous factors. The primary endpoint for the 2 randomized groups was compared using Fisher exact test. Univariate logistic analysis was performed to assess the relationship of baseline (including treatment allocation) and demographic factors on the primary endpoint. A multivariate analysis utilizing only those factors with P < .15 from the univariate analyses, along with the treatment allocation, was then performed. McNemar test was used to compare baseline dichotomous MDRS impairment groups vs the 12-month groups for subjects with both baseline and 12-month assessments.
Ethical and Regulatory Issues
The clinical trial was conducted in accordance with the ethical standards of the Helsinki Declaration. The protocol required approval at each institution by the local institutional review board or ethics committee before enrollment could proceed. Written informed consent was obtained from each patient or legal guardian. Performance of the protocol was overseen by an independent DSMB. North American sites were monitored by CASG personnel under the direction of the Data Coordinating Center; sites in the United Kingdom and Sweden were monitored by a contracted clinical research organization (Pharmaceutical Product Development, LLC).
RESULTS
Population Demographics
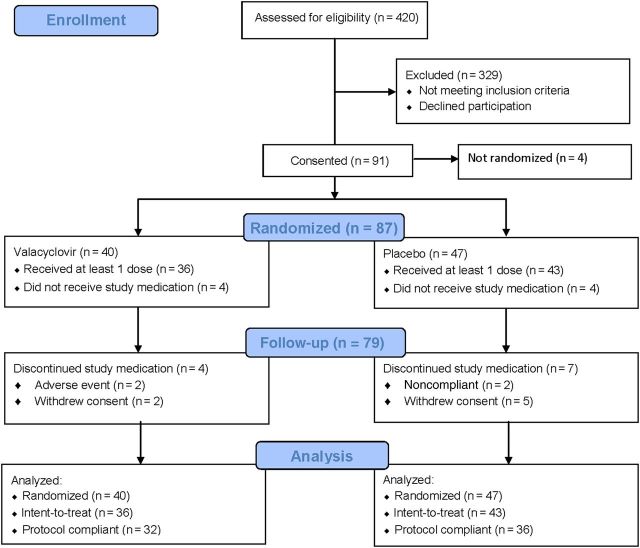
Patients were enrolled at 15 sites (5 in Sweden, 1 in England, 9 in North America) from 2000 to 2009. A total of 420 subjects were screened for eligibility, of whom 91 provided informed consent (Figure 1). Subjects were enrolled from Sweden (57 [63%]), the United States (16 [18%]), Canada (15 [16%]), and the United Kingdom (3 [3%]). The most common criterion for excluding screened patients from enrollment was the absence of HSV DNA in the CSF as measured by PCR. Of the 91 patients consented, 4 were never randomized.
Figure 1.
Consolidated Standards of Reporting of Trials (CONSORT) diagram demonstrating disposition of screened and enrolled subjects.
The DSMB recommended termination of the study for futility after reviewing data on the first 89 enrolled subjects. The DSMB determined that efficacy as defined by the primary endpoint could not be demonstrated within the projected sample size. Thus, these analyses are performed on 87 randomized subjects rather than the planned 100.
Forty subjects were randomized to receive VACV and 47 to receive placebo (Figure 1). Among randomized subjects, 36 of 40 in the VACV arm received at least 1 dose of study medication, as did 43 of 47 in the placebo group; these 79 subjects are included in the intent-to-treat analysis. Eleven subjects discontinued investigational therapy prematurely. Data from these subjects are included in the database until time of termination. Sixty-eight subjects (86% of those who received study medications) completed protocol requirements. Proportions of subjects completing the protocol were not different between the VACV (n = 32) and placebo (n = 36) arms (P = .75). Adherence to study medications, as assessed by pill counts, was >95%.
The majority of subjects were white, reflecting the preponderance of participants from Sweden. The subjects were approximately equally divided between males (54%) and females (46%). The median age was 55 years (Table 1).
Table 1.
Demographic Characteristics
| Characteristics | All Randomized Subjects |
P Value* | Intent-to-Treat Subseta |
|||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 87) | VACV (n = 40) | Placebo (n = 47) | Total (N = 79) | VACV (n = 36) | Placebo (n = 43) | P Value* | ||
| Race, No. (%) | ||||||||
| White, non-Hispanic | 83 (95.4) | 40 (100) | 43 (91.5) | .50 | 75 (94.0) | 36 (100) | 39 (90.7) | .50 |
| African American | 1 (1.1) | 0 (0.0) | 1 (2.1) | 1 (1.3) | 0 (0.0) | 1 (2.3) | ||
| Asian or Pacific Islander | 2 (2.3) | 0 (0.0) | 2 (4.3) | 2 (2.5) | 0 (0.0) | 2 (4.7) | ||
| Native American or Alaskan | 1 (1.1) | 0 (0.0) | 1 (2.1) | 1 (1.3) | 0 (0.0) | 1 (2.3) | ||
| Sex, No. (%) | ||||||||
| Male | 47 (54.0) | 20 (50.0) | 27 (57.4) | .52 | 43 (54.4) | 19 (52.8) | 24 (55.8) | .82 |
| Female | 40 (46.0) | 20 (50.0) | 20 (42.6) | 36 (45.6) | 17 (47.2) | 19 (44.2) | ||
| Age at enrollment, y | ||||||||
| Median | 55.0 | 53.5 | 56.0 | .28 | 54.0 | 52.0 | 55.0 | .41 |
| Range | 14.0–83.0 | 14.0–77.0 | 26.0–83.0 | 14.0–80.0 | 14.0–77.0 | 26.0–80.0 | ||
| Duration of HSE, db | ||||||||
| Median | 4.0 | 4.0 | 4.0 | .20 | 4.0 | 5.0 | 4.0 | .17 |
| Range | 0.0–28.0 | 0.0–23.0 | 0.0–28.0 | 0.0–28.0 | 0.0–23.0 | 0.0–28.0 | ||
| No. | 84 | 38 | 46 | 78 | 35 | 43 | ||
Abbreviations: HSE, herpes simplex encephalitis; VACV, valacyclovir.
a Subjects who received at least 1 dose of study medication.
b Duration of symptoms prior to initiation of intravenous acyclovir therapy.
* P value by Fisher exact test for categorical data and by Kruskal–Wallis test for continuous data.
Baseline Test Results
The baseline scores for the 5 domains of the MDRS are shown in Table 2. At the time of randomization, 68% of VACV recipients and 61% of placebo recipients had no or mild cognitive impairment (P = .63).
Table 2.
Baseline Mattis Dementia Rating Scalea
| Test Domain | Total (N = 79) | VACV (n = 36) | Placebo (n = 43) | P Value* |
|---|---|---|---|---|
| Attention | ||||
| Median | 36.0 | 36.0 | 36.0 | .46 |
| Range | 0.0–37.0 | 0.0–37.0 | 0.0–37.0 | |
| No. | 75 | 34 | 41 | |
| Initiation and perseveration | ||||
| Median | 30.5 | 28.5 | 31.0 | .22 |
| Range | 0.0–99.0 | 0.0–37.0 | 0.0–99.0 | |
| No. | 74 | 34 | 40 | |
| Construction | ||||
| Median | 6.0 | 6.0 | 6.0 | .20 |
| Range | 0.0–6.0 | 0.0–6.0 | 0.0–6.0 | |
| No. | 72 | 33 | 39 | |
| Conceptualization | ||||
| Median | 37.0 | 37.0 | 35.0 | .31 |
| Range | 0.0–39.0 | 0.0–39.0 | 0.0–39.0 | |
| No. | 73 | 34 | 39 | |
| Memory | ||||
| Median | 20.0 | 20.0 | 19.0 | .62 |
| Range | 0.0–25.0 | 0.0–25.0 | 1.0–25.0 | |
| No. | 73 | 34 | 39 | |
| Total score | ||||
| Median | 126.0 | 126.5 | 126.0 | .89 |
| Range | 0.0–144.0 | 0.0–143.0 | 1.0–144.0 | |
| No. | 75 | 34 | 41 | |
| Overall level of impairment, No. (%) | ||||
| Moderate/severe/very severe (<121) | 27 (36.0) | 11 (32.4) | 16 (39.0) | .63 |
| No/mild (score from 121–144) | 48 (64.0) | 23 (67.6) | 25 (61.0) | |
Abbreviation: VACV, valacyclovir.
a Test administered prior to initiation of study medication. Data missing for 4 subjects.
* P value by Kruskal–Wallis for continuous data.
The median baseline score for GCS for the 2 randomization groups was 15, with a range of 12–15 for VACV recipients, and 6–15 for placebo recipients (P = .71). All of the VACV recipients and 41 of 43 placebo recipients scored GCS >12, indicating no or mild impairment. The baseline MMSE demonstrated that 61% (22 of 36) and 63% (27 of 43) of VACV and placebo recipients had no or mild impairment at baseline (score ≥23; P = .99). No differences were detected in baseline status for the NIH Stroke Scale. The median total scores on the SF-36 QOL survey for the 2 randomization groups were 392 and 344, respectively, for VACV and placebo recipients (P = .88). Neither the GCS nor the NIH Stroke Scale provided insight into baseline vs 1-year outcomes and will not be further discussed.
Outcomes
Mattis Dementia Rating Scale
In the follow-up phase, 76 of 79 subjects had MDRS evaluations at 12 months (Table 3). At day 90, 89% of VACV recipients and 81% of placebo recipients had no or mild impairment (score 121–144); at 12 months, the figures were 86% (30 of 35 subjects) and 90% (37 of 41 subjects), respectively. Of note, these percentages did not change significantly between 12 and 24 months of follow-up. None of these differences are statistically significant.
Table 3.
Mattis Dementia Rating Scale Level of Impairment Across Follow-up Visitsa
| Level of Impairment | Total (N = 79) | VACV (n = 36) | Placebo (n = 43) | P Value* |
|---|---|---|---|---|
| Baseline (begin study drug) | ||||
| Moderate/severe/very severe (<121) | 27 (36.0) | 11 (32.4) | 16 (39.0) | |
| No/mild (121–144) | 48 (64.0) | 23 (67.6) | 25 (61.0) | |
| Day 90 (complete study drug) | ||||
| Moderate/severe/very severe (<121) | 12 (15.8) | 4 (11.4) | 8 (19.5) | .37 |
| No/mild (121–144) | 64 (84.2) | 31 (88.6) | 33 (80.5) | |
| 6 mo | ||||
| Moderate/severe/very severe (<121) | 12 (15.8) | 6 (17.1) | 6 (14.6) | NS |
| No/mild (121–144) | 64 (84.2) | 29 (82.9) | 35 (85.4) | |
| 12 mo | ||||
| Moderate/severe/very severe (<121) | 9 (11.8) | 5 (14.3) | 4 ( 9.8) | .72 |
| No/mild (121–144) | 67 (88.2) | 30 (85.7) | 37 (90.2) | |
| 24 mo | ||||
| Moderate/severe/very severe (<121) | 8 (10.5) | 4 (11.4) | 4 ( 9.8) | NS |
| No/mild (121–144) | 68 (89.5) | 31 (88.6) | 37 (90.2) | |
Data are presented as No. (%).
Abbreviations: NS, nonsignificant; VACV, valacyclovir.
a Last or most recent evaluation utilized for missing endpoint. Three subjects had no assessments for all 5 visits.
* P value by Fisher exact test for categorical data.
Thus, the proportion of total subjects with no or mild impairment as measured by MDRS increased from 64% at baseline to 88% at 12 months. The preponderance of the improvement was seen between baseline and day 90.
Mini-Mental State Examination
In the follow-up phase, 68 of 79 subjects were tested by MMSE at 12 months (Table 4). At day 90, 90% of VACV recipients and 86% of placebo recipients had no or mild impairment (MMSE ≥23); at 12 months, the figures were 88% and 86%, respectively. At 24 months, the frequency of no or mild impairment was 88% and 94% in the VACV and placebo groups, respectively. None of these differences were statistically significant.
Table 4.
Mini-Mental State Examination Level of Impairment Across Follow-up Visits
| Level of Impairment | Total (N = 79) | VACV (n = 36) | Placebo (n = 43) | P Value* |
|---|---|---|---|---|
| Baseline: begin study drug | ||||
| Moderate/severe/very severe (<23) | 26 (34.7) | 12 (35.3) | 14 (34.1) | |
| No/mild (23–30) | 49 (65.3) | 22 (64.7) | 27 (65.9) | |
| Day 90: complete study drug | ||||
| Moderate/severe/very severe (<23) | 8 (11.9) | 3 (9.7) | 5 (13.9) | .72 |
| No/mild (23–30) | 59 (88.1) | 28 (90.3) | 31 (86.1) | |
| 6 mo | ||||
| Moderate/severe/very severe (<23) | 8 (13.1) | 4 (14.3) | 4 (12.1) | NS |
| No/mild (23–30) | 53 (86.9) | 24 (85.7) | 29 (87.9) | |
| 12 mo | ||||
| Moderate/severe/very severe (<23) | 9 (13.2) | 4 (12.1) | 5 (14.3) | NS |
| No/mild (23–30) | 59 (86.8) | 29 (87.9) | 30 (85.7) | |
| 24 mo | ||||
| Moderate/severe/very severe (<23) | 6 (9.1) | 4 (12.5) | 2 (5.9) | .42 |
| No/mild (23–30) | 60 (90.9) | 28 (87.5) | 32 (94.1) | |
Data are presented as No. (%).
Abbreviations: NS, nonsignificant; VACV, valacyclovir.
* P value by Fisher exact test for categorical data.
Thus, the proportion of total subjects with no or mild impairment as measured by MMSE increased from 65% at baseline to 87% at 12 months. The greatest incremental improvement was seen between baseline and day 90.
SF-36 Quality-of-Life Survey
In the follow-up phase, 62 of 79 subjects completed QOL evaluations at 12 months. At day 90, the median total QOL scores were 496.3 and 502.5 in the VACV and placebo groups, respectively (P = .76); at 12 months, the scores were 654.0 and 630.0, respectively (P = .98). No differences were noted by analysis of subdomain scores (data not shown).
Indicative of gradual improvement in health following HSE, there was a steady increase in measured QOL over time. For the overall population, positive median score changes from baseline were 85.9 at day 90, 154.5 at 6 months, 268.8 at 12 months, and 311.7 at 24 months. There were no significant differences between treatment groups.
Factors Related to Outcome at 12 Months
Logistic analysis was performed on the following univariate factors: randomized therapy (odds ratio [OR], 0.65; P = .54), age at presentation (OR, 0.96; P = .12), sex (OR, 0.93; P = .92), duration of encephalitis symptoms prior to intravenous ACV therapy (OR, 0.93; P = .24), and baseline MDRS score (OR, 1.05; P ≤ .001;). Factors with P values <.15 were included in a multivariate logistic model in determining the benefit of VACV therapy (Table 5). The placebo arm of the study actually resulted in fewer impaired patients (OR, 0.25 [95% confidence interval, .03–1.95]) after covariate adjustment for baseline MDRS and age, although the finding was not statistically significant (P = .18).
Table 5.
Factors Related to Severity of 1-Year Impairment Assessed by the Mattis Dementia Rating Scale
| Factor | No/Mild Dementia | Moderate/Severe Dementia | Univariate Factors |
Multivariate Factors |
||
|---|---|---|---|---|---|---|
| P Value* | OR (95% CI) | P Value* | OR (95% CI) | |||
| Randomized therapy | ||||||
| Placebo | 37 (55.2) | 4 (44.4) | .54 | 0.65 (.16–2.63) | .18 | 0.25 (.03–1.95) |
| VACV | 30 (44.8) | 5 (55.6) | ||||
| Age | ||||||
| Mean | 52.3 | 60.8 | .12 | .96 | .21 | 0.95 (.88–1.03) |
| SD | 15.2 | 10.4 | ||||
| Median | 53 | 59 | ||||
| Range | 14–80 | 47–73 | ||||
| Sex | ||||||
| Male | 36 (53.7) | 5 (55.6) | .92 | .93 | … | … |
| Female | 31 (46.3) | 4 (44.4) | ||||
| Baseline MDRS | ||||||
| Mean | 122.8 | 59.7 | <.001 | 1.05 | <.001 | 1.05 (1.02–1.08) |
| SD | 22 | 47.7 | ||||
| Median | 127.5 | 69 | ||||
| Range | 29–144 | 0–120 | ||||
| HSV symptom duration | ||||||
| Mean | 5.3 | 7.3 | .24 | .93 | … | … |
| SD | 4.5 | 6.8 | ||||
| Median | 4 | 4 | ||||
| Range | 0–28 | 1–23 | ||||
Data are presented as No. (%) unless otherwise specified.
Abbreviations: CI, confidence interval; HSV, herpes simplex encephalitis; MDRS, Mattis Dementia Rating Scale; OR, odds ratio; VACV, valacyclovir.
* P value by Wald χ2 test.
Collapsed Analysis
Because the multivariate analysis resulted in only the baseline factor of dichotomized MDRS, a paired analysis was undertaken based on that factor and its corresponding 12-month counterpart. For those subjects with no or mild impairment (MDRS score 121–144) at baseline, all remained with no or mild impairment at 12 months (Table 6); none regressed to more severe impairment. Importantly, for those subjects with moderate or greater impairment (score <121) at baseline, 72% improved significantly, performing at a level of no or mild impairment at 12 months (P < .0001). In a similar analysis comparing baseline and 12-month MMSE scores, 4.4% of subjects with no or mild impairment (score 23–30) at baseline regressed to more severe impairment, whereas the other 95.6% of subjects remained in the no or mild impairment category (Table 6). Among those with moderate or worse impairment at the time of presentation (score <23), 31.8% maintained at least moderate impairment, whereas the remaining 68.2% of subjects improved to no or mild impairment at the 12-month follow-up visit (P = .002).
Table 6.
Change in Level of Impairment Between Baseline and 12-Month Follow-up Visita
| Measure | Baseline Score | 12-mo Score |
P Valueb | |
|---|---|---|---|---|
| ≥Moderate | No/Mild | |||
| Mattis Dementia Rating Scale | ≥Moderate impairment (<121) | 7 (28.0%) | 18 (72.0%) | <.0001 |
| No/mild impairment (121–144) | 0 (0.0%) | 43 (100.0%) | ||
| Mini-Mental State Examination | ≥Moderate impairment (<23) | 7 (31.8%) | 15 (68.2%) | .002 |
| No/mild impairment (23–30) | 2 (4.4%) | 43 (95.6%) | ||
Data are presented as No. (%).
a For subjects completing all evaluations.
b By McNemar test for paired data.
Adverse Events
Four subjects died during the course of the study. Deaths were attributed to cardiac failure, pneumonia, cerebral hemorrhage, and pancreatic cancer. None of the deaths were considered to be related to study medication.
A total of 390 adverse events were reported for the 79 randomized subjects in the intent-to-treat population (4.9 adverse events per subject). Eighty-four percent of subjects experienced at least 1 adverse event, with no difference between randomization groups. The most common class of adverse events was gastrointestinal disorders, reported by 49% of subjects overall. The frequency was higher in VACV recipients than in those who received placebo (21 of 36 [58%] vs 18 of 43 [42%]; P = .18). No significant differences in grade 3 or grade 4 laboratory abnormalities were observed between the treatment groups.
DISCUSSION
The design for this study was predicated on the hypothesis that chronic low-level viral replication in the CNS contributes to progressive neurologic morbidity in patients with HSE. The conclusion derived from this study is that a 90-day course of VACV did not improve the outcome, as assessed by serial neuropsychiatric testing. Notably, this is the first large-scale therapeutic study of HSE in adults that utilized PCR detection of HSV DNA in the CSF as the inclusion criterion.
Due to its design, this study focused on a specific subset of HSE patients. To be eligible, the patient first had to survive the initial period of standard-of-care intravenous ACV therapy, which excluded the most seriously ill patients with the highest mortality rate. Second, surviving patients had to be sufficiently neurologically intact at the completion of intravenous ACV therapy to be able to successfully swallow study medications. As a result, the population of patients eligible for participation in this study was a relatively high-functioning subset of HSE survivors. Thus, the conclusions from this study cannot be generalized to all patients with HSE.
Although this study failed to demonstrate outcome differences between recipients of high-dose VACV or placebo, it provided insights into the outcome of adults with HSE. Among the 79 subjects in the intent-to-treat population, 84% and 88% functioned with no or mild impairment at 90 days and 12 months, respectively, after randomization, as assessed by the MDRS. The preponderance of improvement was seen between the time of completion of intravenous ACV and day 90; continued incremental improvement was seen up to 2 years of follow-up. This outcome is better than anticipated, even given the selected nature of this study population. In comparison, among 34 survivors in a New Zealand cohort of 42 HSE patients, 20 (59%) were able to resume activities of daily living, 9 (26%) were impaired but able to live independently, and 5 (15%) had severe neurologic defects at 90 days [17]. Among 85 HSE patients assessed in France, 65% had a “favorable” outcome and 20% had “severe disability” at 6 months [19]. Use of standardized outcomes testing and cut-point interpretations such as those described in this study may make study-to-study comparisons more readily interpretable in future clinical trials.
Given the current limitations of HSE therapy, an important controllable variable to improve clinical outcome is prompt initiation of ACV therapy. Unfortunately, multiple studies have indicated that there is still unacceptable delay in many cases between clinical presentation and antiviral drug therapy. That delay is clearly associated with poorer clinical outcome [19, 22, 26–31]. Clinicians should have a low threshold for performing a lumbar puncture and immediately initiating empiric treatment with ACV (among other diagnostic and therapeutic interventions) in patients presenting with fever and neuropsychiatric abnormalities [32].
In view of the significant morbidity rates still seen with HSE, active investigation of improved therapeutic approaches is warranted. Combination drug therapy or antiviral drugs with alternative mechanisms of action should be explored [33]. In addition to direct HSV-mediated cytolysis, HSE is also characterized by acute and persistent intrathecal inflammatory responses and possibly by autoimmune phenomena [34, 35]. Further studies to define the role of immune responses and to explore the therapeutic potential of adjunctive therapy with anti-inflammatory and immunomodulating agents are essential [36–39].
Notes
Acknowledgments. The authors thank the investigators and coordinators who participated in this study: Johns Hopkins University, Baltimore, Maryland: Susan Rice, RN; Kansas University Medical Center, Kansas City: Stacy McCrea-Robertson, MS, MT; Karolinska Institute, Stockholm, Sweden: Ingrid Harviden, RN, Camilla Olofsson, RN, Martin Glimåker MD, Anders Hjalmarsson, MD; Queen's University, Kingston, Ontario, Canada: Sandy Weatherby, RN; Mayo Clinic, Rochester, Minnesota: Jennie Wilson, Janet Fisher-Dittrich; Royal Free Hospital, London: Andrew Gale, MB, ChB, Anna Stanton, RN, Susanne Luck, MD; Sahlgrenska Östra University Hospital, Gothenburg, Sweden: Lars Hagberg, MD, Romy Koltrand, RN; Skåne University Hospital, Lund, Sweden: Ann Akesson, RN, Margareta Knutsson, RN; Hans Norrgren MD, Henrik Elmrud, MD, Björn Olsén, MD; St Louis University, St Louis, Missouri: Shari Bogovich; Umeå University, Umeå, Sweden: Gunilla Persson, MD, Gunilla Karlsson, RN, Maria Casserdahl, RN, Sonia Lundberg RN; University of Alabama at Birmingham: Nancy Grady, RN; University of Alberta Hospital, Edmonton, Canada: Sharon Roberts, RN, Adeline Reinsma, RN, Carrie Douglas; University of Manitoba, Winnipeg, Canada: Gregory W. Hammond, MD, Pamela H. Orr, MD, Karen Janzen, RN, Teralyne Wilson, RN; University of New Mexico, Albuquerque: Robert Nofchissey; Uppsala University Hospital, Uppsala, Sweden: Fredrik Sund, MD, Anna Dahlstedt, RN; CASG Central Unit: Mary Wyatt Bowers, Sara Davis, Nancy Grady, RN; Linda Austin, Sharon Blount, Fred Lakeman, PhD. The authors also thank Anne Gershon, MD, Paul Albert, MD, Spotswood Spruance, MD, and Yaakov Stern, MD, for serving on the DSMB. This article is dedicated to the memory of Laura N. Riser, MSN, CRNP, who was instrumental in the organization and initiation of this clinical trial.
Disclaimer. The pharmaceutical sponsor had no role in the design or conduct of the clinical trial; the randomization procedures; the collection or analysis of the data; the preparation of the manuscript; or the decision to submit the manuscript for publication.
Financial support. The work was supported by a contract from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (N01A130025). Matched study drug and placebo were kindly provided by GlaxoSmithKline (GSK), Research Triangle Park, North Carolina. All medications were distributed by Fisher Bioservices to site pharmacies in the United States, Canada, and the United Kingdom as well as to Karolinska Pharmacy in Stockholm for distribution to Swedish sites.
Potential conflicts of interest. J. W. G. has received research grants from the NIH; personal fees from Merck (consultant), BioCryst (DSMB member), and GlaxoSmithKline (DSMB member). P. G. has received research grants from Sanofi-Pasteur, Genentech/Roche, and AiCuris, and personal fees from Viropharma and Microbiotix. S. C.-F. has received personal fees (clinical adjudication committee) from Biotronic, Lilly, and Novo Nordisk. R. J. W has received research grants from NIH for the work under consideration. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Whitley RJ, Alford CA, Hirsch MS, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med 1986; 314:144–9. [DOI] [PubMed] [Google Scholar]
- 2.Skoldenberg B, Forsgren M, Alestig K, et al. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet 1984; 2:707–11. [DOI] [PubMed] [Google Scholar]
- 3.Hjalmarsson A, Blomqvist P, Skoldenberg B. Herpes simplex encephalitis in Sweden, 1990–2001: incidence, morbidity, and mortality. Clin Infect Dis 2007; 45:875–80. [DOI] [PubMed] [Google Scholar]
- 4.Skoldenberg B. Herpes simplex encephalitis. Scand J Infect Dis Suppl 1991; 80:40–6. [PubMed] [Google Scholar]
- 5.Skoldenberg B. Herpes simplex encephalitis. Scand Infect Dis Suppl 1996; 100:8–13. [PubMed] [Google Scholar]
- 6.Tyler KL. Update on herpes simplex encephalitis. Rev Neuro Dis 2004; 1:169–78. [PubMed] [Google Scholar]
- 7.Kennedy PG, Steiner I. Recent issues in herpes simplex encephalitis. J Neurovirol 2013; 19:346–50. [DOI] [PubMed] [Google Scholar]
- 8.Studahl M, Lindquist L, Eriksson BM, et al. Acute viral infections of the central nervous system in immunocompetent adults: diagnosis and management. Drugs 2013; 73:131–58. [DOI] [PubMed] [Google Scholar]
- 9.Skelly MJ, Burger AA, Adekola O. Herpes simplex virus-1 encephalitis: a review of current disease management with three case reports. Antivir Chem Chemother 2013; 23:13–8. [DOI] [PubMed] [Google Scholar]
- 10.Aurelius E, Johansson B, Skoldenberg B, Forsgren M. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J Med Virol 1993; 39:179–86. [DOI] [PubMed] [Google Scholar]
- 11.Mateen FJ, Miller SA, Aksamit AJ., Jr Herpes simplex virus 2 encephalitis in adults. Mayo Clin Proc 2014; 89:274–5. [DOI] [PubMed] [Google Scholar]
- 12.Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch'ien LT, Alford CA. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N Engl J Med 1977; 297:289–94. [DOI] [PubMed] [Google Scholar]
- 13.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis 1995; 171:857–63. [DOI] [PubMed] [Google Scholar]
- 14.Aurelius E, Johansson B, Skoldenberg B, Staland A, Forsgren M. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 1991; 337:189–92. [DOI] [PubMed] [Google Scholar]
- 15.Whitley RJ, Lakeman F. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin Infect Dis 1995; 20:414–20. [DOI] [PubMed] [Google Scholar]
- 16.Stahl JP, Mailles A, De Broucker T; Steering Committee and Investigators Group Herpes simplex encephalitis and management of acyclovir in encephalitis patients in France. Epidemiol Infect 2012; 140:372–81. [DOI] [PubMed] [Google Scholar]
- 17.McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry 1997; 63:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 2010; 10:835–44. [DOI] [PubMed] [Google Scholar]
- 19.Raschilas F, Wolff M, Delatour F, et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis 2002; 35:254–60. [DOI] [PubMed] [Google Scholar]
- 20.Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res 2006; 71:141–8. [DOI] [PubMed] [Google Scholar]
- 21.Gordon B, Selnes OA, Hart J, Jr, Hanley DF, Whitley RJ. Long-term cognitive sequelae of acyclovir-treated herpes simplex encephalitis. Arch Neurol 1990; 47:646–7. [DOI] [PubMed] [Google Scholar]
- 22.Utley TF, Ogden JA, Gibb A, McGrath N, Anderson NE. The long-term neuropsychological outcome of herpes simplex encephalitis in a series of unselected survivors. Neuropsychiatry Neuropsychol Behavioral Neurol 1997; 10:180–9. [PubMed] [Google Scholar]
- 23.Skoldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 2006; 253:163–70. [DOI] [PubMed] [Google Scholar]
- 24.Aurelius E, Forsgren M, Skoldenberg B, Strannegard O. Persistent intrathecal immune activation in patients with herpes simplex encephalitis. J Infect Dis 1993; 168:1248–52. [DOI] [PubMed] [Google Scholar]
- 25.Aurelius E, Andersson B, Forsgren M, Skoldenberg B, Strannegard O. Cytokines and other markers of intrathecal immune response in patients with herpes simplex encephalitis. J Infect Dis 1994; 170:678–81. [DOI] [PubMed] [Google Scholar]
- 26.Hughes PS, Jackson AC. Delays in initiation of acyclovir therapy in herpes simplex encephalitis. Can J Neurol Sci 2012; 39:644–8. [DOI] [PubMed] [Google Scholar]
- 27.Poissy J, Champenois K, Dewilde A, et al. Impact of herpes simplex virus load and red blood cells in cerebrospinal fluid upon herpes simplex meningo-encephalitis outcome. BMC InfectDis 2012; 12:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poissy J, Wolff M, Dewilde A, et al. Factors associated with delay to acyclovir administration in 184 patients with herpes simplex virus encephalitis. Clin Microbiol Infect 2009; 15:560–4. [DOI] [PubMed] [Google Scholar]
- 29.Bell DJ, Suckling R, Rothburn MM, et al. Management of suspected herpes simplex virus encephalitis in adults in a U.K. teaching hospital. Clin Med 2009; 9:231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benson PC, Swadron SP. Empiric acyclovir is infrequently initiated in the emergency department to patients ultimately diagnosed with encephalitis. Ann Emerg Med 2006; 47:100–5. [DOI] [PubMed] [Google Scholar]
- 31.Sili U, Kaya A, Mert A; Group HSVES. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol 2014; 60:112–8. [DOI] [PubMed] [Google Scholar]
- 32.Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008; 47:303–27. [DOI] [PubMed] [Google Scholar]
- 33.Prichard MN, Kern ER, Hartline CB, Lanier ER, Quenelle DC. CMX001 potentiates the efficacy of acyclovir in herpes simplex virus infections. Antimicrobial Agents Chemother 2011; 55:4728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruss H, Finke C, Holtje M, et al. N-methyl-d-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012; 72:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armangue T, Leypoldt F, Malaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014; 75:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson KA, Blessing WW, Wesselingh SL. Herpes simplex replication and dissemination is not increased by corticosteroid treatment in a rat model of focal herpes encephalitis. J Neurovirol 2000; 6:25–32. [DOI] [PubMed] [Google Scholar]
- 37.Meyding-Lamade UK, Oberlinner C, Rau PR, et al. Experimental herpes simplex virus encephalitis: a combination therapy of acyclovir and glucocorticoids reduces long-term magnetic resonance imaging abnormalities. J Neurovirol 2003; 9:118–25. [DOI] [PubMed] [Google Scholar]
- 38.Kamei S, Sekizawa T, Shiota H, et al. Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry 2005; 76:1544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Torres F, Menon S, Pritsch M, et al. Protocol for German trial of acyclovir and corticosteroids in herpes-simplex-virus-encephalitis (GACHE): a multicenter, multinational, randomized, double-blind, placebo-controlled German, Austrian and Dutch trial [ISRCTN45122933]. BMC Neurol 2008; 8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]