Abstract
BACKGROUND
Uric acid is associated with increased risk of cardiovascular disease and arterial stiffness in patients with hypertension or stroke. It remains unknown if uric acid is associated with arterial stiffness in the general population.
METHODS
We analyzed the association between serum uric acid levels and measures of arterial stiffness such as carotid-femoral pulse wave velocity (CF PWV), carotid-radial pulse wave velocity (CR PWV) and augmentation index (AI) in 4,140 participants from the Generation 3 Framingham cohort using linear regression.
RESULTS
Mean (SD) age was 40.0 (8.8) years and mean (SD) serum uric acid levels were 5.3 (1.5) mg/dl. Mean (SD) CF PWV was 7.0 (1.4) m/s. Individuals in the highest quartile of uric acid were more likely to be male, have a higher prevalence of hypertension, higher BMI, fasting glucose and insulin, and lower estimated glomerular filtration rate (eGFR). Multivariate adjusted means of CF PWV were 6.90, 6.94, 7.06, and 7.15 m/s for uric acid quartile 1, 2, 3, and 4 respectively. In unadjusted analysis each 1mg/dl increase in uric acid was associated with higher CF-PWV (β = 0.27; 95% CI = 0.25, 0.29; P < 0.0001). This was attenuated but remained significant after adjusting for age, sex, smoking, hypertension, BMI, fasting glucose, insulin, animal protein intake, and eGFR (β= 0.06; 95% CI = 0.02, 0.09; P < 0.0007). There was no association between serum uric acid levels and AI upon adjustment for cardiovascular risk factors.
CONCLUSIONS
Serum uric acid levels are significantly associated with CF PWV and CR PWV in a younger Caucasian population.
Keywords: blood pressure, hypertension, uric acid, vascular stiffness.
Uric acid is a final oxidation product of dietary or endogenous purine metabolism in humans. It is generated by the liver and eliminated by the kidney. Symptomatic hyperuricemia (serum uric acid > 7.0mg/dl in men and >6.0mg/dl in women) is a recognized disease state necessitating treatment such as in the setting of gout and kidney stones. The clinical significance of asymptomatic hyperuricemia, however, remains unknown. Increased serum uric levels are reportedly associated with cardiovascular disease (CVD).1 Yet, it remains unclear if such associations are causal in humans.
Arterial stiffness is a measure of distensibility and compliance of the vessels. Increased arterial stiffness may be an early marker of cardiac disease as it predicts clinical CVD and mortality in patients with essential hypertension,2 diabetes3 and end stage kidney disease.4 There are several causal mechanisms through which uric acid may promote development of stiff arteries. Uric acid promotes proliferative and proinflammatory responses in cultured vascular smooth-muscle cells by inducing monocyte chemoattractant protein-1 (MCP-1)5 and cyclooxygenase2 (COX2).6 When infused into mice, uric acid directly increases cytokine production of tumor necrosis factor-α (TNF-α).7 Consequently, several studies have examined the potential relationship between increased serum uric acid levels and arterial stiffness.8–11 Results of these studies have had conflicting results. In addition, the majority of these reports are in Asian populations, where manifestations of the metabolic syndrome differ from the United States.12–14 To our knowledge, no large epidemiological study has examined the association between serum uric acid levels and arterial stiffness in the United States. We hypothesized that uric acid levels are associated with increased vascular stiffness in US adults. To test our hypothesis, we examined the association between serum uric acid levels and measures of vascular stiffness including: carotid-femoral pulse wave velocity (CF PWV), aortic augmentation index (AI), and carotid-radial pulse wave velocity (CR PWV) in the participants of the Generation 3 Framingham Heart Study.
METHODS
Study population
For this analysis, we utilized data from participants of the Generation 3 Framingham cohort (Gen III). The Third Generation Cohort was recruited to identify risk factors for CVD and to establish a resource of genetic and phenotypic data. The study design for the Generation 3 Framingham Cohort Study has been described previously,15 but briefly, the offspring participants provided updated information about their children at their sixth and seventh exam cycles. Individuals were eligible for enrollment in the Third Generation Cohort if they were ≥20 years old and had one parent in the Offspring Study. Invitation letters and response cards were sent to prospective participants in November 2001. Recruitment occurred between 2002 and 2005 in the order of recruitment prioritization. Data collected during the first examination included: clinical and laboratory assessments of vascular risk factors and imaging for subclinical atherosclerosis and cardiac structure and function. Subjects underwent a medical history, physical examination including blood pressure (BP) measurement, lab tests, and electrocardiography. Arterial tonometry was routinely performed in the Framingham Heart study Gen III participants. The Boston Medical Center Institutional Review Board approved the protocol and each participant gave written informed consent. There were 4,257 individuals who had data on serum uric acid. Of the 4,257, 117 subjects were on diuretics and were excluded from analysis because diuretics are known to raise serum uric acid levels.16Of these, 3,257, 3,145, and 3,291 subjects had complete data on CR PWV, CF PWV, and AI respectively and were included in the analysis.
Outcome
As described previously, arterial stiffness was assessed with the use of a custom tonometer (Cardiovascular Engineering, Norwood, MA). Tonometry was performed on brachial, radial, femoral, and carotid arteries with simultaneous electrocardiography monitoring after 5 minutes of rest with participants in the supine position. The distances from the suprasternal notch to pulse-recording site for each artery were obtained with body surface measurements. CF PWV and CR PWV were calculated using body surface measurements and tonometry data.17
The suprasternal notch was utilized as a fiducial point. Time delay was measured between the foot of the carotid and femoral waveforms. CF transit distance was estimated by measuring the distance from the suprasternal notch to the carotid and femoral sites and taking the difference to account for parallel transmission along the brachiocephalic and carotid arteries and around the aortic arch. To obtain the CF PWV, the corrected distance was divided by transit time delay. A similar procedure was used to measure CR PWV. Calibrated carotid pressure was used as surrogate for central arterial pressure. AI was defined as the proportion of central pulse pressure that is attributable to the augmented pressure.18 Measurement reproducibility for CF PWV was high with correlation coefficient (R) > 0.95 and was comparable to analysis reproducibility. Analysis reproducibility was assessed in a random sample of 50 cases that were blindly reanalyzed by a second observer and noted to have correlation coefficient (R) 0.972 for CF PWV and 0.997 for AI.19
Predictor
Fasting serum uric acid was measured using an autoanalyzer with a phosphotungstic acid reagent.20
Other covariates
Demographic data included age (years), gender (male vs. female) and smoking status. BP was measured in a sitting position after 5 minutes of rest using standard sphygmomanometry method. Hypertension was defined as systolic BP ≥ 140mm Hg or diastolic BP ≥ 90mm Hg or current use of anti-hypertensive medications. Current smoking status was defined as smoking one cigarette/day within the past 1 year before examination. Body mass index (BMI) was defined as weight in kg divided by the square of the height in meters. Fasting plasma glucose, high-density lipoprotein-cholesterol and triglyceride were measured using standard enzymatic method. Estimated glomerular filtration rate (eGFR) was calculated using Chronic Kidney Disease Epidemiology Collaboration equation as follows: GFR = 141 × min (Scr/κ, 1)α × max (Scr/κ, 1)−1.209 × (0.993)age × 1.1018 (if female) × 1.159 (if black), where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.21
Statistical analysis
We conducted a cross-sectional analysis of serum uric acid levels and CF PWV, CR PWV, AI of 4,140 participants. Using the entire population, we initially compared CF PWV, CR PWV and AI with potential covariates across serum uric acid quartiles. P values for linear trend across quartiles were calculated. For the regression analysis, serum uric acid was modeled as a continuous and a categorical variable. CF PWV, CR PWV, and AI were modeled as continuous variable in linear regression analysis. Least square means were compared in the quartile analysis. The shape of the relationship between serum uric acid levels and PWV was explored graphically and through linear regression using appropriate low degree polynomials to fit splines. We then performed multiple linear regression, adjusting for potential covariates found to be significant in simple linear regression, to evaluate the independent effect of serum uric acid levels on measures of arterial stiffness. Analysis of covariance was used to assess the significance of covariates to be included in a final multivariate (multiple regression) model. Variables significant at P < 0.05 in the full multivariate models were included in the final models. For the categorical analysis, least square means of the first 3 uric acid quartiles were compared to the least square mean of 4th quartile. Considering that hypertension plays a major role in vascular stiffness, the analysis was repeated after excluding individuals with history of hypertension.22 Due to the availability of data on 4,140 patients, we had >99% power to detect an R 2 = 0.009 even when adjusting for all covariates.
RESULTS
Characteristics of the subjects
The demographic, biochemical and clinical characteristics of the subjects were stratified into four groups according to median serum uric acid values. The four quartiles of uric acid were defined as follows: quartile 1: ≤4.1mg/dl (n = 1,045), quartile 2: 4.2–5.1mg/dl (n = 1,012), quartile 3: 5.2–6.3mg/dl (n = 1,073), and quartile 4: >6.3mg/dl (n = 979). The clinical characteristics of the study participants are presented in Table 1. 98.4% of participants reported their ethnicity as White.15 Of 4,257 participants, 1,992 were men and 2,265 were women. Mean age of the subjects was 40.0±8.8 years. Those in the higher quartiles of uric acid tended to be male, had lower eGFR and high-density lipoprotein-cholesterol, higher triglycerides, insulin, fasting blood glucose, BP, and BMI. In addition, they tended to drink more alcoholic beverages per/month.
Table 1.
Characteristics of participants according to serum uric acid quartile (mg/dl)
| Variables | 1st quartile ≤ 4.1 (n = 1,045) | 2nd quartile 4.2–5.1 (n = 1,012) | 3rd quartile 5.2–6.3 (n = 1,073) | 4th quartile > 6.3 (n = 979) | Analysis of variance P value |
|---|---|---|---|---|---|
| Age (years) | 39.8±8.2 | 39.6±8.9 | 40.0±9.3 | 40.5±8.8 | 0.117 |
| Sex (males, %) | 5.2 | 27.5 | 66.9 | 91.2 | < 0.0001 |
| High-density lipoprotein (mg/dl) | 62.6±15.2 | 57.9±16.5 | 50.4±14.1 | 46.07±12.7 | < 0.0001 |
| Triglycerides (mg/dl) | 82.2±39.7 | 97.5±53.7 | 122.6±91.2 | 159.3±126.3 | < 0.0001 |
| Smoking status (%) | |||||
| Current | 14.9 | 16.7 | 15.4 | 15.8 | 0.75 |
| Past | 28.9 | 26.9 | 27.1 | 25.9 | |
| Never | 56.2 | 56.4 | 57.5 | 58.4 | |
| Alcoholic drinks (per/month) | 12.9±18.8 | 17.2±22 | 23±31 | 32.5±45.8 | < 0.0001 |
| History of hypertension (%) | 7.3 | 10.4 | 16.3 | 19.6 | < 0.0001 |
| Systolic BP (mm Hg) | 111±14 | 115±14 | 119±14 | 123±13 | < 0.0001 |
| Diastolic BP (mm Hg) | 71±9 | 74±9 | 77±10 | 80±9 | < 0.0001 |
| BMI (kg/m2) | 23.8±4.3 | 25.8±4.9 | 28.0±5.5 | 29.5±5.2a | < 0.0001 |
| Fasting serum glucose (mg/dl) | 91.2±21.0 | 93.1±17.1 | 97.0±16.9 | 98.7±15.6* | < 0.0001 |
| Insulin (pmol/L) | 24.6±11.9 | 28.7±19.2 | 32.8±20.5 | 38.2±24.6a | < 0.0001 |
| eGFR (ml/min/1.73 m 2) | 104.0±21.2 | 103.0±21.8 | 101.2±21.8 | 97.1±23.2a | < 0.0001 |
| Uric acid (mg/dl) | 3.5±0.5 | 4.7±0.3 | 5.7±0.3 | 7.3±0.8a | < 0.0001 |
| CF PWV (m/s) | 6.5±1.0 | 6.7±1.3 | 7.2±1.4 | 7.5±1.5a | <0.0001 |
| CR PWV (m/s) | 9.06±1.34 | 9.29±1.37 | 9.64±1.48 | 9.96±1.50 | <0.0001 |
| Augmentation index % | 9.87±12.14 | 8.24±13.27 | 5.00±14.29 | 2.92±14.75a | <0.0001 |
Values are expressed as means ± standard deviation or % = percent of patients.
Abbreviations: BMI, body mass index; BP, blood pressure; CF PWV, carotid-femoral pulse wave velocity; CR PWV, carotid-radial pulse wave velocity; eGFR, estimated glomerular filtration rate.
aAll pair-wise differences were significant. *P < 0.001 4th quartile compared with 1st and 2nd quartile.
Association between measures of vascular stiffness and serum uric acid levels
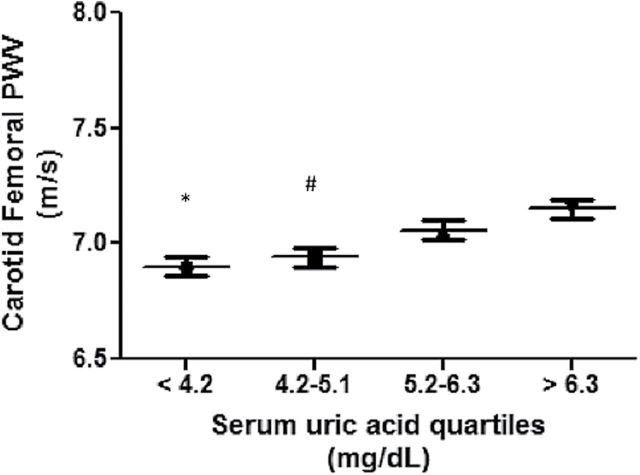
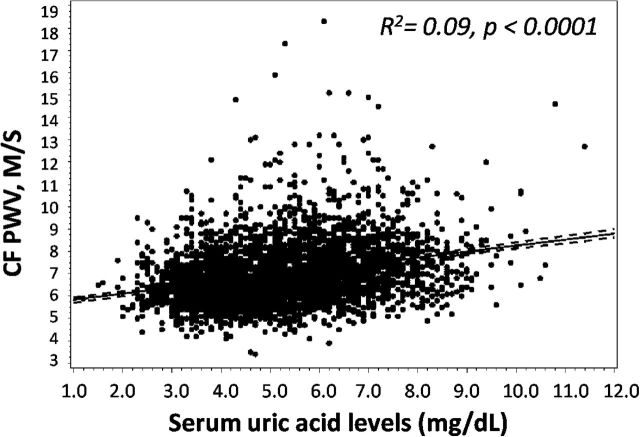
As shown in Table 1, there was a significant difference in CF PWV, CR PWV, and AI across serum uric acid quartiles. CF PWV and CR PWV were both lowest in the 1st quartile of uric acid and highest in the 4th quartile of uric acid (P < 0.0001), whereas AI was higher in lower quartiles of uric acid and lowest in the 4th quartile. Multivariate adjusted means of CF PWV by uric acid quartiles were 6.90, 6.94, 7.06, and 7.15 m/s for uric acid quartiles 1, 2, 3, and 4 respectively (Figure 1). When modeled continuously, each 1mg/dl higher serum uric acid levels was associated with a 0.27 higher CF PWV (95% CI = 0.24, 0.30; P < 0.0001) in unadjusted analysis (Figure 2). This was attenuated but remained significant after adjusting for age, sex, smoking, hypertension, BMI, fasting glucose, insulin, eGFR and animal protein intake (β = 0.06; 95% CI = 0.02, 0.09; P < 0.0001). The full multivariate model is shown in Table 2. Of note, other variables in the multivariate model that associated significantly with CF PWV were age, gender, SBP, insulin and fasting glucose. eGFR, smoking, animal protein intake or BMI were not associated with CF PWV in the multivariate model. When serum uric acid was modeled in quartiles, lower serum uric acid levels were significantly associated with lower CF PWV (β = −0.25; 95% CI = −0.11, −0.38; P = 0.0004 for quartile 1 vs. 4, and β = −0.20; 95% CI = −0.08, −0.32; P = 0.0008 for quartile 2 vs. 4).
Figure 1.
Mean carotid-femoral pulse wave velocity (CF PWV) according to serum uric acid quartiles. *P = 0.002 compared to serum uric acid > 6.3mg/dl; #P = 0.005 compared to serum uric acid > 6.3mg/dl. Adjusted for age, sex, estimated glomerular filtration rate (eGFR), smoking, hypertension, fasting glucose, insulin, and body mass index.
Figure 2.
Linear regression analysis of serum uric acid levels with carotid-femoral pulse wave velocity (CF PWV). Uric acid is positively correlated with CF PWV in linear regression analysis, R 2 represents the Coefficient of determination.
Table 2.
Full multivariate model for the association between serum uric acid levels and CF PWV
| Variables | β estimate (95% Confidence interval) | P value |
|---|---|---|
| Age (per 1 year) | 0.065 (0.059, 0.071) | < 0.0001 |
| Sex (male)a | −0.39 (−0.52, −0.26) | < 0.0001 |
| Smoking status (current)b | −0.006 (−0.05, 0.04) | 0.81 |
| History of hypertension | 0.23 (0.12, 0.35) | < 0.0001 |
| Systolic BP > 140mm Hg | 0.03 (0.028, 0.033) | <0.0001 |
| BMI (kg/m2) | 0.00001 (−0.009, 0.009) | 0.99 |
| Fasting serum glucose (mg/dl) | 0.005 (0.003, 0.007) | < 0.0001 |
| Insulin (pmol/L) | 0.006 (0.004, 0.008) | < 0.0001 |
| eGFR (per 1ml/min/1.73 m2) | 0.002 (−0.003, 0.008) | < 0.42 |
| Uric acid (per 1mg/dl) | 0.06 (0.02, 0.09) | 0.0007 |
| Animal protein intake (gm) | 0.00009 (−0.008, 0.001) | 0.83 |
R 2 for the full model = 0.52.
Abbreviations: BMI, body mass index; BP, blood pressure; CF PWV, carotid-femoral pulse wave velocity; eGFR, estimated glomerular filtration rate.
aFemales as reference. bPast/never as reference.
Linear regression analysis showed a small, but significant association between uric acid and CR PWV (β = 0.24; 95% CI = 0.21, 0.27; P < 0.0001) and AI (β = −1.85; 95% CI = −2.13, −1.56; P < 0.0001). In the final multivariate model, serum uric acid levels were significantly associated with CR PWV (β = 0.05; 95% CI = 0.003, 0.09; P = 0.036). However, there was no association between serum uric acid levels and AI when adjusted for age, sex, smoking, hypertension, BMI, fasting glucose, Insulin, eGFR, and animal protein intake.
Additional analysis
In order to evaluation whether gender influences the association between serum uric acid levels and CF PWV, we conducted multivariate linear regression analysis separately in men and women. We found that serum uric acid was associated with CF PWV in men (β = 0.07, P = 0.002) but not in women (β = 0.04, P = 0.086). There was no association between uric acid and CR PWV and AI in men or women.
In addition, we conducted sensitivity analysis excluding subjects with hypertension. After the exclusion of individuals with hypertension, 1mg/dl increase in uric acid resulted in a 0.04 m/s; (95% CI = 0.008, 0.07) increase in CF PWV (P = 0.016). In quartile analysis, we found that lower serum uric acid levels were significantly associated with lower CF PWV (β= −0.18; 95% CI = −0.32, −0.04; P = 0.007 for quartile 1 vs. 4, and β = −0.18; 95% CI = −0.30, −0.06; P = 0.002 for quartile 2 vs. 4). Similar to the above results, there was a significant association between serum uric acid levels and CR PWV when individuals with hypertension were excluded.
Of note our findings were unchanged when the analysis was done including subjects on diuretics (n = 4,257).
DISCUSSION
We evaluated the association between serum uric acid levels and measures of arterial stiffness in a large cohort of community dwelling adults in the United States. The Gen III Framingham heart study consisted of US adults with a low burden of risk factors for CVD. Our cross-sectional analyses showed that higher serum uric acid levels were significantly associated with arterial stiffness as assessed by CF PWV and CR PWV. The association persisted after adjustment for conventional risk factors, such as age, sex, hypertension, BMI, fasting glucose, insulin levels, animal protein intake, and eGFR.
Bian et al. evaluated the association between serum uric acid levels and vascular stiffness in a Chinese population and demonstrated that serum uric acid levels were directly associated with CF PWV in women. Similar to our findings, this association was independent of cardiovascular risk factors.9 Park et al. conducted in post-menopausal Korean women and noted that increased serum uric acid levels are independently associated with arterial stiffness.23 Tsai and colleagues showed that serum uric acid levels were independently associated with PWV in patients with essential hypertension.11 However, not all the previous studies have shown this association; a recent Italian study of 619 participants showed that after adjusting for several confounding variables, serum uric acid was not associated with PWV. However, their mean uric acid levels were lower and aortic elasticity was relatively preserved which could partly explain the absence of an association between uric acid and CF PWV.24 In another study of Korean adults, serum uric acid levels were not associated with PWV in either men or women.8 The reason behind these discrepant findings may be that that hyperuricemia is a stronger predictor of vascular disease in higher risk populations. In the last study, the authors included only healthy individuals and none of the participants had a history of CVD, renal disease, diabetes, or hypertension. Consistent with this notion, we have previously shown that serum uric acid levels are not associated with endothelial dysfunction in healthy older adults.25 It is possible that high uric acid levels predispose to CVD, but that a second “hit” is necessary for CVD to manifest. Of note, our current findings show a significant association between serum uric acid levels and vascular stiffness, but the magnitude of the association is small suggesting that this association may not be clinically significant in generally healthy community dwelling adults. As such, our data support the current clinical practice that screening for asymptomatic hyperuricemia in generally healthy adults is not warranted. However, the identified association between serum uric acid levels and CF PWV and CR PWV does support the possibility that uric acid contributes to vascular disease. While the potential effects of hyperuricemia as a sole risk factor for vascular disease appears to be small, such detrimental effects are likely augmented in higher risk populations. Consistent with this, observational studies have shown serum uric acid levels to be a stronger predictor of risk in high risk groups such as African Americans,26 patients with underlying hypertension,27 or in patients with congestive heart failure.28
It should be noted that it remains controversial as to whether uric acid itself is a causal risk factor for CVD. On the one hand, under physiological concentrations, urate is a powerful antioxidant that can scavenge superoxide, hydroxyl radicals, and singlet oxygen.29 Intravenous infusion of uric acid has been reported to improve endothelial function in healthy adults30,31 and in patients with type-1 diabetes.32 In some animal studies, hyperuricemia was associated with improved endothelial function.33 Alternatively, uric acid can react with nitric oxide irreversibly in vitro leading nitric oxide depletion34 and it may cause oxidative stress once internalized by the cells.35 In vascular smooth muscle cells, uric acid induces inflammatory mediators and activates NFκB.5,36 In some animal models, mild elevation in uric acid levels has been reported to induce endothelial dysfunction37 and inflammatory cytokines.7 Recently, we have shown that experimental hyperuricemia inhibits vitamin D activation in normal rats.38 Collectively, such data support a potential role for hyperuricemia in disease.
In addition to inhibition of nitric oxide and its pro-inflammatory potential, recent clinical evidence and experimental studies suggest that elevated serum uric acid levels contribute to the development of hypertension independently of other risk factors.39 Consistent with this, lowering serum uric acid levels with allopurinol has been shown to improve BP in newly diagnosed hypertension in adolescents.40 Interestingly, our results indicate that the association between serum uric acid levels and CF PWV is independent of hypertension, as the association remained significant after adjustment for hypertension and despite the exclusion of individuals with hypertension. This suggests that the other mechanisms such as those mentioned above, may play a role in the relation between hyperuricemia and vascular stiffness.
In this study, we found no association between uric acid and AI. AI is a measure of peripheral wave reflection and may not represent as good a measure of vascular stiffness as CF PWV since it can be affected by heart rate and left ventricular function. For example, while vascular stiffness measured by CR PWV increases with age, AI tends to increase until 55 years of age and then plateau41 likely because the impedance mismatch between a normally compliant aorta and peripheral muscular vessels diminishes as aorta stiffens with age.41 Unfortunately, the number of study participants aged >55 years with serum uric acid and AI measurements was too small for us to explore this as a potential confounder.
Strengths of our study include a large, community-based sample, which ensures adequate power to detect moderate effects of serum uric acid on arterial stiffness and interactions with other cardiovascular risk factors. Nevertheless, our study is not without limitations. First, this is a cross-sectional analysis. Therefore, it cannot be inferred that the relationship between serum uric acid and increased arterial stiffness is causal. Second, although we have adjusted for most of the potential confounding factors in our multivariate analyses, it is possible that residual confounding of unmeasured variables might influence the reported association. In addition, our study participants were primarily Caucasian so the associations observed here may not apply to other ethnic groups and further studies in different ethnicities are needed to verify our findings. And finally, while the relation between serum uric acid levels land CF PWV is statistically significant, it remains to be determined if small changes in CF PWV are clinically significant in a population with few cardiovascular risk factors.
In conclusion, in this cross-sectional observational study, serum uric acid was independently associated with CF PWV and CR PWV but not with AI in healthy subjects. This positive correlation between uric acid and CF PWV and CR PWV suggests that uric acid may play a role in increasing arterial stiffness. Further studies are needed to evaluate whether serum uric acid is an independent predictor of increased vascular stiffness in prospective analysis and to define populations in which lowering serum uric acid levels may reduce the cardiovascular burden.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This analysis was conducted on data collected as part of the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No.N01-HC-25195). In addition, we would like to thank Emelia Benjamin. Dr Benjamin is funded by the following grants: RO1 HL70100; R01 HL076784; 1R01AG028321. This work was also supported by Dr Jalal’s grant: 1K23DK088833, as well as K24 DK07820, and by Dr Madero’s grant No. 87236 from the Mexican Council of Science and Technology.
REFERENCES
- 1. Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V. Uric acid and long-term outcomes in CKD. Am J Kidney Dis 2009; 53:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 3. Mansour AS, Yannoutsos A, Majahalme N, Agnoletti D, Safar ME, Ouerdane S, Blacher J. Aortic stiffness and cardiovascular risk in type 2 diabetes. J Hypertens 2013; 31:1584–1592. [DOI] [PubMed] [Google Scholar]
- 4. Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension 1998; 32:570–574. [DOI] [PubMed] [Google Scholar]
- 5. Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003; 41:1287–1293. [DOI] [PubMed] [Google Scholar]
- 6. Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol 2002; 13:2888–2897. [DOI] [PubMed] [Google Scholar]
- 7. Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood 1997; 89:577–582. [PubMed] [Google Scholar]
- 8. Lim JH, Kim YK, Kim YS, Na SH, Rhee MY, Lee MM. Relationship between serum uric Acid levels, metabolic syndrome, and arterial stiffness in korean. Korean Circ J 2010; 40:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bian S, Guo H, Ye P, Luo L, Wu H, Xiao W. Serum uric Acid level and diverse impacts on regional arterial stiffness and wave reflection. Iran J Public Health 2012; 41:33–41. [PMC free article] [PubMed] [Google Scholar]
- 10. Liang J, Li Y, Zhou N, Teng F, Zhao J, Zou C, Qi L. Synergistic effects of serum uric acid and cardiometabolic risk factors on early stage atherosclerosis: the cardiometabolic risk in Chinese study. PloS one 2012; 7:e51101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai WC, Huang YY, Lin CC, Li WT, Lee CH, Chen JY, Chen JH. Uric acid is an independent predictor of arterial stiffness in hypertensive patients. Heart Vessels 2009; 24:371–375. [DOI] [PubMed] [Google Scholar]
- 12. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA: J Am Med Assoc 2012; 307:491–497. [DOI] [PubMed] [Google Scholar]
- 13. Reynolds K, Gu D, Whelton PK, Wu X, Duan X, Mo J, He J; InterASIA Collaborative Group. Prevalence and risk factors of overweight and obesity in China. Obesity (Silver Spring) 2007; 15:10–18. [DOI] [PubMed] [Google Scholar]
- 14. Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, National Heart L, Blood I, American Heart A. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 2004; 24:e13–18. [DOI] [PubMed] [Google Scholar]
- 15. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007; 165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 16. Steele TH, Oppenheimer S. Factors affecting urate excretion following diuretic administration in man. Am J Med 1969; 47:564–574. [DOI] [PubMed] [Google Scholar]
- 17. Zachariah JP, Xanthakis V, Larson MG, Vita JA, Sullivan LM, Smith HM, Safa R, Peng X, Hamburg N, Levy D, Sawyer DB, Mitchell GF, Vasan RS. Circulating vascular growth factors and central hemodynamic load in the community. Hypertension 2012; 59:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012; 308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004; 43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 20. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999; 131:7–13. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 2007; 49:1202–1206. [DOI] [PubMed] [Google Scholar]
- 23. Park JS, Kang S, Ahn CW, Cha BS, Kim KR, Lee HC. Relationships between serum uric acid, adiponectin and arterial stiffness in postmenopausal women. Maturitas 2012; 73:344–348. [DOI] [PubMed] [Google Scholar]
- 24. Cicero AF, Salvi P, D’Addato S, Rosticci M, Borghi C. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens 2014; 32:57–64. [DOI] [PubMed] [Google Scholar]
- 25. Jalal DI, Jablonski KL, McFann K, Chonchol MB, Seals DR. Vascular endothelial function is not related to serum uric acid in healthy adults. Am J Hypertens 2012; 25:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr, Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens 1999; 13:13–21. [DOI] [PubMed] [Google Scholar]
- 27. Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000; 36:1072–1078. [DOI] [PubMed] [Google Scholar]
- 28. Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003; 107:1991–1997. [DOI] [PubMed] [Google Scholar]
- 29. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981; 78:6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond) 2003; 105:425–430. [DOI] [PubMed] [Google Scholar]
- 31. Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol 2001; 38:365–371. [DOI] [PubMed] [Google Scholar]
- 32. Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes 2006; 55:3127–3132. [DOI] [PubMed] [Google Scholar]
- 33. Kurra V, Eräranta A, Jolma P, Vehmas TI, Riutta A, Moilanen E, Tahvanainen A, Kalliovalkama J, Niemelä O, Myllymäki J, Mustonen J, Pörsti I. Hyperuricemia, oxidative stress, and carotid artery tone in experimental renal insufficiency. Am J Hypertens 2009; 22:964–970. [DOI] [PubMed] [Google Scholar]
- 34. Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 2008; 27:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 2008; 295:C1183–C1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 2005; 16:3553–3562. [DOI] [PubMed] [Google Scholar]
- 37. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005; 67:1739–1742. [DOI] [PubMed] [Google Scholar]
- 38. Chen W, Roncal-Jimenez C, Lanaspa M, Gerard S, Chonchol M, Johnson RJ, Jalal D. Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism 2014; 63:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mazzali M, Kanbay M, Segal MS, Shafiu M, Jalal D, Feig DI, Johnson RJ. Uric acid and hypertension: cause or effect? Curr Rheumatol Rep 2010; 12:108–117. [DOI] [PubMed] [Google Scholar]
- 40. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008; 300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C. Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing 2007; 36:43–48. [DOI] [PubMed] [Google Scholar]