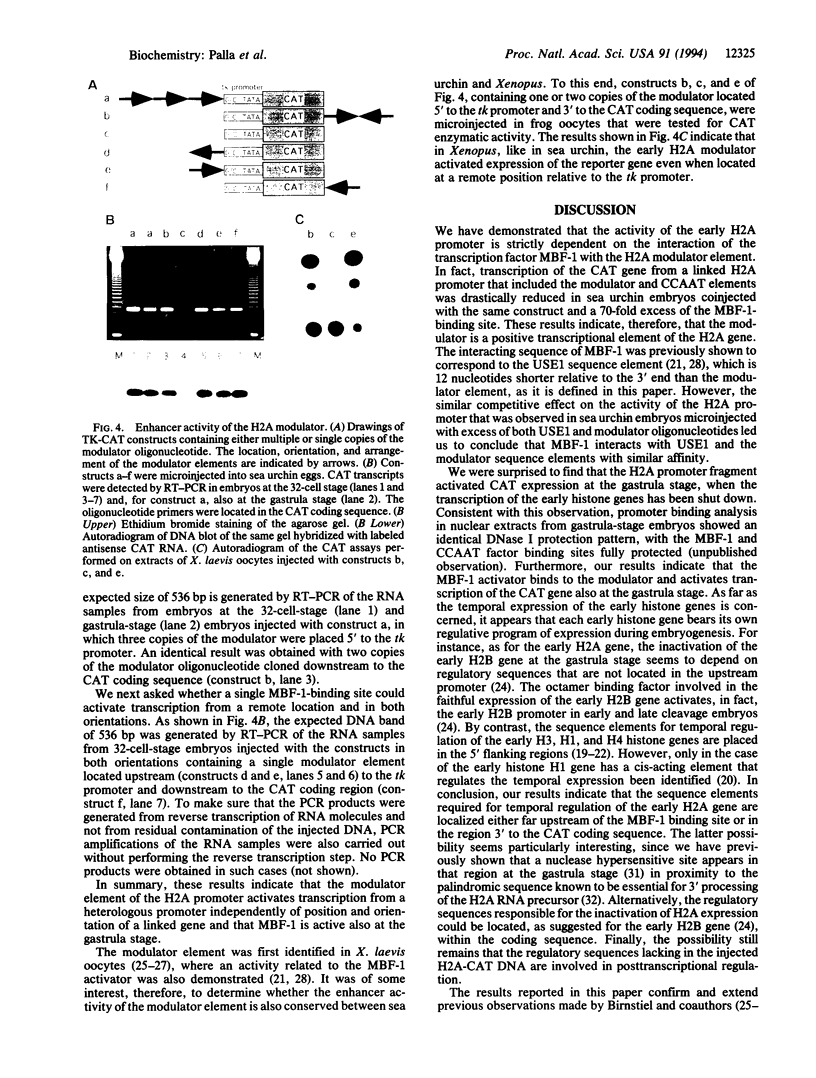
Abstract
The sea urchin early H2A histone gene, like the other four members of the repeating units, is transiently expressed during very early development. To investigate the mechanisms underlying the faithful expression of the early H2A gene, we focused our attention on the modulator element. We showed by DNase I cleavage protection patterns that the modulator includes the upstream sequence element 1 (USE1) and mapped at nucleotides -137 to -108 in the early H2A gene promoter. Functional tests conducted by microinjection into sea urchin embryos then showed that the modulator element binds the transcriptional factor called modulator-binding factor 1 (MBF-1). We found in fact that coinjection of an excess of the MBF-1-binding site, either as the modulator or as the USE1, efficiently impaired the activity of the H2A promoter. An unexpected finding was the expression of the reporter gene from the early H2A promoter at the gastrula stage of embryonic development, when the early histone genes are transcriptionally silent. In addition, we also found that the modulator element was active at the gastrula stage. The potential enhancer activity of the modulator was tested by microinjecting several constructs containing single or multiple copies of the modulator element placed 5' or 3' to a thymidine kinase gene (tk) promoter in both sea urchin embryos and Xenopus laevis oocytes and determining the expression of a reporter chloramphenicol acetyltransferase gene under the control of the linked tk promoter. We found that an oligonucleotide bearing the MBF-1-binding site activates the expression of the reporter gene independently of the position and orientation. We conclude that the modulator binds the MBF-1 activator and that it is a transcriptional enhancer of the early H2A histone gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anello L., Albanese I., Casano C., Palla F., Gianguzza F., Di Bernardo M. G., Di Marzo R., Spinelli G. Different micrococcal nuclease cleavage patterns characterize transcriptionally active and inactive sea-urchin histone genes. Eur J Biochem. 1986 Apr 15;156(2):367–374. doi: 10.1111/j.1432-1033.1986.tb09592.x. [DOI] [PubMed] [Google Scholar]
- Bell J., Char B. R., Maxson R. An octamer element is required for the expression of the alpha H2B histone gene during the early development of the sea urchin. Dev Biol. 1992 Apr;150(2):363–371. doi: 10.1016/0012-1606(92)90248-f. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Buratowski S. The basics of basal transcription by RNA polymerase II. Cell. 1994 Apr 8;77(1):1–3. doi: 10.1016/0092-8674(94)90226-7. [DOI] [PubMed] [Google Scholar]
- DiLiberto M., Lai Z. C., Fei H., Childs G. Developmental control of promoter-specific factors responsible for the embryonic activation and inactivation of the sea urchin early histone H3 gene. Genes Dev. 1989 Jul;3(7):973–985. doi: 10.1101/gad.3.7.973. [DOI] [PubMed] [Google Scholar]
- Dynan W. S. Modularity in promoters and enhancers. Cell. 1989 Jul 14;58(1):1–4. doi: 10.1016/0092-8674(89)90393-0. [DOI] [PubMed] [Google Scholar]
- Fei H., Childs G. Temporal embryonic expression of the sea urchin early H1 gene is controlled by sequences immediately upstream and downstream of the TATA element. Dev Biol. 1993 Feb;155(2):383–395. doi: 10.1006/dbio.1993.1037. [DOI] [PubMed] [Google Scholar]
- Giese K., Cox J., Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992 Apr 3;69(1):185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Delimitation of far upstream sequences required for maximal in vitro transcription of an H2A histone gene. Proc Natl Acad Sci U S A. 1982 Jan;79(2):297–301. doi: 10.1073/pnas.79.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Mächler M., Rohrer U., Birnstiel M. L. A functional component of the sea urchin H2A gene modulator contains an extended sequence homology to a viral enhancer. Nucleic Acids Res. 1983 Dec 10;11(23):8123–8136. doi: 10.1093/nar/11.23.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hori R., Carey M. The role of activators in assembly of RNA polymerase II transcription complexes. Curr Opin Genet Dev. 1994 Apr;4(2):236–244. doi: 10.1016/s0959-437x(05)80050-4. [DOI] [PubMed] [Google Scholar]
- Lai Z. C., DeAngelo D. J., DiLiberto M., Childs G. An embryonic enhancer determines the temporal activation of a sea urchin late H1 gene. Mol Cell Biol. 1989 Jun;9(6):2315–2321. doi: 10.1128/mcb.9.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. J., Tung L., Bumcrot D. A., Weinberg E. S. UHF-1, a factor required for maximal transcription of early and late sea urchin histone H4 genes: analysis of promoter-binding sites. Mol Cell Biol. 1991 Feb;11(2):1048–1061. doi: 10.1128/mcb.11.2.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Flytzanis C. N., Hough-Evans B. R., Katula K. S., Britten R. J., Davidson E. H. Introduction of cloned DNA into sea urchin egg cytoplasm: replication and persistence during embryogenesis. Dev Biol. 1985 Apr;108(2):420–430. doi: 10.1016/0012-1606(85)90045-4. [DOI] [PubMed] [Google Scholar]
- Müller M. M., Gerster T., Schaffner W. Enhancer sequences and the regulation of gene transcription. Eur J Biochem. 1988 Oct 1;176(3):485–495. doi: 10.1111/j.1432-1033.1988.tb14306.x. [DOI] [PubMed] [Google Scholar]
- Palla F., Bonura C., Anello L., Casano C., Ciaccio M., Spinelli G. Sea urchin early histone H2A modulator binding factor 1 is a positive transcription factor also for the early histone H3 gene. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6854–6858. doi: 10.1073/pnas.90.14.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palla F., Casano C., Albanese I., Anello L., Gianguzza F., Di Bernardo M. G., Bonura C., Spinelli G. Cis-acting elements of the sea urchin histone H2A modulator bind transcriptional factors. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6033–6037. doi: 10.1073/pnas.86.16.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Schatt M. D., Rusconi S., Schaffner W. A single DNA-binding transcription factor is sufficient for activation from a distant enhancer and/or from a promoter position. EMBO J. 1990 Feb;9(2):481–487. doi: 10.1002/j.1460-2075.1990.tb08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli G., Albanese I., Anello L., Ciaccio M., Di Liegro I. Chromatin structure of histone genes in sea urchin sperms and embryos. Nucleic Acids Res. 1982 Dec 20;10(24):7977–7991. doi: 10.1093/nar/10.24.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli G., Gianguzza F., Casano C., Acierno P., Burckhardt J. Evidences of two different sets of histone genes active during embryogenesis of the sea urchin Paracentrotus lividus. Nucleic Acids Res. 1979 Feb;6(2):545–560. doi: 10.1093/nar/6.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Tung L., Lee I. J., Rice H. L., Weinberg E. S. Positive and negative transcriptional regulatory elements in the early H4 histone gene of the sea urchin, Strongylocentrotus purpuratus. Nucleic Acids Res. 1990 Dec 25;18(24):7339–7348. doi: 10.1093/nar/18.24.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Schaffner W. A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J. 1988 Dec 1;7(12):3763–3770. doi: 10.1002/j.1460-2075.1988.tb03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]
- Zhao A. Z., Colin A. M., Bell J., Baker M., Char B. R., Maxson R. Activation of a late H2B histone gene in blastula-stage sea urchin embryos by an unusual enhancer element located 3' of the gene. Mol Cell Biol. 1990 Dec;10(12):6730–6741. doi: 10.1128/mcb.10.12.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A. Z., Vansant G., Bell J., Humphreys T., Maxson R. Activation of the L1 late H2B histone gene in blastula-stage sea urchin embryos by Antennapedia-class homeoprotein. Mech Dev. 1991 Mar;34(1):21–28. doi: 10.1016/0925-4773(91)90088-n. [DOI] [PubMed] [Google Scholar]