HTLV-1–infected individuals who do not fulfill diagnostic criteria for HTLV-1–associated myelopathy/tropical spastic paraparesis had high incidence rates of neurologic symptoms and signs, especially involving the sensory, urinary, and motor tracts.
Keywords: HTLV-1, HAM/TSP, neurologic manifestations, cohort, incidence
Abstract
Background. Human T-cell lymphotropic virus type 1 (HTLV-1) is the agent of HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP), observed in up to 5% of infected individuals. Despite low prevalence, many HTLV-1–infected patients who do not fulfill criteria for HAM/TSP present with neurological complaints related to sensory, motor, urinary, or autonomic manifestations. The aim of this study was to determine the incidence of neurologic manifestations and risk factors associated with these outcomes.
Methods. The incidence of HAM/TSP and new signs and neurologic symptoms were computed in a group of patients enrolled in a cohort study.
Results. Of 414 subjects, 76 had definite HAM/TSP, 87 had possible or probable HAM/TSP, and 251 subjects had no neurologic manifestation and were selected for analysis. Definite HAM/TSP developed in 5 (1.47%) patients. Follow-up of at least 3 years was achieved in 51% of patients. The incidence rate was computed in 1000 person-years (206 for hand numbness, 187 for feet numbness, 130 for nocturia, and 127 for urgency). Average incidence rate in neurological exam was 76 for leg hyperreflexia, 53 for leg weakness, and 37 for Babinski sign. In the applied Expanded Disability Status Scale, the incidence rate of worsening 1 point was 134 per 1000 person-years. Kaplan–Meier curves stratified by sex and proviral load showed that females and patients with proviral load >50 000 copies/106 peripheral blood mononuclear cells had a higher risk of progression.
Conclusions. Development of neurological symptoms or signs occurred in up to 30% of asymptomatic subjects during 8 years of follow-up.
(See the Editorial Commentary by Taylor on pages 57–8.)
The human T-cell lymphotropic virus type 1 (HTLV-1) was the first human retrovirus described, initially found in a patient with cutaneous lymphoma [1]. Subsequently, the virus was also associated with an ancient neurological condition called tropical spastic paraparesis (TSP) that was prevalent in Central America [2]. In Japan, a similar disease was found related to the virus and was called HTLV-1–associated myelopathy (HAM) [3]. After these 2 neurological conditions were found to be the same, an integrated name—HAM/TSP—was proposed to describe the entity [4].
HTLV-1 infects about 20 million people worldwide with a high prevalence in Japan, the Caribbean islands, Central and South America, and North and West Africa [5]. It is estimated that about 3%–5% of infected individuals develop myelopathy [6], but the annual incidence of HAM/TSP among HTLV-1–infected subjects is not well established. As classic clinical disease occurs mainly after the sixth decade, the incubation time has been considered to be in the range of 20–40 years [7].
Despite the low prevalence of HAM/TSP, HTLV-1–infected individuals can present with neurological or clinical symptoms related to the virus even without significant motor abnormalities (paraparesis). In cross-sectional studies comparing neurologic manifestations in HTLV-1–infected subjects who did not fulfill the criteria for diagnosis of HAM/TSP, numbness and weakness in the lower limbs, urinary symptoms of overactive bladder, erectile dysfunction, hyperreflexia in the lower limbs, and Babinski sign were found to be higher among HTLV-1 carriers than in seronegative individuals [8, 9].
Urinary manifestations are very common in HTLV-1 infection without HAM/TSP, with a reported prevalence that varies from 15% to 17% [8, 10]. In urodynamic studies, most of these alterations are due to detrusor overactivity and sphincter-detrusor dyssynergy [11]. Urinary symptoms may precede the diagnosis of HAM/TSP by years, suggesting that these symptoms may represent an oligosymptomatic manifestation of HAM/TSP [12].
There are few cohort studies in HTLV infection, and most are in patients with HAM/TSP [7, 13, 14]. Only 1 study followed up both HTLV-1– and HTLV-2–infected subjects without myelopathy and, although the incidence of urinary, motor, and sensory symptoms was high, none of the subjects developed definitive HAM/TSP [15]. The aim of this study was to determine the incidence rate of neurologic symptoms and signs in a cohort of HTLV-1–infected individuals without definitive HAM/TSP at baseline who were followed for up to 8 years.
METHODS
This is a cohort study with 414 HTLV-1–infected individuals followed in the HTLV-1 clinic at Hospital Universitário Prof. Edgard Santos, Salvador, Bahia, Brazil. The period of study was from 2004 to 2011. HTLV-1 infection was diagnosed by detection of antibodies against viral antigens by enzyme-linked immunosorbent assay (ELISA) (Cambridge Biotech Corp, Worcester, Massachusetts) and confirmed by Western blot (HTLV blot 2.4, Genelab, Singapore). Subjects were referred mainly by blood banks at the time of attempted blood donation. Subjects were seen at 6- to 12-month intervals by a multidisciplinary team consisting of neurologists, urologists, rheumatologists, psychologists, and dentists. A standardized assessment was conducted each year with questions about clinical, neurological, and urological symptoms. Physical and neurologic exams were also performed. Because of the complexity of coordinating multiple subspecialty visits, data for certain symptoms/signs were not available at each study visit.
Case Definition
HAM/TSP diagnostic criteria were based on recommendations from an international consortium [16]. In brief, definite HAM/TSP is a nonremitting progressive spastic paraparesis with sufficiently impaired gait to be perceived by the patient. Sensory symptoms or signs may not be present but, when present, they are subtle and without a clear-cut sensory level. Urinary and anal sphincter signs or symptoms may or may not be present. Probable HAM/TSP was defined by a monosymptomatic presentation: spasticity or hyperreflexia in the lower limbs or isolated Babinski sign with or without subtle sensory signs or symptoms, or neurogenic bladder only confirmed by urodynamic tests. For both definite and probable definitions, clinicians must exclude an array of disorders that can mimic HAM/TSP. Possible HAM/TSP was defined by complete or incomplete clinical presentation in a setting where disorders resembling HAM/TSP have not been excluded. Anti–HTLV-1 antibodies in serum and/or cerebrospinal fluid are part of all of the above-mentioned criteria.
Neurologic Evaluation
Muscular strength was evaluated with the Medical Research Council criteria [17] as follows: 0 = no muscular contraction; 1 = little contraction; 2 = active movement with no gravity; 3 = active movement against gravity; 4 = active movement against gravity and resistance; 5 = normal. Leg strength was evaluated on both sides in proximal and distal muscles. If the score was <4 in one muscle, the segment was considered weak for our analysis. Reflexes were defined according to the Campbell score [18]: 0 = none; 1 = present but diminished; 2 = normal; 3 = enhanced; 4 = hyperactive, with clonus. The routinely evaluated reflexes were biceps, triceps, brachioradialis in both arms, and patellar and ankle jerks in the legs. Any segment with a reflex graded 3 or 4 was considered abnormal. The time in seconds to walk 8 meters (m) was utilized in survival analysis, and increase in at least 10 seconds in time was the definition for failure. The Expanded Disability Status Scale (EDSS) scores [19] were computed each year. Sensory symptoms were self-reported (numbness, prickling, or tingling). These could be occasional or frequent and of any intensity, but they had to appear in the prior 12 months.
Overactive bladder syndrome was considered to be present if individuals had symptoms of urgency with or without incontinence, according to international criteria [20].
Immunological Assays
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples by density gradient centrifugation using Ficoll-Hypaque. A total of 3 × 106 cells/mL was cultured in RPMI 1640 supplemented with 2 mM l-glutamine, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10% heat-inactivated fetal bovine serum, and 0.05% gentamicin. Cells were incubated only with medium for 72 hours at 37°C in 5% carbon dioxide. Determination of interferon gamma (IFN-γ) and tumor necrosis factor (TNF) levels in cell supernatants were performed by ELISA using reagents purchased from BD Bioscience Pharmingen (San Jose, California) as previously detailed [21].
Proviral Load
The DNA was extracted from 106 PBMCs using proteinase K and salting-out procedure. HTLV-1 proviral load was determined using real-time TaqMan polymerase chain reaction method (Applied Biosystems) as described previously [22]. There is no consensus regarding the proviral load cutoff related to HAM/TSP. Some authors have used 1% (Furtado et al [23]) and 5% (Grassi et al [24]), corresponding to 10 000 or 50 000 copies per 106 cells, respectively. We chose the cutoff of 5%.
Statistical Analysis
Survival analysis was performed with individuals without the symptom or sign at study entry as the at-risk population. Failure was defined as the development of the corresponding symptom or sign.
Censoring was applied to those lost to follow-up or who died of causes not related to HAM/TSP. Subjects were administratively censored on February 2012.
Kaplan–Meier curves were utilized to estimate the proportion without signs/symptoms, and the log-rank test was used to compare curves. Cox regression was performed to identify risk factors for neurologic worsening by EDSS score.
Ethics
The ethical committee of the Federal University of Bahia and the Weill Cornell Medical College Institutional Review Board approved the study, and all participants provided written informed consent.
RESULTS
Table 1 summarizes the baseline characteristics of the study participants. Of the 414 study subjects, 236 (59.2%) were referred from blood banks, 129 (32.3%) from other sources (television and radio advertisements, patient associations, and community associations), 17 (4.3%) from the neurology clinic, and 17 (4.3%) from family members. None of the patients referred from the neurology clinic were asymptomatic. Patients with definite HAM/TSP were older than subjects in the other groups, and no difference was found regarding sex. There was no difference regarding history of blood transfusion and intravenous drug use in the 3 groups.
Table 1.
Demographic and Epidemiologic Characteristics, Proviral Load, and Immunological Data of 414 HTLV-1–Infected Subjects, by Neurologic Status at Study Entry
| Characteristic | Without HAM/TSP (n = 251) | Probable or Possible HAM/TSP (n = 87) | HAM/TSP (n = 76) | P Value |
|---|---|---|---|---|
| Median age, y | 44.6 ± 12.2 | 47.9 ± 11.6 | 52.4 ± 14.0 | <.01a |
| Female sex | 143 (57.0) | 58 (66.7) | 51 (67.1) | .13b |
| Breastfed as infant (n = 203) | .15b | |||
| <1 y | 72 (58.5) | 24 (51.0) | 17 (51.5) | |
| 1–3 y | 39 (31.7) | 13 (27.7) | 14 (42.4) | |
| >3 y | 12 (9.8) | 10 (21.3) | 2 (6.1) | |
| Blood transfusion (n = 409) | 38 (15.4) | 22 (25.3) | 16 (21.3) | .09b |
| Intravenous drug use (n = 414) | 8 (3.2) | 4 (4.6) | 2 (2.7) | .76b |
| Homosexual activity (n = 285) | 16 (8.9) | 3 (5.5) | 2 (4.0) | .27b |
| HBV infection (n = 277) | 14 (7.7) | 8 (13.8) | 2 (5.3) | .26b |
| HCV infection (n = 276) | 10 (5.7) | 5 (8.5) | 3 (7.5) | .65b |
| Baseline proviral load (n = 251)c | 25 109 (1924–79 234) | 39 644 (3488–2701) | 148 938 (84 465–278 815) | <.01a |
| Baseline TNF-α (n = 257)d | 209 (0–769) | 346 (12–804) | 719 (240–1574) | <.01a |
| Baseline IFN-γ (n = 257)d | 866 (96–2036) | 711 (45–1678) | 2195 (773–3620) | <.01a |
Data are expressed as No. (%) unless otherwise specified.
Abbreviations: HAM/TSP, HTLV-1–associated myelopathy/tropical spastic paraparesis; HBV, hepatitis B virus; HCV, hepatitis C virus; HTLV-1, human T-cell lymphotropic virus type 1; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha.
a One-way analysis of variance.
b χ2 test.
c Expressed as number of HTLV-1 copies per 106 peripheral blood mononuclear cells, median (interquartile range).
d Expressed as pg/mL, median (interquartile range).
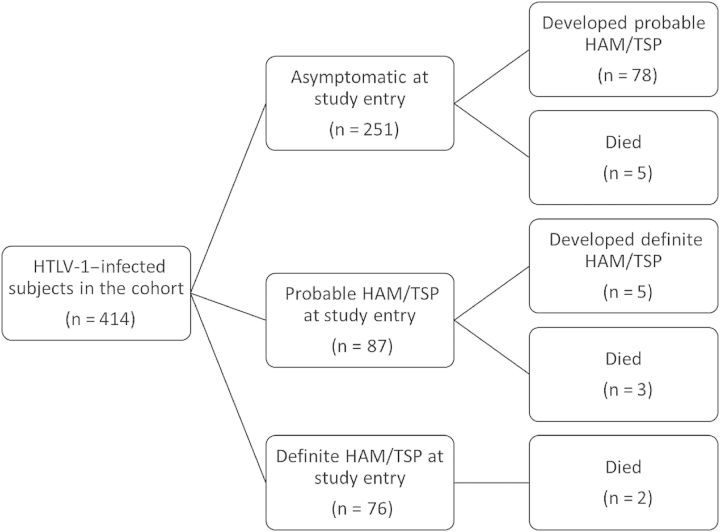
Figure 1 summarizes the outcomes of cohort participants. Of 414 HTLV-1–infected individuals evaluated, 76 had definite HAM/TSP (18.4%), 87 had possible or probable HAM/TSP (21%), and 251 (60.6%) patients did not have neurological disease related to HTLV-1 infection at study entry. During the study, 5 of 338 individuals (1.47%) without HAM/TSP at entry developed definite HAM/TSP. All of them had intermediate symptoms that were classified as probable HAM/TSP at entry, primarily neurogenic bladder confirmed by urodynamic study. In the group of asymptomatic individuals, 78 of 251 (31.07%) developed probable HAM/TSP.
Figure 1.
Outcomes of cohort participants. Abbreviations: HAM/TSP, human T-cell lymphotropic virus type 1–associated myelopathy/tropical spastic paraparesis; HTLV-1, human T-cell lymphotropic virus type 1.
Ten subjects died during follow-up of the following causes: 3 (30%) had acute T-cell lymphoma/leukemia (ATL), 2 (20%) other cancers, 2 (20%) myocardial infarction, 2 (20%) liver failure in the setting of hepatitis C virus (HCV) coinfection, and 1 (10%) accidental death (electrocution). Death rates were similar between the groups (Figure 1).
The asymptomatic group served as the study population for evaluating incident neurologic manifestations in subsequent analyses. From the sample of 251 carriers, 73.7%–100% did not have symptoms in the cohort at entry, depending on the variable analyzed, and 69.6%–75% had follow-up registered (Tables 2 and 3).
Table 2.
Survival Analysis of Self-Reported Symptoms in HTLV-1–Infected Subjects Without Human T-Cell Lymphotropic Virus Type 1 (HTLV-1)–Associated Myelopathy/Tropical Spastic Paraparesis
| Symptom | Initial Population Without Respective Symptom, no./No. (%) | Sample With Follow-up, no./No. (%) Follow-up Data Available | Proportion of Failures, no./No. (%) | Median Person-time Observation, y (Max-Min) | Incidence Rate/1000 Person-years (95% Confidence Interval) |
|---|---|---|---|---|---|
| Sensory symptoms | |||||
| Hand numbness | 173/251 (68.9) | 129/173 (74.5) | 73/129 (56.6) | 2 (1–7) | 206.2 (163–259) |
| Foot numbness | 188/251 (73.7) | 141/188 (75.0) | 76/141 (53.9) | 2 (1–7) | 186.7 (149–233) |
| Urinary symptoms | |||||
| Nocturia | 214/251 (85.2) | 160/214 (74.7) | 65/160 (40.6) | 2 (1–7) | 129.7 (101–165) |
| Urgency | 251/251 (100) | 187/251 (74.5) | 73/187 (19.0) | 2 (1–7) | 126.9 (100–159) |
| Retention | 238/251 (94.8) | 178/238 (74.7) | 50/178 (28.0) | 3 (1–7) | 79.1 (59–104) |
| Incontinence | 226/251 (90.0) | 169/226 (74.7) | 69/169 (40.8) | 5 (1–7) | 95.8 (75–121) |
| Motor symptoms | |||||
| Difficulty walking | 221/251 (88.0) | 164/221 (74.2) | 64/164 (39.0) | 2 (1–7) | 124.0 (97–158) |
| Difficulty running | 215/251 (85.6) | 159/215 (73.9) | 77/159 (48.4) | 2 (1–7) | 177.4 (141–221) |
Table 3.
Survival Analysis of Neurological Signs in Human T-Cell Lymphotropic Virus Type 1 (HTLV-1)–Infected Subjects Without HTLV-1–Associated Myelopathy/Tropical Spastic Paraparesis
| Sign | Initial Population Without Respective Symptom, no./No. (%) | Sample With Follow-up, no./No. (%) | Proportion of Failures, no./No. (%) | Median Person-time Observation, y, (Max-Min) | Incidence Rate/1000 Person-years (95% CI) |
|---|---|---|---|---|---|
| Neurological exam | |||||
| Arm hyperreflexia | 194/236 (82.2) | 135/194 (69.6) | 20/135 (14.8) | 2 (1–7) | 45.0 (30–72) |
| Leg hyperreflexia | 198/244 (81.1) | 148/198 (74.7) | 34/148 (22.9) | 2 (1–7) | 75.8 (54–106) |
| Leg weakness | 240/240 (100) | 180/240 (75.0) | 34/180 (18.8) | 3 (1–7) | 52.5 (37–73) |
| Babinski sign | 243/243 (100) | 181/243 (74.5) | 25/181 (13.8) | 3 (1–7) | 36.6 (25–55) |
| Neurological scales | |||||
| EDSS | … | 185/251 (73.7) | 78/185 (42.1) | 2 (1–7) | 133.7 (107–167) |
| Delay in 10 s in walking 8 m | … | 184/237 (77.6) | 9/184 (4.8) | 4 (1–7) | 12.1 (6–23) |
Abbreviations: CI, confidence interval; EDSS, Expanded Disability Status Scale.
When self-reported symptoms were evaluated, sensory complaints had the highest annual incidence rates, computed in events per 1000 person-years: 206 for hand numbness and 187 for foot numbness. For urinary symptoms, nocturia had an incidence rate of 130, urgency of 127, retention 79, and incontinence 96. For motor symptoms, difficulty walking and difficulty running had incidence rates of 124 and 177, respectively (Table 2).
Among neurological signs, leg hyperreflexia had an incidence rate of 76 per 1000 person-years, followed by leg weakness (53), arm hyperreflexia (45), and Babinski sign (37). The incidence rate of increasing at least 1 point on the EDSS was 134 and 12 for increasing at least 20 seconds in an 8-m walk in the ambulatory index (Table 3).
The prevalence of symptoms described above did not differ significantly after stratification on baseline proviral load using a cutoff of 50 000 copies per 106 PBMCs and varied from 0.87%–24.4% in subjects with low proviral loads to 0%–26.9% in those with high proviral loads.
When Cox regression was applied using an EDSS increase of 1 point as the outcome, female sex and high proviral load (>50 000 copies/106 PBMCs) were predictors of neurological worsening (hazard ratio [95% confidence interval], 1.80 [1.09–2.97], P = .02 and 1.70 [.30–.98], P = .05, respectively). Other parameters including coinfections (hepatitis B virus, HCV, and syphilis) and comorbidities (hypertension, diabetes mellitus, and hypothyroidism) were not statistically significant. Of note, age was not associated with neurologic worsening or progression to definite HAM/TSP (P = .66).
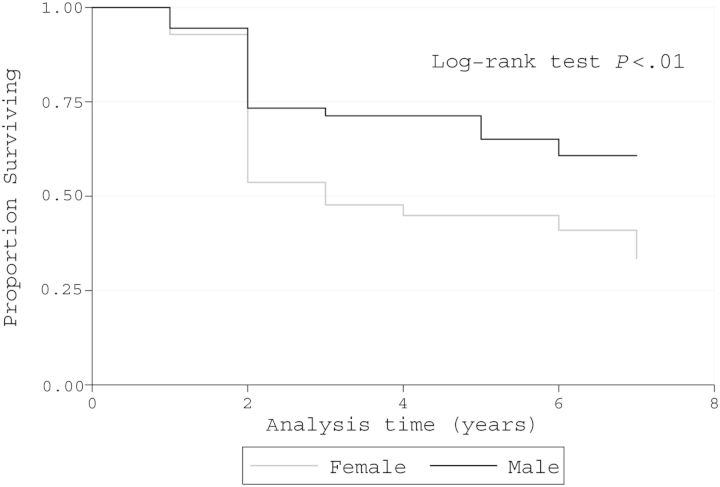
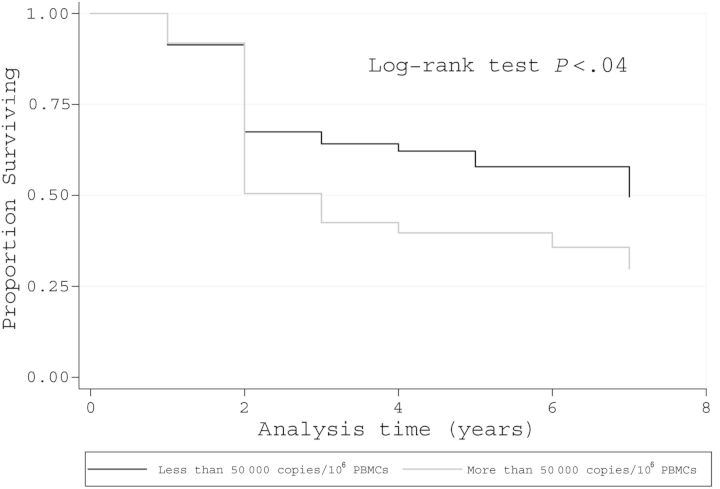
Kaplan–Meier analyses demonstrated that women had a higher incidence of neurologic progression by EDSS, as did subjects with high baseline proviral loads (Figures 2 and 3). Moreover, there was no association between TNF and IFN-γ production and neurologic progression (Supplementary Figure 1). The Kaplan–Meier adjusted incidences of neurologic manifestations showed that during up to 8 years of follow-up, the more common new symptoms and signs were (in decreasing frequency) leg paresthesias, urgency, incontinence, hyperreflexia, leg weakness, and Babinski sign (Supplementary Figure 2).
Figure 2.
Kaplan–Meier survival curve. Decrease in at least 1 point in Expanded Disability Status Scale in human T-cell lymphotropic virus type 1–asymptomatic individuals according to sex (n = 251).
Figure 3.
Kaplan–Meier survival curve. Decrease in at least 1 point in Expanded Disability Status Scale in human T-cell lymphotropic virus type 1–asymptomatic individuals according to proviral load (n = 130). Abbreviation: PBMCs, peripheral blood mononuclear cells.
DISCUSSION
HTLV-1 has been a neglected disease mainly due to the concept that it is a low-morbidity infection, as ATL and HAM/TSP are observed in <5% of infected subjects [25]. In this cohort study, we observed a high cumulative incidence of neurological symptoms and signs of up to 56% of HTLV-1–infected individuals despite our low HAM/TSP rate. This indicates that despite the rarity of HAM/TSP, morbidity associated with HTLV-1 is high.
The appearance of symptoms in HTLV-1 patients seems to follow a particular order, beginning with mild sensorimotor manifestations (hyperreflexia/numbness) and urinary disturbances with evolution to leg weakness, and Babinski sign. Of the 5 patients who developed definite HAM/TSP in our cohort, all had urinary symptoms at study entry, mostly urgency with incontinence. All 5 patients had lower leg hyperreflexia (grade 3 or 4), none had leg weakness or Babinski sign, and all were considered to have probable HAM/TSP. The median time to onset of definite HAM/TSP was 4 years (range, 1–7 years).
Like the other HTLV cohort in non-HAM/TSP patients [15], our study showed a high incidence of symptoms. Moreover, our study calculated individual incidence rates and person-time at risk for each neurological symptom. Although we did not follow a healthy control group to compare the incidence of symptoms and signs, the high rates and association of worsening neurological symptoms with high proviral load strongly suggest a causal relationship with HTLV-1 infection. Giving support to this finding, Biswas et al found that subjects with either HTLV-1 or HTLV-2 had higher prevalences of symptoms, especially involving the urinary and motor pyramidal systems, compared with healthy subjects [15].
The EDSS scale was designed for use in patients with multiple sclerosis [19]. However, it is useful for HTLV-1 infection because it can capture several neurologic domains including sensory, motor, and urinary. We have previously shown the association of EDSS with urinary manifestations, erectile dysfunction, and neurologic symptoms in HTLV-1 infection [10, 26, 27]. Our Cox regression analysis found 2 important risk factors at baseline for worsening EDSS—sex and HTLV-1 proviral load, both of which have been previously found to be associated with worsening neurological symptoms in patients with HTLV-1 without HAM/TSP [7, 13]. Neither age nor race was associated with neurologic progression in our study. The majority of our subjects (78%) were black or of mixed race and did not differ from whites with regard to progression, in keeping with literature data comparing blacks and whites [28].
In a previous study comparing neurologic symptoms and signs in HTLV-1 carriers and in uninfected individuals, complaints of difficulty walking were present in 11% of HTLV-1–infected subjects [8]. Here, although subjective complaints of difficulty walking were reported by >30% of the HTLV-1 carriers, objective determination by increased time to walk 8 m resulted in a lower incidence of gait difficulties. As prolongation of walking time was documented less frequently than leg weakness and hyperreflexia, and about as frequently as HAM/TSP itself, we may conclude that only severe pyramidal involvement appears to influence walking time.
Overactive bladder syndrome was found in at least 19% of the individuals, based on international criteria including the presence of urgency. Most of our patients were also evaluated with urodynamic studies, and detrusor overactivity was the most frequent finding. A prior study from our group showed that these urinary symptoms are not likely to be due to urinary tract infection [29]. We have recently shown that patients with overactive bladder have similar immunologic abnormalities and proviral load as patients with HAM/TSP and that production of proinflammatory cytokines (TNF-α and IFN-γ) and proviral load are higher than that observed in HTLV-1 carriers [30]. It is known that proviral load and production of proinflammatory cytokines are increased in patients with HAM/TSP and that these play a role in the pathogenesis of HTLV-1 infection [13, 31]. These data reinforce the concept that overactive bladder in these individuals is a manifestation related to HTLV-1. These symptoms have an important impact on the quality of life of these individuals.
There are multiple lines of evidence indicating that immunologic responses participate in the pathogenesis of diseases associated with HTLV-1. Children with infective dermatitis, a disease that is recognized as a risk factor for HAM/TSP, have high production of proinflammatory cytokines [32]. Furthermore, patients with periodontal disease associated with HTLV-1 have increased expression of proinflammatory cytokines and a decrease in regulatory T cells and the regulatory cytokine interleukin 10 (IL-10) [31]. Here we evaluated if the production of TNF-α and IFN-γ at entry in the study was associated with development of neurologic symptoms. Our cytokine analysis did not show statistically significant differences between high and low producer groups in Kaplan–Meier curves; there was a trend, however, for an association between high TNF-α levels and neurologic progression (P = .07). Recent data have suggested that impaired ability of regulatory cytokines such as IL-10 and TGF-β to downregulate the inflammatory response may be a better marker for development of HAM/TSP than proinflammatory cytokine production per se [30].
Of note, whereas 19% of HTLV-1 carriers in our cohort developed overactive bladder, only 1.47% developed HAM/TSP. At study entry, 62 HTLV-1–infected individuals already had overactive bladder, but only 3 of them developed definitive HAM/TSP. Taken together, these data suggest that the majority of HTLV-1–infected subjects with overactive bladder do not progress to HAM/TSP in the short term, indicating that in addition to the exaggerated production of proinflammatory cytokine and high proviral load, other factors participate in the development of HAM/TSP. The cumulative incidence of HAM/TSP in our study (1.47%) was less than that found in the literature (3%–5%), but the duration of follow-up (maximum 8 years) was too short compared with the historic average time of 20 years to develop the disease [7].
Regarding ATL development, very few patients in our cohort had the disease (3 patients, all of whom died). Two had histories of relapsing strongyloidiasis, a condition that is associated with ATL [33]. The other patient had HAM/TSP and developed ATL during follow-up. Although this sequence is uncommon, it has been reported in Brazil [34].
Our study has several noteworthy strengths and limitations. The relatively large sample size and comprehensive multidisciplinary evaluations of study subjects are key strengths. The duration of follow-up, however, is short relative to the prolonged natural history of HTLV-1 infection. Furthermore, our findings may not be generalizable to other geographic regions and are limited by potential selection biases related to which patients are referred to and continue to be followed at an academic center. Given that a substantial proportion of study subjects were asymptomatic blood donors found to be HTLV-1 infected at the time of attempted donation, the potential for bias is somewhat reduced.
In conclusion, the findings of this clinic-based cohort study show that HTLV-1–infected individuals had high rates of developing neurologic symptoms and signs, especially involving the sensory, urinary, and motor tracts. These findings have the potential to adversely affect quality of life in patients with HTLV-1 infection. Moreover, although there is no established therapy for HAM/TSP, symptomatic treatment of these manifestations may improve quality of life and the ability to work.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Counsel of Technological and Scientific Development and the US NIH (grant number R01 AI079238).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A 1980; 77:7415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985; 2:407–10. [DOI] [PubMed] [Google Scholar]
- 3.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet 1986; 1:1031–2. [DOI] [PubMed] [Google Scholar]
- 4.Osame M. Review of WHO Kagoshima meeting and diagnostic guidelines for HAM/TSP. In: Human Retrovirology HTLV. Blattner WA, ed. New York: Raven Press, 1990:191–7. [Google Scholar]
- 5.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 2012; 3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan JE, Osame M, Kubota H, et al. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr 1990; 3:1096–101. [PubMed] [Google Scholar]
- 7.Martin F, Fedina A, Youshya S, Taylor GP. A 15-year prospective longitudinal study of disease progression in patients with HTLV-1 associated myelopathy in the UK. J Neurol Neurosurg Psychiatry 2010; 81:1336–40. [DOI] [PubMed] [Google Scholar]
- 8.Caskey MF, Morgan DJ, Porto AF, et al. Clinical manifestations associated with HTLV type I infection: a cross-sectional study. AIDS Res Hum Retroviruses 2007; 23:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poetker SKW, Porto AF, Giozza SP, et al. Clinical manifestations in individuals with recent diagnosis of HTLV type I infection. J Clin Virol 2011; 51:54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro NM, Rodrigues W, Freitas DM, Muniz A, Oliveira P, Carvalho EM. Urinary symptoms associated with human T-cell lymphotropic virus type I infection: evidence of urinary manifestations in large group of HTLV-I carriers. Urology 2007; 69:813–8. [DOI] [PubMed] [Google Scholar]
- 11.Castro NM, Freitas DM, Rodrigues W, Muniz A, Oliveira P, Carvalho EM. Urodynamic features of the voiding dysfunction in HTLV-1 infected individuals. Int Braz J Urol 2007; 33:238–44; discussion 244–5. [DOI] [PubMed] [Google Scholar]
- 12.Imamura A, Kitagawa T, Ohi Y, Osame M. Clinical manifestation of human T-cell lymphotropic virus type-I-associated myelopathy and vesicopathy. Urol Int 1991; 46:149–53. [DOI] [PubMed] [Google Scholar]
- 13.Olindo S, Cabre P, Lézin A, et al. Natural history of human T-lymphotropic virus 1-associated myelopathy: a 14-year follow-up study. Arch Neurol 2006; 63:1560–6. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki T, Nakagawa M, Nagai M, et al. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol 2001; 7:228–34. [DOI] [PubMed] [Google Scholar]
- 15.Biswas HH, Engstrom JW, Kaidarova Z, et al. Neurologic abnormalities in HTLV-I- and HTLV-II-infected individuals without overt myelopathy. Neurology 2009; 73:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Castro-Costa CM, Araújo AQC, Barreto MM, et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses 2006; 22:931–5. [DOI] [PubMed] [Google Scholar]
- 17. Medical Research Council. Aids to examination of the peripheral nervous system. London: Her Majesty's Stationary Office, 1976. [Google Scholar]
- 18.Campbell W. DeJong's the neurologic examination. 6th ed Philadelphia, PA: Lippincott; Williams & Wilkins, 2005:641. [Google Scholar]
- 19.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33:1444–52. [DOI] [PubMed] [Google Scholar]
- 20.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function : report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn 2002; 178:167–78. [DOI] [PubMed] [Google Scholar]
- 21.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis 2004; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehée A, Césaire R, Désiré N, et al. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J Virol Methods 2002; 102:37–51. [DOI] [PubMed] [Google Scholar]
- 23.Furtado MDSBS, Andrade RG, Romanelli LCF, et al. Monitoring the HTLV-1 proviral load in the peripheral blood of asymptomatic carriers and patients with HTLV-associated myelopathy/tropical spastic paraparesis from a Brazilian cohort: ROC curve analysis to establish the threshold for risk disease. J Med Virol 2012; 84:664–71. [DOI] [PubMed] [Google Scholar]
- 24.Grassi MFR, Olavarria VN, Kruschewsky Rde A, et al. Human T cell lymphotropic virus type 1 (HTLV-1) proviral load of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients according to new diagnostic criteria of HAM/TSP. J Med Virol 2011; 1274:1269–74. [DOI] [PubMed] [Google Scholar]
- 25.Proietti FA, Carneiro-Proietti ABF, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005; 24:6058–68. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira P, Castro NM, Muniz AL, et al. Prevalence of erectile dysfunction in HTLV-1-infected patients and its association with overactive bladder. Urology 2010; 75:1100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro N, Oliveira P, Freitas D, Rodrigues W, Muniz A, Carvalho E. Erectile dysfunction and HTLV-I infection: a silent problem. Int J Impot Res 2005; 17:364–9. [DOI] [PubMed] [Google Scholar]
- 28.Viana GM, Nascimento Mdo D, de Oliveira RA, Dos Santos AC, Galvão Cde S, da Silva MACN. Seroprevalence of HTLV-1/2 among blood donors in the state of Maranhão, Brazil. Rev Bras Hematol Hemoter 2014; 36:50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha PN, Rehem AP, Santana JF, et al. The cause of urinary symptoms among human T lymphotropic virus type I (HLTV-I) infected patients: a cross sectional study. BMC Infect Dis 2007; 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos SB, Oliveira P, Luna T, et al. Immunological and viral features in patients with overactive bladder associated with human T-cell lymphotropic virus type 1 infection. J Med Virol 2012; 84:1809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garlet GP, Giozza SP, Silveira EM, et al. Association of human T lymphotropic virus 1 amplification of periodontitis severity with altered cytokine expression in response to a standard periodontopathogen infection. Clin Infect Dis 2010; 50:e11–8. [DOI] [PubMed] [Google Scholar]
- 32.Primo J, Siqueira I, Nascimento MCF, et al. High HTLV-1 proviral load, a marker for HTLV-1 associated myelopathy/tropical spastic paraparesis, is also detected in patients with infective dermatitis associated with HTLV-1. Braz J Med Biol Res 2009; 42:761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh M, Toma H, Sugahara K, et al. Involvement of IL-2/IL-2R system activation by parasite antigen in polyclonal expansion of CD4(+)25(+) HTLV-1-infected T-cells in human carriers of both HTLV-1 and S. stercoralis . Oncogene 2002; 21:2466–75. [DOI] [PubMed] [Google Scholar]
- 34.Farre L, de Fátima Paim de Oliveira M, Primo J, Vandamme A-M, Van Weyenbergh J, Bittencourt AL. Early sequential development of infective dermatitis, human T cell lymphotropic virus type 1-associated myelopathy, and adult T cell leukemia/lymphoma. Clin Infect Dis 2008; 46:440–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.