Effectiveness of antiretroviral therapy (ART) for preventing sexual HIV transmission in resource limited settings is debated. Longitudinal follow-up of 4916 serodiscordant couples in rural China found that ART provided substantial protection against sexual transmission, an effect that evolved over time.
Keywords: HIV treatment as prevention, HIV/AIDS, China, serodiscordant couples, sexual transmission
Abstract
Background. Antiretroviral therapy (ART) administered in clinical trial settings virtually eliminates the sexual transmission of human immunodeficiency virus (HIV) in serodiscordant couples, but effectiveness of treatment as prevention in the community is debated. Conflicting results from previous analyses in a Chinese cohort underscore the importance of determining effectiveness of ART delivered in resource limited settings.
Methods. All available years of data (2006–2012) from local disease control records of HIV patients and their seronegative spouses in Henan Province, China, were analyzed using marginal structural Cox models to estimate the effect of ART in the initially infected partner his or her partner's HIV seroconversion risk.
Results. We observed 157 seroconversion events in 4916 serosdiscordant couples, for an incidence rate of 0.59 cases per 100 person-years (PY) (95% confidence interval [CI], .51–.70). Of these, 84 occurred after the index partner had initiated ART (0.43/100PY; 95% CI, .35–.53) and 73, whereas index partners were untreated (5.87/100 PY; 95% CI, 4.65–7.42). In a marginal structural Cox model weighted for confounding and censoring, the hazard ratio (HR) for HIV transmission was 0.52 (95% CI, .34–.82). ART efficacy varied significantly by time period; least effective in the early phase from 2006 to 2008 (HR, 0.68; 95% CI, .34–1.36) but far more protective from 2009 onward (HR, 0.33; 95% CI, .20–.55).
Conclusions. ART can provide HIV-infected persons in resource-limited setting substantial protection against sexual transmission. Effectiveness in the Henan cohort appears to have increased over time, suggesting that quality of care and service infrastructure may be integral to successful use of treatment for prevention.
Since 2012, several human immunodeficiency virus (HIV) treatment guidelines have begun calling for immediate treatment of all HIV-infected persons regardless of CD4 cell count [1–3]. The guideline changes are attributable, in part, to results of a clinical trial showing that antiretroviral treatment (ART) can stop sexual HIV transmission [4], as well as evidence that earlier ART reduces HIV morbidity and mortality [5].
Supporters of rapid rollout of “treatment as prevention” strategies argue that the effect is already visible in areas where broader ART access has coincided with declines in measured sexual transmission of HIV [6, 7]. However exceptions to this pattern [8] and the potential risks of rapid rollout such as widespread emergence of drug resistance [9] or changes in risk behaviors [10], have tempered enthusiasm. In resource-poor settings, limited access to the resources required for durable viral suppression—optimal drug regimens and routine laboratory monitoring, for example—have prompted calls for research to inform treatment as prevention guidelines adapted to these locations [11].
Of about a dozen observational studies reporting on the real world effect of ART on sexual HIV transmission by following serodiscordant couples, in which only 1 partner is infected [12, 13], only 3 have followed those receiving routine care in resource-limited settings (the remainder tracked patients in wealthy countries or who received specialized care as part of scientific research studies). One of these 3 studies reported that ART prevented linked HIV transmission in couples from the southern Chinese province of Yunnan, though the uniqueness of the cohort—the initially infected (or “index”) partners were overwhelming male (78.7%), with about half reporting historic drug use (45.1%)—and high loss of follow-up among untreated couples, limited generalizability of the results [13]. Notably, the remaining 2 reports from Uganda [14] and Henan Province in China [15], both reported a lack of protective immunity attributable to the index partner's ART use. Follow-up of the Henan cohort has been ongoing, and an analysis of a later phase of follow-up showed that ART was instead highly protective against transmission [16], raising further questions about the relationship between ART exposure and HIV transmission in nontrial settings.
The Chinese government provides free HIV testing and universal ART through the existing healthcare system, in many cases managed by nonphysician clinicians [17], which provides a realistic version of proposed test and treat strategies [18]. The purpose of the current report is to investigate how well ART can work under such conditions so as to inform future programming for the use of treatment as prevention, particularly in resource poor settings.
METHODS
Study Setting and Data Sources
The Henan HIV serodiscordant couple cohort resulted from a regional HIV epidemic largely attributed to unsanitary blood and plasma selling practices prevalent in the mid-1990s [19]. Government crackdowns ended these practices by 1997, at which time between 50 and 170 thousand persons in the province were estimated to be living with HIV [20, 21]. Over one-third of HIV-infected persons in Henan live in the prefecture where the data used in this report were collected [15].
In 2006 the prefectural disease control center began enrollment of an open cohort of HIV serodiscordant couples to track HIV transmission in married couples. According to local guidelines, eligible couples were (1) registered residents of the study prefecture, (2) over 16 years of age (the age of legal consent in China), (3) in a stable marriage (no separation nor divorce), (4) in a HIV serodiscordant couple at the time of enrollment, and (5) willing to provide informed consent. HIV status of both partners was confirmed at enrollment through enzyme-linked immunosorbent assay (ELISA; Lizhu, Zhuhai, Guangdong Province; Xinzhuang, Xiamen, Fujian Province), and positive test results were confirmed by Western blot assay (Ou'ya, Hangzhou, Zhejiang Province), both of which were carried out at the county or prefectural level disease control center laboratories.
Eligible couples participated in annual surveys consisting of private and separate face-to-face interviews for each partner in their native dialect with trained county-level disease control staff. Participants provided information on demographics and behaviors over the previous year including sexual contact inside and outside the primary partnership, diagnosis of sexually transmitted infections, drug use, and history of migration for work. The initially HIV-infected index partners provided additional medical history including ART use, and initially uninfected (or “nonindex”) partners were screened for antibodies to HIV, with those testing positive referred to their local county disease control center for confirmatory testing and treatment eligibility screening.
Exposure, Outcome, and Other Covariates
The primary outcome for this analysis was time to HIV seroconversion in the initially uninfected partner of couples participating in at least 2 study visits between 2006 and 2012. Seroconversion was calculated as the midpoint between the date of the last HIV-negative or indeterminate test, and three months before the date of the first HIV-positive test to provide an average window period for seroconversion. Couples experiencing the outcome were censored in the interval in which estimated seroconversion occurred; those who remained discordant throughout the study were censored on the date of their last HIV-negative test date. Seroconversion events were manually validated by comparing test results and dates in the national HIV surveillance database, a centralized web-based system to which local disease control authorities report all newly identified HIV cases. If results differed between the 2 sets of records, information from the national surveillance database was used.
The primary exposure was time-varying ART use by the index partner. As with the outcome, we validated treatment status using records in the national ART database, information from which was given priority where discrepancies occurred. Additional index partner covariates including disease stage, AIDS related signs and symptoms, and CD4 cell count were linked from the national epidemiology and treatment databases using a unique identifier. Disease stage was determined using all other clinical information available when CD4 count was not recorded.
Statistical Analyses
To minimize potential bias induced by differences in transmission risk among patients initiating therapy at different times, we restricted analysis to those unexposed to ART at baseline. This new user design [22] eliminates the bias induced by under-ascertainment of events likely to occur early in the course of therapy, as well as by our inability to control for baseline factors that are themselves affected by the treatment (eg, disease stage). Due to very low rates of treatment termination (0.06%), all new users of ART were assumed to continue therapy until they were censored through an event, loss to follow-up, couple breakup, or death of either partner. Variables for which information was missing for more than 20% of the sample were multiply imputed using Markov Chain Monte Carlo simulation [23].
We used marginal structural Cox proportional hazard models [24] to estimate the association between index partner ART use and time to HIV seroconversion in nonindex partners. To mitigate bias from time-varying confounding variables that are also potentially affected by prior treatment, we weighted our model with the inverse probability of treatment and censoring. Stabilization with baseline indicators yielded an appropriate weight distribution (mean, 1.04; standard deviation; 0.24; range, 0.27–13.02) [25]. The final structural model was estimated using pooled logistic regression to approximate a discrete time hazards model [26] using robust standard errors and independent correlation matrices. Note that our marginal structural model included adjustment for baseline covariates in the final model as well as in the weights (which is typical [25–27]); as such, comparisons between the baseline-adjusted-and-weighted marginal structural model and the baseline-adjusted-but-unweighted model allowed us to assess the extent of confounding attributable to time-varying confounders alone.
Hazard ratios (HRs) from the marginal structural model were compared with those of a crude unweighted model and an adjusted unweighted model for residual confounding by baseline variables identified by directed acyclic graphs [28]. Additional analyses stratified estimates using interacting terms for index partner characteristics including time period (2006–2008 vs 2009 onward, to reflect periods before and after availability of second line ART), biological sex, baseline CD4, and type of treatment clinic. The assumption of proportional hazards was relaxed after inspection of the log-log survival curve by interacting our time and exposure variables.
Ethical Approval
All data used for this analysis were collected as part of prefectural disease control efforts. Ethical approval for the analysis of this data for research purposes was provided by the Institutional Review Board of the National Center for AIDS/STD Control and Prevention (NCAIDS) at the Chinese Center for Disease Control and Prevention.
RESULTS
Study Population
The final analysis included 4916 couples with a mean follow-up of 5.4 years (Table 1). The median age of index partners was 44 years (interquartile range: 40–49), and 45.7% were men. About half of index patients (46.8%) were thought to have been infected through blood or plasma donation. Over 90% of subjects reported “farmer” as the primary occupation. Most subjects (68.9%) had 6 or fewer years of education. Of the 94.8% (N = 4662) couples who reported any sex in the past year at most recent visit, 63.3% of them (N = 3115) reported “always” using condoms. All HIV-infected index partners were treatment naive at baseline (by study design), but by 2012 over 80% were receiving ART.
Table 1.
Characteristics of the 4916 HIV Serodiscordant Couples Included in the Final Analysis
| Seroconversion | No Seroconversion | Total | |
|---|---|---|---|
| N = 157 (%) | N = 4759 (%) | N = 4916 (%) | |
| Male index partner | 69 (43.9) | 2177 (45.7) | 2246 (45.7) |
| Missing | 2 (1.3) | 95 (2.0) | 97 (2.0) |
| Index partner age over 45 | 84 (53.5) | 2255 (47.4) | 2339 (47.6) |
| Index partner HIV transmission route | |||
| Blood/plasma donation | 101 (64.3) | 2301 (48.4) | 2402 (46.8) |
| Blood transfusion | 10 (6.4) | 705 (14.8) | 715 (14.5) |
| Injection drug use | 4 (2.5) | 122 (2.6) | 126 (2.6) |
| Hetero or homosexual sex | 18 (11.5) | 1270 (26.7) | 1288 (26.2) |
| Missing | 24 (15.3) | 461 (9.7) | 485 (9.9) |
| Index partner occupation as “farmer” | 153 (97.5) | 4307 (90.5) | 4460 (90.7) |
| Missing | 2 (1.3) | 100 (2.1) | 102 (2.1) |
| Index partner education level | |||
| Primary or less | 86 (54.8) | 3299 (69.3) | 3385 (68.9) |
| More than primary | 58 (36.9) | 948 (19.9) | 1006 (20.5) |
| Missing | 13 (8.3) | 512 (10.8) | 525 (10.7) |
| Average monthly frequency of intra-couple sex in the past year | |||
| 0–2 times | 11 (.7.0) | 1429 (30.0) | 1440 (29.3) |
| 3 or more times | 140 (89.2) | 3209 (67.4) | 3349 (45.7) |
| Missing | 6 (3.8) | 272 (5.7) | 278 (5.7) |
| Condom use in last year | |||
| “Always” | 36 (22.9) | 2871 (60.3) | 2907 (59.1) |
| “Sometimes” or “never” | 18 (11.5) | 179 (3.8) | 197 (4.0) |
| Missing | 103 (65.6) | 1709 (35.9) | 1812 (36.9) |
| Index partner baseline CD4 <250 cells/mL | 66 (42.0) | 1520 (31.9) | 1586 (32.3) |
| Missing | 25 (15.9) | 504 (10.6) | 529 (10.8) |
| Time since index HIV diagnosis under 5 years | |||
| <5 yr since last follow-up | 5 (3.2) | 496 (10.4) | 501 (10.2) |
| Missing | 19 (12.1) | 105 (2.2) | 124 (2.5) |
| Index partner ART use (ever) | 101 (64.3) | 3851 (80.9) | 3952 (80.4) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Few nonindex partners reported any risks associated with HIV acquisition risk outside the partnership. Over 7 years of follow-up, only 32 (0.7%) reported any episode of extramarital sex, in which condom use was reported as perfect in all but 1 case. Information on past diagnoses of sexually transmitted infections was collected for the first time in 2012, which showed that only 13 nonindex partners (0.4%) had received such a diagnosis in the previous year. No nonindex partners reported any type of illicit drug use throughout the study.
Main Analyses
In 26 389 years of follow-up, we observed 157 seroconversions (overall incidence rate, 0.59 cases per 100 person-years [PY]; 95% confidence interval [CI], .51–.70; Table 2). Of these seroconversions, 84 occurred after the index partner had initiated ART (incidence rate, 0.43 per 100 PY; 95% CI, .35–.53) and 73 before (incidence rate, 5.87 per 100 PY; 95% CI, 4.65–7.42; Table 2). Couples who seroconverted were more likely to be farmers, report more frequent sex (3 or more episodes a month over the last year vs fewer) and report “sometimes” or “never” having used condoms (vs “always”) in the past year. Couples whose index partners were diagnosed with HIV longer than 5 years before their most recent visit, and whose partner seroconverted before 2009 experienced higher rates of HIV transmission.
Table 2.
Incidence of HIV Seroconversion in Nonindex Partners by Key Covariates
| Events | Person Years | Cases/100 PY (95% CI) | |
|---|---|---|---|
| Sex of index partner | |||
| Male | 69 | 12 045 | 0.57 (.45–.72) |
| Female | 86 | 13 915 | 0.62 (.50–.76) |
| Missing | 2 | 429 | 0.47 (.12–1.86) |
| Age of index partner | |||
| <45 | 73 | 14 072 | 0.52 (.41–.65) |
| ≥45 | 84 | 12 317 | 0.68 (.55–.84) |
| Index partner HIV transmission route | |||
| Blood/plasma donation | 101 | 12 115 | 0.83 (.69–1.01) |
| Blood transfusion | 10 | 4084 | 0.24 (.13–.45) |
| Injection drug use | 4 | 710 | 0.56 (.21–1.50) |
| Hetero or homosexual sex | 18 | 7294 | 0.25 (.16–.39) |
| Missing | 24 | 2186 | 1.10 (.73–1.63) |
| Index partner occupation | |||
| Farmer | 153 | 24 015 | 0.64 (.54–.75) |
| Nonfarmer | 2 | 1923 | 0.10 (.02–.42) |
| Missing | 2 | 451 | 0.44 (.11–1.77) |
| Index partner education level | |||
| Primary or less | 73 | 18 454 | 0.40 (.31–.50) |
| More than primary | 13 | 2699 | 0.48 (.28–.83) |
| Missing | 71 | 5236 | 1.36 (1.08–1.71) |
| Average monthly frequency of intra-couple sex in the past year | |||
| 0–2 times | 124 | 15 456 | 0.80 (.67–.96) |
| 3 or more times | 29 | 9370 | 0.31 (.22–.45) |
| Missing | 4 | 1563 | 0.26 (.10–.68) |
| Condom use in last year | |||
| “Always” | 54 | 15 981 | 0.34 (.26–.44) |
| “Sometimes or never” | 25 | 840 | 2.98 (2.02–4.38) |
| Missing | 78 | 9568 | 8.15 (.65–1.02) |
| Index partner baseline CD4 | |||
| <250 | 66 | 8651 | 0.76 (.60–.97) |
| ≥250 | 66 | 15 747 | 0.42 (.33–.53) |
| Missing | 25 | 1991 | 1.26 (.85–1.85) |
| Estimated time of index HIV diagnosis | |||
| <5 yr | 5 | 1923 | 0.26 (.10–.62) |
| ≥5 yr | 133 | 22 978 | 0.58 (.49–.69) |
| Missing | 19 | 488 | 3.89 (2.51–6.05) |
| Index ART user (ever) | |||
| Yes | 90 | 20 769 | 0.43 (.35–.53) |
| No | 66 | 1124 | 5.87 (4.65–7.42) |
| Time period | |||
| 2008 and earlier | 86 | 10 160 | 0.85 (.69–1.04) |
| After 2008 | 71 | 16 229 | 0.44 (.35–.55) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; PY, person-year.
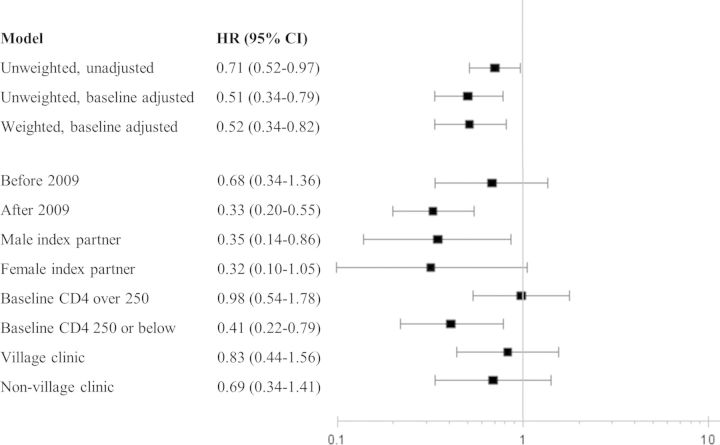
In unadjusted Cox models, ART reduced the risk of HIV in treated couples by 29% (HR, 0.71; 95% CI, .52–.97); however, weighting couples by their inverse probability of treatment and censoring weights increased this protective effect to and 48% (HR, 0.52; 95% CI, .34–.82). Most of the change in estimate was due to adjustment for baseline factors rather than from control of time-varying confounding through weighting, as suggested by similarity of results between weighted (HR, 0.52) and unweighted (HR, 0.51; 95% CI, .34–.79) models, both of which adjusted for baseline covariates (Figure 1).
Figure 1.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for crude, sensitivity analysis, and stratified analyses. All stratified analyses (including by time period) are weighted by inverse probabilities of treatment and censoring. All models use multiply imputed data for variables missing more than 20%. Adjustment for the baseline characteristics of the index partner including age, sex, education, disease stage, and time period.
Stratified Analyses
In our stratified analyses, in which models were weighted and adjusted for baseline covariates, we observed the most substantial difference in effect of ART over time. Specifically, we saw a modest benefit from ART on transmission in the early period from 2006 to 2008 (HR, 0.68; 95% CI, .34–1.36), and a far stronger effect in the later period from 2009 to 2012 (HR, 0.33, 95% CI, .20–.55). This evolving effect is also captured in the divergent survival curves of Figure 2, which are adjusted with the same inverse probability of treatment and censoring weights as in the marginal structural model [27]. ART also appeared to lower transmission risk more for index partners initiating ART at CD4 cell count at 250 or higher (HR, 0.41; 95% CI, .22–.79) rather than below 250 (HR, 0.98; 95% CI, .54–1.78).
Figure 2.
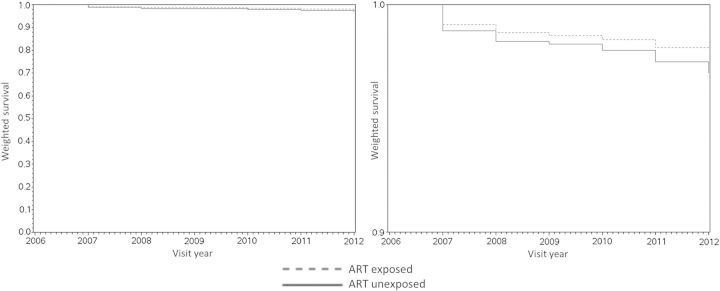
Inverse probability of treatment and censoring weighted Kaplan–Meir survival curves comparing treated and untreated experiences of couples. The same curves are shown on 2 different scales (0 to 1 on the left and 0.9 to 1.0 on the right) to better display trends. Abbreviation: ART, antiretroviral therapy.
We saw no substantive differences across index partner sex or type of treatment center. Stratified results are summarized in Figure 1.
DISCUSSION
We observed that ART reduced the overall HIV transmission risk in serodiscordant couples in rural China by 48%. Crucially, this effect evolved over time, with minimal protection from ART in the earlier phase of the study (2006–2008), and a strong preventive effect taking hold from 2009 to 2012. Notably, our time-specific results were consistent with findings from the 2 previous analyses of the same cohort, the first which found a modest effect in the early phase (2006–2008) [15], and the second which reported a strong effect in the later phase (2007–2011) [16]. We posit 2 plausible explanations for the dramatic change in effect over time: (1) reduced use of less tolerable antivirals such as didanosine, which were common in the early years of the ART program [29] (but which could not be directly assessed in this study given the low prevalence of use in later years of observation); and (2) systems-level improvements over time, such as enhancements in the ART delivery system [30] or increased medication adherence support [31].
The dramatic improvement in the protectiveness of ART over time also highlights a key pitfall of causal conclusions drawn from single summary HR estimates, which are often poor representations of period-specific effects, particularly if ratios shift over time [32]. Our plot of weighted survival curves (Figure 2) showing divergent, nonparallel curves, confirms that this in fact the case of our study, which not only undermines the proportional hazards assumption necessary for valid use of Cox models but also calls into question the value of interpreting effects that span time periods during which treatment environments underwent substantial changes. Nevertheless, the apparent improvement in ART effectiveness in Henan in spite of its resource limitations is encouraging, and whether this might be due to factors such as cumulative clinician experience or innovations in low cost service delivery is a possible area for further research. In other settings where treatment conditions may have improved as rapidly as in rural China, we urge consideration of weighted survival curves [27] over single HRs to disseminate observational findings on treatment for HIV prevention.
The lack of a protective effect of ART in couples whose index partner initiated therapy with a CD4 cell count below 250 is also noteworthy, though further research will need to explore possible explanations, such as compromised suppressive potential of ART in sicker patients, or systematically poorer drug adherence in this group. In the meantime these results support programming decisions to provide specialized monitoring for such couples to receive additional drug adherence support and safe sex counseling.
Our report expands on 2 previous analyses of the same population from Henan [15, 16], with several key differences. By using all available years of data (2006–2012) and by including 65% more eligible couples than either study, our study mitigated selection bias. We also minimized misclassification by validating exposure and outcome data using national disease control databases and by considering ART use as a time varying exposure (vs status at last visit). We also restricted our analysis to couples still unexposed to ART at enrollment with a new user design in order to establish clear temporality between baseline confounding variables, ART use, and transmission. Finally, weighting of models with visit-specific inverse probabilities of treatment and censoring mitigated bias due to time-varying confounding.
Our estimate of the effect of ART on HIV transmission risk was far less optimal than that reported by a meta-analysis of couples taking ART in other low-income countries (48% vs 86%) [33], and even our most optimal estimate of a 67% reduction in the later years of observation is still more modest than effects observed elsewhere. The difference is most likely because the latter studies reflected experiences of couples managed under special conditions as part of scientific research studies [34–36]. By contrast, couples in our study were treated through China's government administered ART program, in which many received care from nonphysicians in basic rural healthcare clinics. Differences across these 2 types of settings may matter for successful use of treatment as prevention, and underscore the need for more investigations in settings like Henan and Uganda [14] to inform real world implementation challenges.
The consistency of our results with couples elsewhere in China are noteworthy (Table 3), less for the fact that they are all from the same country than for the fact that all were recipients of government healthcare. In a nationally representative sample of over 38 000 couples, for example, ART was shown to have reduced transmission by 26% between 2003 and 2011 [37]. In a second report from Yunnan province, investigators reported a 66% reduction of virologically linked transmissions between 2009 and 2011 attributable to ART in index partners [13]. Results from each of these reports roughly correspond to our results from the earlier and later phases of follow-up (32% and 67% reduction, respectively).
Table 3.
Comparison of Longitudinal Studies Assessing the Effect the ART on HIV Transmission in Serodiscordant Couples in China
| Author [Year] | Number of Couples | Region | Time Period | Exposure Assessment in Index Partnera | Methods for Control of Confounding Bias | Estimate of Effect of ART in Index Partner on Risk of HIV Transmission HR (95% CI) | Conclusion |
|---|---|---|---|---|---|---|---|
| Wang L et al (2010) [14] | 1927 | Henan Province | 2006–2008 | Ever vs never exposed to ART. | Longitudinal follow-up of couples in a region where most indexes were infected through blood donation. | Unadjusted HR = 0.76 (.45–1.28). | ART did not effectively prevent HIV transmission over two years of follow-up. |
| Wang L et al (2013) [26] | 4499 | Henan Province | 2007–2011 | 1) Ever vs never exposed to ART; | Longitudinal follow-up of couples in a region where most indexes were infected through blood donation. | 1) Ever v never: adjusted HR = 0.01 (.00–.12); | ART was protective against HIV transmission over four years of follow-up. |
| 2) Exposed or unexposed to ART at most recent visit. | 2) ART at last visit: adjusted HR = 0.05 (.01–.16).b | ||||||
| He N et al (2013) [35] | 813 | Yunnan Province | 2009–2011 | ART initiated before midpoint of study, vs initiated after or not at all. | Multivariable models retained variables significantly associated with outcome (P < .10); forced entry of frequency of sex in 12 mo and HSV-2 status . | Unadjusted HR = 0.34 (.12–.97); | ART was protective against HIV transmission over two years of follow-up. |
| Adjusted HR = 0.30 (.10–.86).c | |||||||
| Jia Z et al (2013) [34] | 38 862 | National | 2003–2011 | ART exposed at baseline visit, vs not exposed at baseline visit. | Retrospective cohort assembled from national epidemiology and treatment databases. | Unadjusted HR = 0.61 (.55–.67); | ART was protective against HIV transmission. Durability of ART protective benefit wanes after 4 years. |
| Adjusted HR = 0.74 (.65–.84).d | |||||||
| Current analysis | 4916 | Henan Province | 2006–2012 | ART exposure as a time-varying variable. | Inverse probability of treatment and censoring weighting to address time-varying confounders identified through use of directed acyclic graphs. | Overall HR is not proportional | ART efficacy for HIV prevention varied over time. |
| Weight-adjusted HR for 2006 to 2008 effect, HR = 0.86 (.69–1.04); for 2009 to 2012 effect, HR = 0.44 (.35–.55). |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; HSV, herpes simplex virus.
a Outcome for all models is HIV seroconversion estimated as midpoint between last negative and first positive HIV antibody test.
b Adjusted for duration of follow-up, sex, age, education, marital status, occupation, route of HIV infection, and baseline CD4 cell count of the index patient.
c Adjusted for education, sexual frequency, condom use, last recorded CD4 cell count, AIDS, last recorded viral load, and ever taken ART.
d Adjustment for age, sex, education, seropositivity for herpes simplex virus 2, and frequency of sex.
HIV treatment as administered in resource limited settings like rural China appears to be an important means of HIV prevention in the real world. The public health applicability of these results will need to consider the uniqueness of this population and our limited ability to verify some measures. The HIV population of Henan is largely made up of older persons with low reported rates of drug use or sexual promiscuity [30] (corroborated by low rates of syphilis or reported sexually transmitted infection-like symptoms in our cohort), limiting generalizability to groups with more frequent or multiple sources of HIV exposure, such as sex workers who use drugs. These same features, however, also make seroconversions observed in our study population likely true representations of HIV transmission between primary partners, a hypothesis that could ideally have been confirmed through phylogenetic linkage analysis had useable samples of stored plasma been available. Other data limitations precluded investigation of hypotheses about differential ART effectiveness across subgroups. Future investigations will therefore benefit from collection of more details on index partner treatment experiences (ie, drug regimens, viral load, or medication adherence) or evolution of the local healthcare environment (ie, healthcare budget expenditures or staffing) to explore mechanisms driving potential effect modification.
In summary, we find that ART treatment of infected patients reduced HIV transmission. The magnitude and durability of benefit will depend on availability of well tolerated antiretroviral agents, a sufficient healthcare infrastructure, and constant adherence to medication. The experience in China suggests that these requirements can be met even in poor and remote locations.
Notes
Acknowledgments. We thank Zeng Ge, Shuai Ming, Fang Fang Chen, and Juan Han for their help with data collection and linkage work. We also thank the countless clinicians and staff involved in surveillance, laboratory testing, and treatment for human immunodeficiency virus (HIV) in China, as well as the HIV patients and their families for participating in this research.
Disclaimer. The funding sources had no involvement in the development of this work.
Financial support. This work was supported by Chinese Government grant under the 12th Five-Year Plan (2012ZX10001-002), the National Institute of Allergy and Infectious Disease (T32 AI007001; T32 AI102623) and the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR; P30 AI50410). M. K. S. was also supported by the Fulbright-Hayes Fellowship for Dissertation Research Abroad.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Guidance on Couples HIV Testing and Counselling including Antiretroviral Therapy for Treatment and Prevention in Serodiscordant Couples Recommendations for a public health approach, 2010. [PubMed]
- 2. Panel on Antiretroviral Guidelines for Adolescents and Adults, Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents, 2013.
- 3.Hoen B, Bonnet F, Delaugerre C, et al. Review article French 2013 guidelines for antiretroviral therapy of HIV-1 infection in adults. J Int AIDS Soc 2014; 17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitahata MM, Gange S, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das M, Chu PL, Santos G-M, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montaner JSG, Lima V, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dukers N, Spaargaren J, Geskus R, Beijnen J, Coutinho R, Fennema H. HIV incidence on the increase among homosexual men attending an Amsterdam sexually transmitted disease clinic: using a novel approach for detecting recent infections. AIDS 2002; 16:F19–24. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Wainberg M, Brun-Vezinet F, et al. Oral antiretroviral drugs as public health tools for HIV prevention: global implications for adherence, drug resistance, and the success of HIV treatment programs. J Infect Dis 2013; 207:S101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crepaz N, Lyles C, Wolitski R, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS 2006; 20:143–57. [DOI] [PubMed] [Google Scholar]
- 11.Vitoria M, Vella S, Ford N. Scaling up antiretroviral therapy in resource-limited settings: adapting guidance to meet the challenges. Curr Opin HIV AIDS 2013; 8:12–8. [DOI] [PubMed] [Google Scholar]
- 12.Muessig KE, Cohen MS. Advances in HIV prevention for serodiscordant couples. Curr HIV/AIDS Rep 2014; 11:434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He N, Duan S, Ding Y, et al. Antiretroviral therapy reduces HIV transmission in discordant couples in rural Yunnan, China. PLoS One 2013; 8:e77981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birungi J, Wang H, Ngolobe MH, et al. Lack of effectiveness of antiretroviral therapy (ART) as an HIV prevention tool for serodiscordant couples in a rural ART program without viral load monitoring in Uganda. In: 19th International AIDS Conference, 2012. [Google Scholar]
- 15.Wang L, Ge Z, Jing L, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr 2010; 55:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Wang L, Smith MK, et al. Heterosexual transmission of HIV and related risk factors among serodiscordant couples in Henan province, China. Chin Med J (Engl) 2013; 126:3694–700. [PubMed] [Google Scholar]
- 17.Ma Y, Zhang F, Zhao Y, et al. Cohort profile: the Chinese national free antiretroviral treatment cohort. Int J Epidemiol 2010; 39:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granich R, Gilks C, Dye C, Cock K, Williams B. Universal voluntary HIV testing and immediate antiretroviral therapy—author's reply. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, Rou K, Detels R. Prevalence of HIV infection among former commercial plasma donors in rural eastern China. Health Policy Plan 2001; 16:41–6. [DOI] [PubMed] [Google Scholar]
- 20.Wang L. Overview of the HIV/AIDS epidemic, scientific research and government responses in China. AIDS 2007; 21(Suppl 8):S3–7. [DOI] [PubMed] [Google Scholar]
- 21.AIDS in China: anatomy of an epidemic. Economist 2005; 53–5. [Google Scholar]
- 22.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003; 158:915–20. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991; 10:585–98. [DOI] [PubMed] [Google Scholar]
- 24.Robins JM. Association, causation, and marginal structural models. Synthese 1999; 121:151–79. [Google Scholar]
- 25.Cole SR, Hernán M. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008; 168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernán M, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 96:440–8. [DOI] [PubMed] [Google Scholar]
- 27.Westreich D, Cole SR, Tien PC, et al. Time scale and adjusted survival curves for marginal structural cox models. Am J Epidemiol 2010; 171:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10:37–48. [PubMed] [Google Scholar]
- 29.Liu H, Ma Y, Su Y, et al. Emerging Trends of HIV Drug Resistance in Chinese HIV-Infected Patients Receiving First-Line Highly Active Antiretroviral Therapy: A Systematic Review and Meta-Analysis. Clin Infect Dis 2014; 10:1495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dou Z, Chen RY, Xu J, et al. Changing baseline characteristics among patients in the China National Free Antiretroviral Treatment Program, 2002–09. Int J Epidemiol 2010; 39(Suppl 2):ii56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Wu Z. Factors associated with adherence to antiretroviral therapy among HIV/AIDS patients in rural China. AIDS 2007; 21:S149–55. [DOI] [PubMed] [Google Scholar]
- 32.Hernán M. The hazards of hazard ratios. Epidemiology 2010; 21:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baggaley R, White R, Hollingsworth T, Boily M-C. Heterosexual HIV-1 infectiousness and antiretroviral use: systematic review of prospective studies of discordant couples. Epidemiology 2013; 24:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnell D, Baeten J, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds S, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS 2011; 25:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia Z, Mao Y, Zhang F, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet 2013; 382:1195–203. [DOI] [PubMed] [Google Scholar]