Abstract
This protocol specifically focuses on tools for assessing phrenic motor neuron (PhMN) innervation of the diaphragm at both the electrophysiological and morphological levels. Compound muscle action potential (CMAP) recording following phrenic nerve stimulation can be used to quantitatively assess functional diaphragm innervation by PhMNs of the cervical spinal cord in vivo in anesthetized rats and mice. Because CMAPs represent simultaneous recording of all myofibers of the whole hemi-diaphragm, it is useful to also examine the phenotypes of individual motor axons and myofibers at the diaphragm NMJ in order to track disease- and therapy-relevant morphological changes such as partial and complete denervation, regenerative sprouting and reinnervation. This can be accomplished via whole-mount immunohistochemistry (IHC) of the diaphragm, followed by detailed morphological assessment of individual NMJs throughout the muscle. Combining CMAPs and NMJ analysis provides a powerful approach for quantitatively studying diaphragmatic innervation in rodent models of CNS and PNS disease.
Keywords: Neuroscience, Issue 99, Compound muscle action potential, phrenic motor neuron, phrenic nerve, diaphragm, innervation, denervation, sprouting, neuromuscular junction, NMJ, spinal cord injury, SCI, amyotrophic lateral sclerosis, ALS, respiratory function
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a debilitating motor neuron disease associated with the loss of both upper and lower motor neurons and consequent muscle paralysis. Upon diagnosis, patient survival is on average only 2-5 years1. Phrenic motor neuron (PhMN) loss is a critical component of the pathogenesis of ALS. Patients ultimately die due to loss of PhMN innervation of the diaphragm, the primary muscle of inspiration2,3. Traumatic spinal cord injury (SCI) is also a serious problem with associated breathing difficulties. Approximately 12,000 new cases of SCI occur each year4 due to traumatic damage to the spinal cord. Despite disease heterogeneity with respect to location, type and severity, the majority of SCI cases involve trauma to the cervical spinal cord, which often results in debilitating and persistent respiratory compromise. In addition to ALS and SCI, other central nervous system (CNS) diseases can be associated with diaphragmatic respiratory dysfunction5,6.
The phrenic nerve is an efferent motor nerve that innervates the ipsilateral hemi-diaphragm and that originates from PhMN cell bodies located in the C3-C5 levels of the ipsilateral cervical spinal cord. PhMN output is controlled by descending bulbospinal input from the brainstem in an area known as the rostral ventral respiratory group (rVRG)7. The rVRG-PhMN-diaphragm circuit is central to the control of inspiratory breathing, as well as other non-ventilatory diaphragm behaviors. Various traumatic injuries and neurodegenerative disorders that affect this circuitry can lead to a profound decline in respiratory function and patient quality of life. Descending input to PhMNs from the rVRG, PhMN survival, phrenic nerve integrity and proper innervation at the diaphragm neuromuscular junction (NMJ) are all necessary for normal diaphragm function. It is therefore important to employ techniques that can quantitatively evaluate this circuit in vivo in rodent models of ALS, SCI and other CNS diseases.
With this protocol, the goal is to describe experimental tools for assessing PhMN innervation of the diaphragm at both the electrophysiological and morphological levels. Compound muscle action potentials (CMAPs) are recorded by stimulating all efferent motor neuron axons of a given motor nerve and then analyzing the elicited depolarization response of the target myofibers. This technique can be used in vivo in anesthetized rats and mice to quantify functional innervation of the hemi-diaphragm by PhMNs8. Due to the fact that CMAPs represent simultaneous recording of all (or at least many/most) myofibers of the whole hemi-diaphragm, it is useful to also examine the phenotypes of individual motor axons and myofibers at the diaphragm NMJ in order to track disease- and therapy-relevant morphological changes such as partial and complete denervation, regenerative sprouting and reinnervation. This can be accomplished via whole-mount immunohistochemistry (IHC) of the diaphragm, followed by detailed morphological assessment of individual NMJs throughout the muscle9. Combining CMAPs and NMJ analysis provides a powerful approach for quantitatively studying diaphragmatic innervation in rodent models of CNS and PNS disease.
Protocol
Experimental procedures were approved by the Thomas Jefferson University institutional animal care and use committee and conducted in compliance with the European Communities Council Directive (2010/63/EU, 86/609/EEC and 87-848/EEC), the NIH Guide for the care and use of laboratory animals, and the Society for Neuroscience’s Policies on the Use of Animals in Neuroscience Research.
1. Compound Muscle Action Potentials (CMAPs)
- Preparing the animal:
- Anesthetize the rat using an inhalant (isoflurane) at 1-2% delivered to effect or by injection (ketamine, xylazine, acepromazine). Dosage of injectable anesthesia is as follows: 95.0 mg/kg of ketamine, 10.0 mg/kg of xylazine, 0.075 mg/kg of acepromazine. NOTE: We successfully use both inhalant and injectable anesthetic approaches when conducting this CMAP recording protocol, with no preference.
- The animal must be maintained at the appropriate anesthetic depth before the surgery is started, through the conclusion of surgery, and until post-operative buprenorphine has taken effect. Confirm proper anesthetization by brief toe pinch to determine that a withdrawal response is not elicited. Additionally, test the corneal reflex with a cotton swab. Throughout the surgical procedure, determine that heart rate and respiratory rate have not increased, which suggests that anesthetic depth has become too light.
- Apply a vet ointment on the animal’s eyes to prevent dryness while under anesthesia.
- The surgeon should wash her/his hands with a disinfectant (chlorhexidine scrub) before beginning surgery. The surgeon should wear sterile gloves, facemask, and gown or clean lab coat. All instruments should be washed and autoclaved before surgery. Add a sterilization indicator strip inside the instrument kit before autoclaving at the level of the instruments to verify that sterilization is complete. The surgeon should verify that the entire line on the strip is black before beginning surgery. Tape the outside of the box with indicating autoclave tape as well. If surgery is to be performed on several animals on the same day, clean the instruments between surgeries and sterilize in a glass bead sterilizer. If necessary, sterilize both ends of the instrument in the glass bead sterilizer by placing the handle of the instrument in the sterilizer for the appropriate time, removing the instrument with a sterile hemostat, cooling it in a sterile field and then placing the functional end of the instrument into the glass bead sterilizer for the appropriate period of time.
- Once animal is anesthetized, place on surgical board with dorsal surface down and abdomen facing up so that tape can be used to secure animal’s forelimbs to surgical board.
- Shave ventral surface caudally beginning at the base of the skull, and ensure that the area around abdomen on either side of the midline is shaved depending on which hemi-diaphragm is being recorded. Apply povidone-iodine to the shaved surface of the skin.
- Preparation for CMAP recordings:
- Following animal preparation, place ground electrode subcutaneously into tail (Figure 1).
- Place the reference electrode subcutaneously into the contralateral lower abdominal region.
- Place conductive gel onto self-adhesive surface strip or disk recording electrode (dimensions of surface strip electrode: 0.5 x 3 cm), and then place surface electrode transversely along the unilateral costal margin.
- Place stimulating electrodes transcutaneously 0.5 cm apart lateral to trachea and superior to the clavicle. Insert the electrodes approximately 1.0 cm deep through the skin. Secure the electrodes by hand so that their location does not move with subsequent stimulations. NOTE: Angle stimulating electrodes 90° relative to the skin at the site of insertion.
- Compound Muscle Action Potential (CMAP) recordings:
- Obtain electrophysiological recordings using a stimulator/amplifier followed by computer assisted data analysis.
- The stimulus parameters of single pull stimulation should be 0.5 msec, 1.0 Hz supramaximal pulses. The amplitude of the stimulus should be between 6.0 and 8.0 V (Figure 2A). NOTE: Within an experiment, use a stimulus intensity with the same amplitude across animals. The objective is to maximize the response amplitude by using the minimal amplitude of stimulation. It is important to note that the initial response that occurs at the time of stimulation is a stimulation artifact. In addition, care should be taken to obtain a CMAP response that is preceded by a stable/flat baseline after the stimulation artifact, which is then followed by the rapid CMAP response. In order to obtain such a response, it may be necessary to reposition the stimulation and/or recording electrodes.
- Wait 30 sec between stimulations, and repeat 10 stimulations in a row to obtain the average response (Figure 2B-C). Measure the amplitude from baseline to peak (Figure 2A). Conduct phrenic nerve stimulation during the phase of the animal’s respiratory cycle when the phrenic nerve/diaphragm is not activated. The procedure can be repeated on the same animal for the contralateral hemi-diaphragm.
- After the final CMAP recording session, perfuse the animal if NMJ staining will be conducted. Conduct CMAP recordings repeatedly on the same animal approximately once every week (or at interval of choice).
- Following survival CMAP recordings, allow animal to recover on a circulating warm water heating pad, and continuously monitor during recovery until awake and sternally recumbent. Do not return an animal that has undergone surgery to the company of other animals until fully recovered.
- Once sternally recumbent, monitor the animal every 12 hr for the first 48 hr (once daily thereafter). If the animal appears to be dehydrated (flaccid skin, listlessness), administer fluids (lactated ringers solution; subcutaneously; 1-2 ml per injection, with the sites rotated; 2 times per day) until post-surgical weight and fluid loss stabilizes. Provide animals with softened food in small dishes during the acute post-operative period if necessary to encourage eating if they cannot reach the wire bar lid feeder.
- Administer buprenorphine at a dose of 0.05 mg/kg before the end of surgery and then at 12 hr intervals for the first 24 hr (and dependent upon signs of pain/stress thereafter) via subcutaneous injection (site rotated). If signs of pain or distress are noticed thereafter, treat animals with buprenorphine. Signs indicative of pain/distress include lack of activity, vocalizations, lack of eating or drinking (dehydration), excessive weight loss, guarding, excessive gnawing or scratching at site of incision. Additional criteria used in determining pain and distress include unresponsiveness to extraneous stimuli, transient or unprovoked vocalization, labored or abnormal breathing, persistent reddish-brown naso-occular discharge, marked piloerection. Also monitor evidence of poor hygiene that would suggest ill animals. If animal is still observed to be in distress after 72 hr of treatment (according to the pain/stress criteria outline above, and after receiving the analgesic protocol described above) euthanize the animal.
2. Diaphragm Neuromuscular Junction (NMJ) Analysis
- Animal dissection:
- Administer the animal an overdose of injectable anesthesia (3 times dose used above: ketamine at 285.0 mg/kg, xylazine at 30.0 mg/kg, acepromazine at 0.225 mg/kg; administered intraperitoneally). Following overdose, all animals should have a second method of euthanasia (thoracotomy) to ensure death. Death should be confirmed by observing cardiac and respiratory arrest and by noting the animal's fixed and dilated pupils.
- Conduct a laparotomy with scalpel or dissection scissors extending the excision from the zyphoid process caudally along the midline. Carefully separate skin and connective tissue from underlying muscle using scalpel and forceps.
- While pulling up on the zyphoid process, use dissection scissors to remove whole diaphragm, ensuring that the diaphragm remains attached to surrounding bone. This is important as it will prevent damage to the diaphragm muscle, will allow for successful dissection of the entire diaphragm and will aid in cleaning muscle for subsequent staining. NOTE: At this point, animal can be perfused if necessary.
- Cleaning and staining of the diaphragm:
- Pin down the whole dissected diaphragm, superior surface facing up, to silicone rubber in a glass 100 mm Petri dish in 4% paraformaldehyde (made in 1% phosphate buffered saline - PBS) for 20 min using a stereomicroscope. Stretch the diaphragm to make it taut, and using insect pins, pin down the surrounding tissue to avoid pinning the diaphragm muscle itself (Figure 3).
- Carefully clean off connective tissue from only the superior surface of muscle with #5 forceps and small scissors. NOTE: Carry out all subsequent steps of this staining protocol slowly rocking at room temperature covered from light unless otherwise specified. Ensure that the diaphragm muscle is always completely submerged in liquid to avoid drying out.
- Prior to staining, prepare all necessary reagents: 1x PBS, 2% bovine serum albumin (BSA)/PBS, 0.1 M glycine made in 2% BSA/PBS, 4% paraformaldehyde (PFA) in 1x PBS, and 0.2% TBP (0.2% Triton X100 in 2% BSA/PBS). Be sure to pre-cool methanol at -20 °C.
- Wash 3x for 10 min in 1x PBS.
- Incubate in 0.1 M glycine (made in 2% BSA/PBS) for 30 min.
- Incubate in RBTX (rhodamine-conjugated α-bungarotoxin) solution for 15 min. Solution for RBTX is as follows: Rhodamine-conjugated alpha bungarotoxin 1:400 dilution in 1x PBS. NOTE: In this protocol, Rhodamine is used, but any fluorophore of choice can be used for staining.
- Wash 3x for 10 min in 1x PBS.
- In -20 °C freezer, incubate with MeOH for 5 min. NOTE: Do not add MeOH before placing muscle in the freezer, and immediately remove solution following the incubation period.
- Wash 3x for 10 min in 1x PBS.
- Block in 0.2% TBP for 1 hr (0.2% Triton made in 2% BSA/PBS).
- Incubate with primary antibody solution (in 0.2% TBP) at 4 °C overnight while rocking. Dilutions of antibodies as follows: SV2 (1:10) and SMI-312 (1:1,000).
- Wash 3x for 10 min with 0.2% TBP.
- Incubate with secondary antibody using 1:100 dilution of FGM1 (FITC-conjugated goat anti-mouse IgG1; made in 0.2% TBP) while rocking for 1 hr. NOTE: Protect from light for the rest of the protocol for staining.
- Wash 3x for 10 min with 1x PBS.
- Re-clean surrounding connective tissue on the superior (see above) surface of muscle prior to mounting and coverslipping. NOTE: It is recommended to clean and then mount sample immediately after staining. However, if necessary, sample may be covered in 1x PBS, wrapped in parafilm to prevent evaporation and stored at 4 °C for up to 1 week, with changing of PBS daily.
- Upon mounting on glass slide, coverslip with non-hardset Vectashield. Seal edges of coverslip with clear nail polish to prevent drying out. Store slides lying flat in cardboard slide folders in -20 °C for long term.
- Analysis and Confocal Imaging:
- Quantitatively analyze the morphology of the stained and mounted hemi-diaphragm under 20X and 40X magnification using an upright epifluorescence microscope. NOTE: Because the diaphragm is stained and analyzed in a whole-mount fashion, the muscle is quite thick. Consequently, most of the fluorescence staining is completely out of focus when a static image is taken using non-confocal fluorescence microscopy. For this reason, it is best to conduct the NMJ analysis in real-time on the microscope as this allows you to constantly focus “up” and “down” through the muscle to properly identify the morphology of each of the individual NMJs. Using this approach, one can easily follow the trajectory of individual motor axons as they move through the tissue in spatial relationship to the post-synaptic receptor clusters.
- By measuring the ventral-to-dorsal length of the muscle, divide the muscle into 3 separate sections for analysis based on topographic innervation patterns from specific spinal cord levels (Figure 4H).
- Beginning at the ventral region of the muscle and moving dorsally, analyze all en face NMJs located on surface muscle fibers. Do not analyze muscles fibers deeper than the first 3-4 fibers from the surface as interpretation may be difficult due to poorer antibody penetration (RBTX penetrates much deeper into the muscle due to the small size of the toxin compared to the larger antibodies used to label neurons).
- Identify each NMJ as intact if the pre-terminal axon of the neuron is thick in diameter and all regions of the nerve terminal completely overlap with the underlying muscle acetylcholine receptors (AChRs).
- Identify each NMJ as partially denervated if any region of the underlying muscle AChRs is unoccupied with corresponding nerve terminal.
- Identify each NMJ as completely denervated if the muscle AChRs have no corresponding nerve terminal (it is also important to have other NMJs with labeled neurons in the same field of focus, so that one can be sure the lack of nerve terminal is not simply due to poor antibody penetration in that region of muscle).
- Identify each NMJ as multiply innervated if there is more than one pre-terminal axon innervating an individual NMJ (this indicates that there has been some level of denervation and likely attempts of reinnervation at this junction).
- Identify each NMJ as thinly innervated if the NMJ has complete overlap between nerve terminal and underlying AChRs but has a thin pre-terminal axon (this also is indicative of some level of denervation and attempts at reinnervation).
- Conduct analysis for each of the 3 identified regions for all of the above NMJ categories for each animal. Average the data from each animal with results from other animals from the same experimental group. Approximately 200-300 junctions in rat hemi-diaphragm muscle and 150-200 in mouse hemi-diaphragm should be quantified in at least 3 (preferably 5-6 or more) animals per experimental group.
- Obtain high-resolution confocal images, used for publication, with a confocal microscope.
- Obtain Z-stacks at 0.3 μm step size for 20-40 μm depths, and collect additional optical sections above and below each junction to ensure that the entire synaptic profile is included.
- Use acquisition software to reconstruct z-series images into maximum intensity projections.
Representative Results
Adult Sprague-Dawley rats received either laminectomy only (uninjured control) or unilateral hemi-contusion SCI at the C4 spinal cord level10-12. At 5 weeks post-surgery, peak CMAP amplitude recorded from the hemi-diaphragm ipsilateral to the laminectomy/injury site was significantly reduced in SCI rats (Figure 2C) compared to laminectomy-only control (Figure 2B). All NMJs in the hemi-diaphragm were completely intact in control non-diseased wild-type rats (Figure 4A,C). On the contrary, SOD1G93A rats (a rodent model of ALS) showed significant pathology at hemi-diaphragm NMJs (Figure 4B), including complete denervation (Figure 4G, arrowhead), partial denervation (Figure 4E, arrowhead; Figure 4G, NMJ on right), multiple innervation (Figure 4D) and thin pre-terminal axons (Figure 4F, arrowhead).
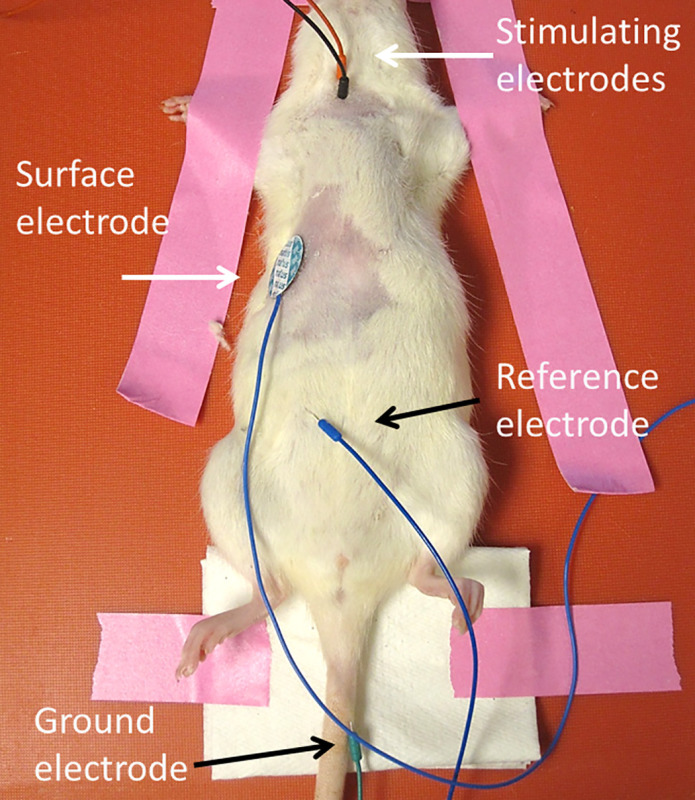 Figure 1: CMAP electrode placement. The ground electrode is placed subcutaneously into the tail. The reference electrode is placed into the abdomen. The surface recording electrode is placed transversely along the unilateral costal margin. Stimulating electrodes are depicted as both red and black. Please click here to view a larger version of this figure.
Figure 1: CMAP electrode placement. The ground electrode is placed subcutaneously into the tail. The reference electrode is placed into the abdomen. The surface recording electrode is placed transversely along the unilateral costal margin. Stimulating electrodes are depicted as both red and black. Please click here to view a larger version of this figure.
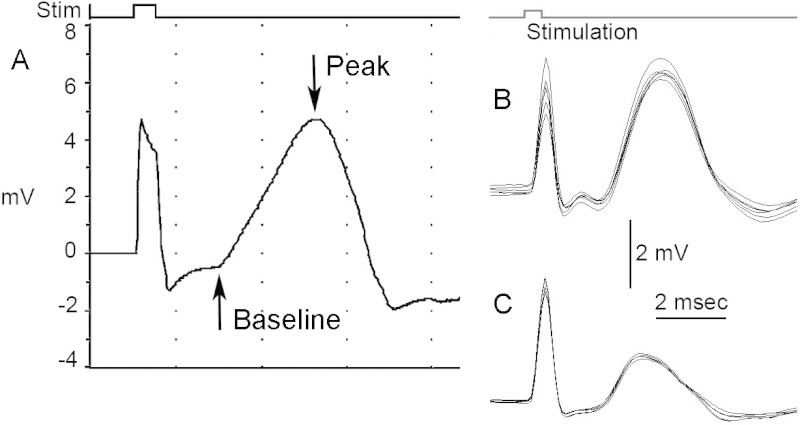 Figure 2: CMAP recordings and analysis. (A) The amplitude of CMAP response can be measured from baseline to peak. A typical CMAP response should show both the stimulus artifact and response curve. (B-C) Representative average CMAP responses. Stimulation should be carried out at least ten consecutive times to obtain a repeated average of the response. Shown here are a laminectomy-only uninjured rat (B) and a comparison of CMAP responses for an injured rat that received a unilateral C4 hemi-contusion (C). Please click here to view a larger version of this figure.
Figure 2: CMAP recordings and analysis. (A) The amplitude of CMAP response can be measured from baseline to peak. A typical CMAP response should show both the stimulus artifact and response curve. (B-C) Representative average CMAP responses. Stimulation should be carried out at least ten consecutive times to obtain a repeated average of the response. Shown here are a laminectomy-only uninjured rat (B) and a comparison of CMAP responses for an injured rat that received a unilateral C4 hemi-contusion (C). Please click here to view a larger version of this figure.
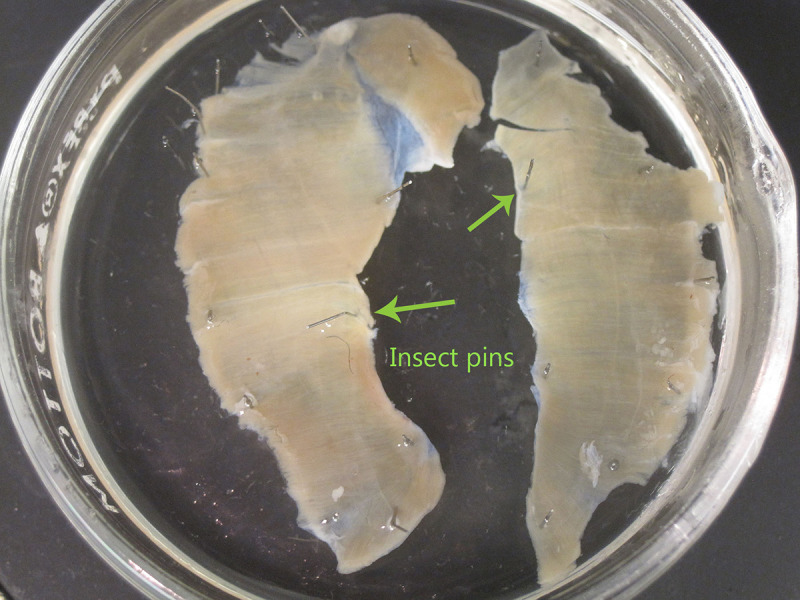 Figure 3: Preparation of diaphragm for whole-mount staining. Insect pins should be placed into surrounding tissue to secure the hemi-diaphragm to Sylgard. Avoid placing pins into the muscle itself. In this image, both hemi-diaphragm muscles are shown and pinned down separately. Please click here to view a larger version of this figure.
Figure 3: Preparation of diaphragm for whole-mount staining. Insect pins should be placed into surrounding tissue to secure the hemi-diaphragm to Sylgard. Avoid placing pins into the muscle itself. In this image, both hemi-diaphragm muscles are shown and pinned down separately. Please click here to view a larger version of this figure.
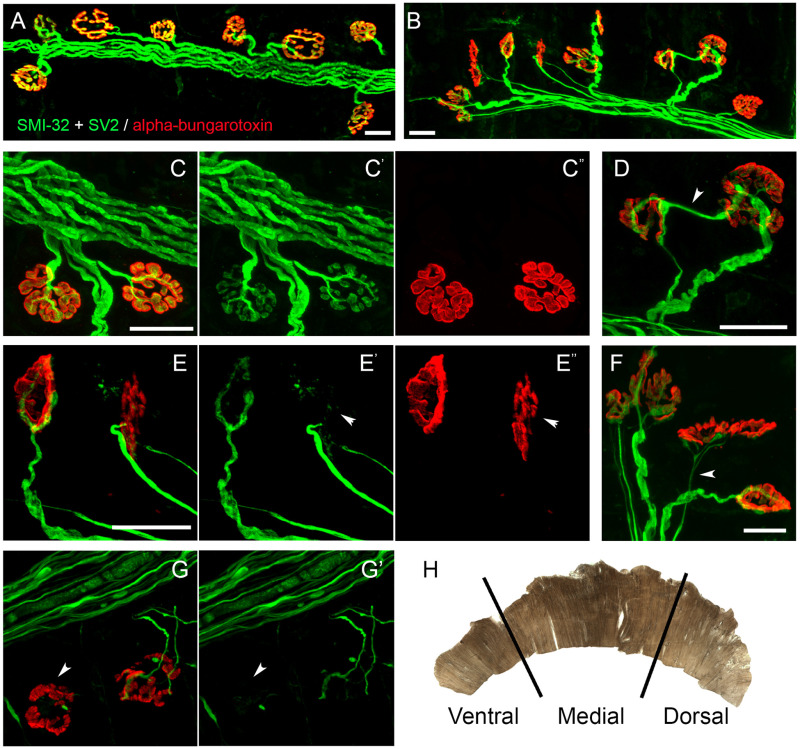 Figure 4: NMJ phenotypes. Confocal imaging was conducted to visualize post-synaptic acetylcholine receptors (in red labeled with rhodamine-conjugated α bungarotoxin) and axonal fibers and pre-synaptic terminals (in green labeled with SMI-32 and SV2 antibodies, respectively). All NMJs in hemi-diaphragm were completely intact in control non-diseased wild-type rats (A, C). On the contrary, SOD1G93A rats (a rodent ALS model) showed significant pathology at hemi-diaphragm NMJs (B), including complete denervation (G, arrowhead), partial denervation (E, arrowhead; G, NMJ on right), multiple innervation (D) and thin pre-terminal axons (F, arrowhead). The diaphragm muscle is sub-divided into three separate sections for NMJ morphology analysis (H)13. Scale bar: 25 µm. Please click here to view a larger version of this figure.
Figure 4: NMJ phenotypes. Confocal imaging was conducted to visualize post-synaptic acetylcholine receptors (in red labeled with rhodamine-conjugated α bungarotoxin) and axonal fibers and pre-synaptic terminals (in green labeled with SMI-32 and SV2 antibodies, respectively). All NMJs in hemi-diaphragm were completely intact in control non-diseased wild-type rats (A, C). On the contrary, SOD1G93A rats (a rodent ALS model) showed significant pathology at hemi-diaphragm NMJs (B), including complete denervation (G, arrowhead), partial denervation (E, arrowhead; G, NMJ on right), multiple innervation (D) and thin pre-terminal axons (F, arrowhead). The diaphragm muscle is sub-divided into three separate sections for NMJ morphology analysis (H)13. Scale bar: 25 µm. Please click here to view a larger version of this figure.
Discussion
As respiratory function is compromised in both traumatic SCI and ALS, developing therapies that target breathing and specifically diaphragm innervation are clinically relevant5,6. In order to comprehensively study respiratory function, a combined approach method should be used. CMAPs measure the degree of functional innervation of the diaphragm by way of external phrenic nerve stimulation, but not endogenous bulbospinal respiratory drive8. In addition, these recordings do not allow for examination of morphological changes at the NMJ, particularly at the level of individual motor axons and post-synaptic acetylcholine receptors. By using the described staining protocol, one can quantitatively assess relevant morphological phenotypes such as complete denervation, partial denervation and even regenerative sprouting and reinnervation at individual NMJs of the diaphragm muscle9.
Nevertheless, these two protocols still do not fully capture all aspects of respiratory pathology. For example, if injury occurs to the descending bulbospinal axons without any damage to the PhMN pool or phrenic nerve, CMAP amplitudes may still appear normal even though there is significant impairment in this circuitry because the phrenic nerve is externally stimulated14. Importantly, CMAPs do not allow for measurement of endogenous supraspinal respiratory drive. Furthermore, CMAPs do not provide a measure of overall ventilation. It may be beneficial to conduct, for example, whole body plethysmography to examine overall breathing behavior and spontaneous intra-muscular EMG recordings and phrenic nerve recordings to test endogenous PhMN activation. In addition, other approaches such as measuring blood gas levels can provide important information about respiratory function15. It may also be possible to employ the described diaphragm CMAP recording technique for motor unit number estimation16, though we have never attempted this. It is also important to note that these two protocols were described for only one respiratory muscle, the diaphragm. However, there are other inspiratory and expiratory respiratory muscles such as the intercostals that can be studied and that are affected by diseases such as SCI and ALS.
In this protocol, we describe diaphragm CMAP and NMJ analysis specifically in the rat model. Similarities between rats and mice make transfer of both these techniques to mice straightforward. We previously showed that partial loss of PhMNs following cervical contusion SCI does result in quantifiable decreases in diaphragm CMAP amplitude that correlate with morphological NMJ denervation. Furthermore, we have demonstrated these deficits in both mouse17 and rat10-12 SCI models. In addition, we have shown quantifiable diaphragm CMAP reduction in both mouse18 and rat13,19 models of ALS (i.e., the SOD1G93A model) that are associated with more subtle diaphragm denervation by PhMNs compared to cervical SCI, and we have demonstrated that we can detect relatively smaller therapeutic effects on CMAP amplitude of interventions such as stem cell transplantation in these ALS models19. Collectively, these findings demonstrate the utility of using these analyses to quantitatively assess both functional and morphological assessment of diaphragm innervation by PhMNs in both rat and mouse models of SCI and ALS.
By combining the histological and functional techniques detailed in this protocol to examine breathing and innervation at the NMJ, better insight can be gained into nervous system diseases associated with diaphragm dysfunction, thus providing greater awareness of how to target therapies in animal models and ultimately in patients.
Disclosures
We have nothing to disclose.
Acknowledgments
This work was supported by the NINDS (grant #1R01NS079702 to A.C.L.) and the SURP Program at Thomas Jefferson University (M.M.).
References
- Miller RG, et al. Practice parameter: the care of the patient with amyotrophic lateral sclerosis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology: ALS Practice Parameters Task Force. Neurology. 1999;52:1311–1323. doi: 10.1212/wnl.52.7.1311. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, et al. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169:94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan LM, Hollander D. Respiratory dysfunction in amyotrophic lateral sclerosis. Clin Chest Med. 1994;15:675–681. [PubMed] [Google Scholar]
- Holtz A, Levi R. Spinal Cord Injury. Oxford: 2010. Available from: http://www.alibris.com/ [Google Scholar]
- Bruijn LI, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Sharma H, Alilain WJ, Sahdu A, Silver J. Treatments to restore respiratory function after spinal cord injury and their implications for regeneration, plasticity and adaptation. Experimental Neurology. 2012;235:18–25. doi: 10.1016/j.expneurol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourévitch B, Mellen N. The preBötzinger complex as a hub for network activity along the ventral respiratory column in the neonate rat. Neuroimage. 2014;98:460–474. doi: 10.1016/j.neuroimage.2014.04.073. [DOI] [PubMed] [Google Scholar]
- Strakowski JA, Pease WS, Johnson EW. Phrenic nerve stimulation in the evaluation of ventilator-dependent individuals with C4- and C5-level spinal cord injury. Am J Phys Med Rehabil. 2007;86:153–157. doi: 10.1097/PHM.0b013e31802edce9. [DOI] [PubMed] [Google Scholar]
- Wright MC, et al. Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses. J Neurosci. 2009;29:14942–14955. doi: 10.1523/JNEUROSCI.2276-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, et al. Overexpression of the astrocyte glutamate transporter GLT1 exacerbates phrenic motor neuron degeneration, diaphragm compromise, and forelimb motor dysfunction following cervical contusion spinal cord injury. J Neurosci. 2014;34:7622–7638. doi: 10.1523/JNEUROSCI.4690-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, et al. Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion. Journal of neurotrauma. 2013;30:1092–1099. doi: 10.1089/neu.2012.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, et al. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol. 2012;235:539–552. doi: 10.1016/j.expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Lepore AC, et al. Peripheral hyperstimulation alters site of disease onset and course in SOD1 rats. Neurobiol Dis. 2010;39:252–264. doi: 10.1016/j.nbd.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BMF, Cummings KJ, Frappell PB, Wilson RJ. Novel method for conscious airway resistance and ventilation estimation in neonatal rodents using plethysmography and a mechanical lung. Respir Physiol Neurobiol. 2014;201:75–83. doi: 10.1016/j.resp.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Ngo ST, Bellingham MC. Neurophysiological recording of the compound muscle action potential for motor unit number estimation in mice. Neuromethods. 2013;78:225–235. [Google Scholar]
- Nicaise C, et al. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. Journal of neurotrauma. 2012;29:2748–2760. doi: 10.1089/neu.2012.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, et al. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1G93A mouse model of ALS. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nature neuroscience. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]


