Infective endocarditis (IE) is a serious complication of Staphylococcus aureus bacteremia (SAB). We propose 2 scoring systems that can accurately predict IE in patients with SAB and complement decision-making by clinicians for optimal use of transesophageal echocardiography in these patients.
Keywords: Staphylococcus aureus, bacteremia, echocardiography, endocarditis, risk score
Abstract
Background. Infective endocarditis (IE) is a serious complication of Staphylococcus aureus bacteremia (SAB). There is limited clinical evidence to guide use of echocardiography in the management of SAB cases.
Methods. Baseline and 12-week follow-up data of all adults hospitalized at our institution with SAB from 2006 to 2011 were reviewed. Clinical predictors of IE were identified using multivariable logistic regression analysis.
Results. Of the 757 patients screened, 678 individuals with SAB (24% community acquired, 56% healthcare associated, and 20% nosocomial) met study criteria. Eighty-five patients (13%) were diagnosed with definite IE within the 12 weeks of initial presentation based on modified Duke criteria. The proportion of patients with IE was 22% (36/166) in community-acquired SAB, 11% (40/378) in community-onset healthcare-associated SAB, and 7% (9/136) in nosocomial SAB. Community-acquired SAB, presence of cardiac device, and prolonged bacteremia (≥72 hours) were identified as independent predictors of IE in multivariable analysis. Two scoring systems, day 1 (SAB diagnosis day) and day 5 (when day 3 culture results are known), were derived based on the presence of these risk factors, weighted in magnitude by the corresponding regression coefficients. A score of ≥4 for day 1 model had a specificity of 96% and sensitivity of 21%, whereas a score of <2 for day 5 model had a sensitivity of 98.8% and negative predictive value of 98.5%.
Conclusions. We propose 2 novel scoring systems to guide use of echocardiography in SAB cases. Larger prospective studies are needed to validate the classification performance of these scoring systems.
(See the Editorial Commentary by Kaasch and Jung on pages 29–30.)
Staphylococcus aureus is a highly virulent pathogen that has become a leading cause of bacteremia in both community and healthcare settings, accounting for 20% of all healthcare-associated bacteremias [1, 2]. The mortality attributed to S. aureus bacteremia (SAB) and sepsis ranges from 20% to 30%. Mortality in S. aureus infective endocarditis (IE) is even higher and ranges from 19% to 65% [3–5]. In a large prospective study of SAB, 34% of cases developed 1 or more metastatic infections involving joints, kidneys, brain, bones, liver, spleen, or spine [6]. Therefore, timely diagnosis of IE in patients with SAB is critical as it has important implications regarding choice of antibiotic therapy, duration of treatment, and need for surgical intervention in patients with complicated IE.
Endocarditis may be either the primary source of SAB or a secondary metastatic complication resulting from hematogenous seeding of cardiac valves from an extracardiac infectious focus. Clinical manifestations of S. aureus IE are nonspecific [7], and classic stigmata of IE are typically absent early in the course of infection. Transesophageal echocardiogram (TEE) is considered the imaging test of choice as it is superior to transthoracic echocardiogram (TTE) to evaluate for IE in adults, especially in the setting of prosthetic heart valves and implanted cardiac devices [8–13]. However, the evidence that TEE should be performed in every patient with SAB is limited [8, 14, 15]. TEE is an expensive and invasive test, is not readily available in all medical centers, and is performed in a fraction of patients with SAB in the usual course of patient care [16, 17].
The usefulness of TEE in SAB patients depends on the pretest probability of IE. The prevalence of IE among patients with SAB varies depending on the study population. While earlier investigations reported a wide range (5%–64%) of IE among SAB patients, more recent studies have reported 5%–17% prevalence [6, 18–22]. Therefore, while TEE is appropriate in patients with high likelihood of IE, it is probably not necessary for every patient with SAB. Recently, simple criteria to guide use of echocardiography were proposed [23–25]. However, a minority of patients in these studies underwent TEE. Our aim in this study was to develop a simple and easy-to-use clinical scoring system to guide use of TEE in the management of both community- and healthcare-associated SAB cases.
METHODS
Study Overview
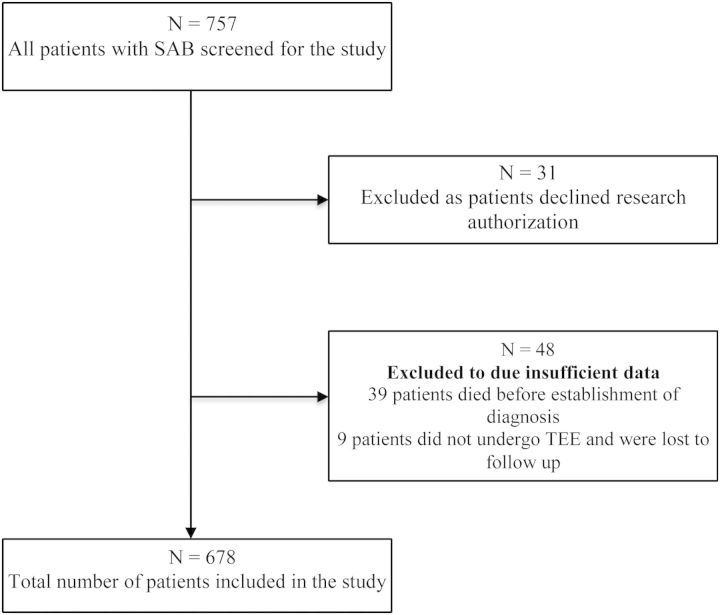
All adults (age ≥18 years) hospitalized at our institution with SAB from July 2006 to June 2011 were included in the study. Cases of SAB were identified in the Department of Laboratory Medicine and Microbiology database. Demographic, microbiologic, echocardiographic, and follow-up (at least 12 weeks) data were collected. Patients who did not undergo TEE and for whom follow-up data at 12 weeks were unavailable were excluded from the analysis (Figure 1). Patients who did not undergo TEE but had 12-week follow-up were included. Patients who died or chose palliative care/hospice prior to diagnostic evaluation for IE were also excluded. Electronic medical records were reviewed to collect follow-up data for 12 weeks or longer. The Mayo Foundation Institutional Review Board approved the study.
Figure 1.
Study population. Figure shows total number of patients screened and those who were excluded from the study. Abbreviations: SAB, Staphylococcus aureus bacteremia; TEE, transesophageal echocardiogram.
Definitions
Cases of SAB were classified as nosocomial, healthcare associated, or community acquired, as defined by Friedman et al [26]. Nosocomial bacteremia was defined as positive blood culture result first obtained from patients who had been hospitalized for ≥48 hours. Healthcare-associated bacteremia was defined as a positive blood culture result obtained from a patient at the time of hospital admission or within 48 hours of admission if the patient (a) received intravenous therapy at home, received wound care or specialized nursing care through a healthcare agency, or had intravenous medical therapy in the previous 30 days; patients whose only home therapy was oxygen use were excluded; (b) attended a hospital or hemodialysis clinic or received intravenous chemotherapy in the previous 30 days; (c) was hospitalized in an acute care hospital for 2 days in the previous 90 days; or (d) resided in a nursing home or long-term care facility. Community-acquired bacteremia was defined as a positive blood culture result obtained at the time of hospital admission or within 48 hours after hospital admission. Prolonged bacteremia was defined as persistently positive blood cultures for ≥72 hours. IE was defined by modified Duke criteria [27]. Echocardiographic evidence of IE was defined as presence of an oscillating intracardiac mass, myocardial abscess, new valvular regurgitation, or new dehiscence of a prosthetic valve. Only patients who met the modified Duke criteria for definite endocarditis were included.
Statistical Methods
Model Derivation
Descriptive statistics on study variables are presented as median (range or interquartile range), mean (standard deviation [SD]), or frequency count (percentage), as appropriate. The associations between candidate risk factors and IE were measured using logistic regression and summarized with odds ratios and 95% confidence intervals. Each factor was screened for an association with IE via univariate logistic regression; those with an alpha level of 0.1 or less were carried forward in multivariable analyses. The multivariable logistic model was reduced to the most important risk factors using stepwise variable selection with backward elimination (alpha level of 0.05). Two-way interactions between candidate risk factors were also tested for importance. The day 1 scoring model was derived based on the modeling steps listed above using baseline risk factors available at the time of diagnosis. Excluding a small minority of patients who were lost to short-term follow-up, the algorithm was then repeated to derive a second scoring system, day 5, that incorporated “prolonged bacteremia.”
Model Validation
We internally validated the fit and performance of both final models using the bootstrap method with 400 resamples. In a given resample, a new set of patients is randomly sampled (with replacement) from the original set, on whom a new model is fit using the same stepwise criteria. The model derived on each bootstrap resample was then tested on the original set of patients, as the difference in performance (eg, c-statistic) between this bootstrap and the test model provides a sense of overfitting in the original model selection. For both final models, we estimated the bias due to overfitting, or “optimism,” and recalculated the c-statistic correcting for this bias. A detailed description of this statistical methodology is provided in the Supplementary Material.
Risk Stratification
From the final models, regression coefficients were used to derive the 2 separate risk scores for predicting IE. In particular, points were assigned for each risk factor in the model and weighted approximately by the respective regression coefficients. For optimal scoring, regression coefficients were rescaled by first dividing by the minimum absolute value among all coefficients and then multiplying each rescaled coefficient by a constant (such as 2 or 3, choosing the one producing the most optimal weighting scheme), and finally rounding the rescaled values to the nearest integer. Using these point values, a patient's risk score was simply computed as the aggregate number of points from his or her risk factor profile.
RESULTS
Study Population
A total of 757 patients with SAB were hospitalized at Mayo Clinic Rochester from July 2006 to June 2011. Thirty-one patients were excluded as they had declined research authorization; 48 patients were excluded due to insufficient data (Figure 1); 39 patients died during the follow-up period before evidence of IE could be established; 9 patients did not undergo TEE and were lost to follow-up; and 678 individuals with SAB (24% community onset, 56% healthcare associated, and 20% nosocomial) who met the study criteria were included in the final analysis. Baseline characteristics are summarized in Table 1. The majority of patients were male (65%) and mean age (SD) was 64.8 (5.7) years. Twelve percent of the patients had a cardiovascular implantable electronic device (CIED). Of these, 7% were permanent pacemakers (PPMs) and 5% were implantable cardioverter defibrillators (ICDs). Only 6% of patients had a prosthetic heart valve. Thirty-nine percent of the S. aureus isolates were methicillin resistant.
Table 1.
Distribution of Candidate Variables for Overall Staphylococcus aureus Bacteremia Cohort and by Infective Endocarditis Status
| Variable | Overall | IE | No IE | Univariate Association Odds Ratio (95% Confidence Interval) [P Value] |
|---|---|---|---|---|
| Baseline Variables | N = 678 | N = 85 | N = 593 | |
| Age at baseline (yr) | 64.8 ± 15.7 | 64.6 ± 17.9 | 64.9 ± 15.4 | 0.99 (.86, 1.14) [.879] |
| Male gender | 442 (65%) | 59 (69%) | 383 (65%) | 1.24 (.76, 2.03) [.383] |
| White ethnicity | 648 (96%) | 81 (95%) | 567 (96%) | 0.93 (.32, 2.73) [.893] |
| Onset of SAB | ||||
| Community | 166 (24%) | 36 (42%) | 130 (22%) | 2.34 (1.43, 3.83) |
| Healthcare | 378 (56%) | 40 (47%) | 338 (57%) | 1.0 (ref) [F test P < .001] |
| Nosocomial | 134 (20%) | 9 (11%) | 125 (21%) | 0.61 (.29, 1.29) |
| Duration of symptoms (d) | ||||
| 0 | 292 (43%) | 32 (38%) | 260 (44%) | 1.0 (ref) [F test P = .383] |
| 1–3 | 210 (31%) | 26 (31%) | 184 (31%) | 1.15 (.66, 1.99) |
| >3 | 176 (26%) | 27 (32%) | 149 (25%) | 1.47 (.85, 2.55) |
| Hypotension at presentation | 133 (20%) | 22 (26%) | 111 (19%) | 1.52 (.89, 2.57) [.122] |
| Diabetes mellitus | 250 (37%) | 33 (39%) | 217 (37%) | 1.10 (.69, 1.75) [.690] |
| Hemodialysis dependent | 80 (12%) | 14 (16%) | 66 (11%) | 1.57 (.84, 2.95) [.156] |
| Central intravenous catheter prior to SAB | 171 (25%) | 16 (19%) | 155 (26%) | 0.66 (.37, 1.16) [.149] |
| Prosthetic joint | 99 (15%) | 17 (20%) | 82 (14%) | 1.56 (.87, 2.78) [.134] |
| Cardiac implantable electronic device | ||||
| Neither | 594 (88%) | 55 (65%) | 539 (91%) | 1.0 (ref) [F test P < .001] |
| Permanent pacemaker | 49 (7%) | 20 (24%) | 29 (5%) | 6.76 (3.59, 12.74) |
| Implantable cardioverter defibrillator | 35 (5%) | 10 (12%) | 25 (4%) | 3.92 (1.79, 8.59) |
| Prosthetic valve | 43 (6%) | 13 (15%) | 30 (5%) | 3.39 (1.69, 6.79) [<.001] |
| Prosthetic vascular graft | 41 (6%) | 5 (6%) | 36 (6%) | 0.97 (.37, 2.54) [.946] |
| Chronic Immunosuppressive therapy | 158 (23%) | 8 (9%) | 150 (25%) | 0.31 (.14, .65) [.002] |
| Intravenous drug use | 15 (2%) | 5 (6%) | 10 (2%) | 3.64 (1.21, 10.93) [.021] |
| Recent Staphylococcus aureus infections | 34 (5%) | 9 (11%) | 25 (4%) | 2.69 (1.21, 5.98) [.015] |
| Deep-seated abscesses | 165 (24%) | 12 (14%) | 153 (26%) | 0.47 (.25, .89) [.021] |
| Skin/skin structure infection | 185 (27%) | 13 (15%) | 172 (29%) | 0.44 (.24, .82) [.009] |
| Antibiotic duration (d)a | (N = 632) | 28.0 (14.0, 42.0) | 28.0 (14.0, 42.0) | 42.0 (42.0, 42.0) |
| Underwent TEE within 12 wk | 482 (71%) | 83/85 (98%) | 399 / 593 (67%) | |
| Time to first TEE (d)a | (N = 482) | 4.0 (3.0, 6.0) | 4.0 (2.0, 6.0) | 4.0 (2.0, 6.0) |
| Cases that underwent TEE within first 5 d | 351 (52%) | 293 / 593 (49%) | 58 / 85 (68%) | |
| Timing of first TEE among those tested (d) | (N = 482) | |||
| ≤5 | 351 (73%) | 293 / 399 (73%) | 58 / 83 (70%) | |
| >5 | 131 (27%) | 106 / 399 (27%) | 25 / 83 (30%) | |
| Day 5 Variable | (N = 662) | N = 83 | N = 579 | |
| Prolonged bacteremia (≥72 h) | 323 (49%) | 68 (82%) | 255 (44%) | 5.76 (3.22, 10.32) [<.001] |
Effects for all variables tested in univariable logistic regression models.
Abbreviations: IE, infective endocarditis; SAB, Staphylococcus aureus bacteremia; TEE, transesophageal echocardiogram.
a Median (interquartile range).
Overall, 71% of the patients underwent TEE within 12 weeks of SAB diagnosis. In 68% of patients, TEE was performed within 14 days after first blood culture. A total of 85 patients (13%) fulfilled the modified Duke criteria for definite IE, of whom 56 (66%) had native valve endocarditis, 13 (15%) had prosthetic valve IE, and 25 (29%) had endocarditis related to a CIED. These included 8 (9%) patients with ICD-related IE and 17 (20%) with PPM-related IE. The proportion of patients with IE was 22% in community-acquired SAB, 11% in community-acquired healthcare-associated SAB, and 7% in nosocomial SAB. The proportion with IE was 18% in patients with hemodialysis, 30% in patients with prosthetic heart valves, 41% in patients with PPMs, 29% in patients with ICDs, and 21% in those with prolonged bacteremia lasting longer than 72 hours.
Model Derivation
Two scoring systems were derived for the clinical purpose of risk stratification at 2 time points: day 1 score (SAB diagnosis day) and day 5 score. Among the baseline factors identified from univariable screening and carried forward into multivariable modeling, the following were selected in the original model fit and corresponded to increased risk of IE: onset of SAB, CIED, prior intravenous drug abuse (IVDA), recent S. aureus infection, and absence of a skin or surgical site as a source of infection (Table 1). However, based on internal validation of the model fit via bootstrap resampling, only onset of SAB and CIED were deemed robust predictors of IE. To derive the day 5 model, the effect of prolonged bacteremia, along with baseline variables, was assessed for 662 patients with SAB (7 patients who died within 5 days of an SAB diagnosis and 9 patients who lacked a negative culture were excluded). Prolonged bacteremia was found to be predictive of IE in the original sample and was internally validated as a predictor after being selected in 100% of the bootstrap models. The final day 5 model therefore included onset of SAB, presence of CIED, and prolonged bacteremia.
Risk Stratification
On the basis of final day 1 and day 5 models, regression coefficients were used to derive 2 risk scores for predicting IE in patients hospitalized for SAB. Points were assigned for each risk factor, weighted in magnitude by the corresponding regression coefficients, and summed together to define an individual's risk score (Table 2).
Table 2.
Risk Factors for Infective Endocarditis and Development of Infective Endocarditis Risk Scores
| Model/Variable | Multivariable Result Odds Ratio (95% Confidence Interval) [P Value] | β | β′ | c × β′ | Points^ |
|---|---|---|---|---|---|
| Day 1 Model | c = 2 | ||||
| CIED | |||||
| ICD | 4.58 (2.03, 10.35) [<.001] | 1.52 | 1.00 | 2.00 | 2 |
| PPM | 7.94 (4.08, 15.44) [<.001] | 2.07 | 1.36 | 2.72 | 3 |
| Neither | 1.0 (reference) | … | … | 0 | |
| Onset of SAB | |||||
| Community | 5.01 (2.22, 11.31) [<.001] | 1.61 | 1.06 | 2.12 | 2 |
| Healthcare | 1.91 (.87, 4.19) [.104] | 0.65 | 0.43 | 0.85 | 1 |
| Nosocomial | 1.0 (reference) | … | … | 0 | |
| Model C Statistic | 0.723 | Score = ∑ Points | |||
| Bias-Corrected C Statistic | 0.693 | Range, 0–5 | |||
| Day 5 Model | c = 2 | ||||
| CIED | |||||
| ICD | 5.00 (2.09, 11.94) [<.001] | 1.61 | 1.20 | 2.40 | 2 |
| PPM | 8.60 (4.18, 17.67) [<.001] | 2.15 | 1.60 | 3.21 | 3 |
| Neither | 1.0 (reference) | … | … | 0 | |
| Onset of SAB | |||||
| Community | 3.83 (1.64, 8.96) [.002] | 1.34 | 1.00 | 2.00 | 2 |
| Healthcare | 1.79 (.80, 4.03) [.159] | 0.58 | 0.43 | 0.87 | 1 |
| Nosocomial | 1.0 (reference) | … | … | 0 | |
| Prolonged bacteremia (≥72 h) | 5.23 (2.85, 9.61) [<.001] | 1.65 | 1.23 | 2.47 | 2 |
| Model C Statistic | 0.792 | Score = ∑ Points | |||
| Bias-Corrected C Statistic | 0.761 | Range, 0–7 | |||
Abbreviations: CIED, cardiovascular implantable electronic device; ICD, implantable cardioverter defibrillator; PPM, permanent pacemaker; SAB, Staphylococcus aureus bacteremia.
DISCUSSION
There is limited evidence regarding optimal use of TEE in patients with SAB to evaluate for underlying IE (Table 4). While some studies have suggested that TEE is dispensable in patients with uncomplicated SAB [23–25], these studies focused on nosocomial SAB and <30% of cohorts had TEE performed, increasing the risk of verification bias. Considering that patients with sustained bacteremia (>72 hours) in these studies received prolonged (3–4 weeks) courses of antimicrobial therapy, it is conceivable that some patients with endocarditis were cured without an IE diagnosis being secured.
Table 3.
Test Performance Characteristics of Two Independent Infective Endocarditis Risk Prediction Models
| Risk Score | Sensitivity (%) | Specificity (%) | False-Positive Rate (%) | False-Negative Rate (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|---|
| Day 1 Score ≥ | ||||||
| High Risk 5 | 9.4 | 99.5 | 0.5 | 90.6 | 72.7 | 88.5 |
| ↑ 4a | 21.2 | 95.6 | 4.4 | 78.8 | 40.9 | 89.4 |
| 3 | 35.3 | 92.4 | 7.6 | 64.7 | 40.0 | 90.9 |
| 2b | 64.7 | 70.2 | 29.8 | 35.3 | 23.7 | 93.3 |
| ↓ 1 | 95.3 | 18.4 | 81.6 | 4.7 | 14.3 | 96.5 |
| Low Risk 0 | 100 | 0.0 | 100 | 0.0 | 12.5 | … |
| Day 5 Score ≥ | ||||||
| High Risk 7 | 7.2 | 99.7 | 0.3 | 92.8 | 75.0 | 88.2 |
| ↑ 6 | 19.3 | 97.9 | 2.1 | 80.7 | 57.1 | 89.4 |
| 5 | 30.1 | 96.7 | 3.3 | 69.9 | 56.8 | 90.6 |
| 4 | 54.2 | 83.1 | 16.9 | 45.8 | 31.5 | 92.7 |
| 3b | 86.7 | 59.2 | 40.8 | 13.3 | 23.4 | 96.9 |
| 2c | 94.0 | 41.1 | 58.9 | 6.0 | 18.6 | 97.9 |
| ↓ 1 | 98.8 | 11.4 | 88.6 | 1.2 | 13.8 | 98.5 |
| Low Risk 0 | 100 | 0.0 | 100 | 0.0 | 12.5 | … |
a A stringent day 1 score ≥4 for screening would have resulted in 44 patients tested, in whom 18 (positive predictive value [PPV] 40.9%) had endocarditis (567 of the 634 not tested did not have endocarditis negative predictive value [NPV] 89.4%), leaving 67 endocarditis cases that would not have been tested.
b Cutpoint from receiver-operating characteristic curve analysis that jointly optimizes sensitivity and specificity.
c A conservative day 5 score ≥2 for screening would have resulted in 419 patients tested, in whom 78 (PPV 18.6%) had endocarditis (238 of the 243 not tested did not have endocarditis [NPV 97.7%]), leaving only 5 endocarditis cases that would have been missed.
Table 4.
Key Studies Evaluating the Role of Transesophageal Echocardiography in Patients With Staphylococcus aureus Bacteremia
| Study | Study Period | Study Design | No. of Patients | Study Findings/Conclusion | IE (%) | Comments |
|---|---|---|---|---|---|---|
| Joseph et al [25] | 2006–2011 | Retrospective study of all cases of SAB | 306 | Patients with underlying prosthetic intracardiac material were higher risk of IE; authors concluded the cardiac imaging should be prioritized to high-risk patients | 10.1 | Of 668 eligible patients with SAB, only 82 (12.7%) underwent TEE |
| Khatib and Sharma [24] | 2002–2009 | Included patients from 3 previous prospective observational studies (2002–2003, 2005–2006, 2008–2009) | 379 | Authors concluded that TEE is dispensable in patients with uncomplicated SAB | 7.3 | 498 of 877 eligible patients (57%) did not undergo echocardiography (TTE or TEE) and were excluded; of 877 eligible patients with SAB, only 177 (20%) underwent TEE |
| Incani et al [7] | 1998–2006 | Retrospective study of all adult patients who were hospitalized with SAB and underwent TEE | 144 | 46% of IE patients were not suspected to have IE based on clinical findings alone; authors concluded that TEE is indicated in all patients with SAB as clinical findings are not sensitive | 29 | 83% of eligible patients underwent TEE and were included in the study |
| Rasmussen et al [28] | 2009–2010 | Prospective observational study of SAB patients | 244 | Noted high rates of IE; clinical symptoms and findings were noted to be insensitive and nonspecific; authors concluded that echocardiography should always be considered in early evaluation of patients with SAB | 22 | Limited by selection bias; 92 eligible patients (27%) did not undergo echocardiography (TTE or TEE) and were excluded; 62% of study patients underwent TEE |
| Kaasch et al [23] | 1994–2009 | Post hoc analysis of 2 prospective SAB cohorts (INSTINCT and SABG) | 736 nosocomial | TEE may not be required in a subset of low-risk nosocomial SAB patients identified by using a simple criteria set | 4.3 (INSTINCT) 9.3 (SABG) | Only 18.5% of INSTINCT and 27.6% of SABG cohorts underwent TEE; included only nosocomial SAB |
| Van Hal et al [29] | 1996–2000 | Retrospective study of patients who underwent both TTE and TEE | 125 | TEE may not be required in a subgroup of low-risk SAB patients if TTE is normal and there are no embolic signs | 18 | Of 808 eligible patients with SAB, 641 (79%) had no TEE and were excluded; limited by sampling bias; excluded patients with prosthetic valves, annuloplasty rings, implantable cardioverter defibrillator, or permanent pacemaker |
| Abraham et al [15] | 1999–2002 | Retrospective review of SAB cases who were referred for either TTE or TEE | 104 | Noted high rates of endocarditis; SAB patients should be aggressively evaluated for endocarditis | 31.7 | Limited by referral bias as only patients with high pretest clinical probability were likely referred for echocardiography |
| Fowler et al [8] | 1994–1996 | Prospective study of SAB patients who underwent both TTE and TEE | 103 | Only 7% of patients had clinical evidence of IE; sensitivity of TTE was only 32%; TEE had 100% sensitivity as none met Duke criteria for definite IE without positive TEE; authors concluded that TEE should be considered in early evaluation of patients with SAB | 25.2 | Only 59% of eligible patients with SAB underwent both TTE and TEE; limited by sampling bias as physicians tended to refer patients with higher likelihood of IE for TEE |
Abbreviations: IE, infective endocarditis; INSTINCT, invasive Staphylococcus aureus infection cohort; SAB, Staphylococcus aureus bacteremia; SABG, Staphylococcus aureus bacteremia group; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram.
The current investigation is one of the largest studies to assess the role of TEE in patients with SAB in which the majority (68% underwent TEE within 14 days, 71% underwent TEE within 12 weeks) of patients underwent TEE, decreasing the risk of verification bias and therefore a low likelihood of missing IE. Based on the results of the multivariable analysis, we propose 2 scoring systems to predict the risk of IE and, thus, the need for TEE on SAB diagnosis day (day 1) and when prolonged bacteremia is documented (day 5).
In the multivariable analysis, community-acquired SAB, presence of CIED or prosthetic heart valve, and prolonged bacteremia were independently associated with IE in patients with SAB. This is congruent with earlier publications aimed at identifying risk factors of complicated SAB [15, 19, 30–35]. For the day 1 risk score (range, 0–5), screening individuals with a score of 2 or higher was the optimal threshold in terms of both sensitivity and specificity (65% and 70%, respectively). Choosing a higher cutoff value to target high-risk patients would improve specificity but at the expense of reduced sensitivity. For example, a screening cutoff of ≥4 would have selected 44 patients for TEE and correctly excluded a very high proportion of those without IE (specificity, 96%), while yielding only a small percentage of IE cases (sensitivity, 21%). Using the day 5 score (range 0–7) to inform an independent screening decision (assuming low-risk patients are not screened for IE based on the day 1 risk score), a cutpoint of ROC (receiver-operating characteristic curve) ≥3 had optimal diagnostic power based jointly on sensitivity and specificity (87% and 59%, respectively) with equal weight. Opting for a risk score more preferential to sensitivity, such as a score ≥2, would ensure that a higher proportion of all IE cases (sensitivity, 94%) are identified, although with a trade-off cost of increased TEE screening (specificity, 41%).
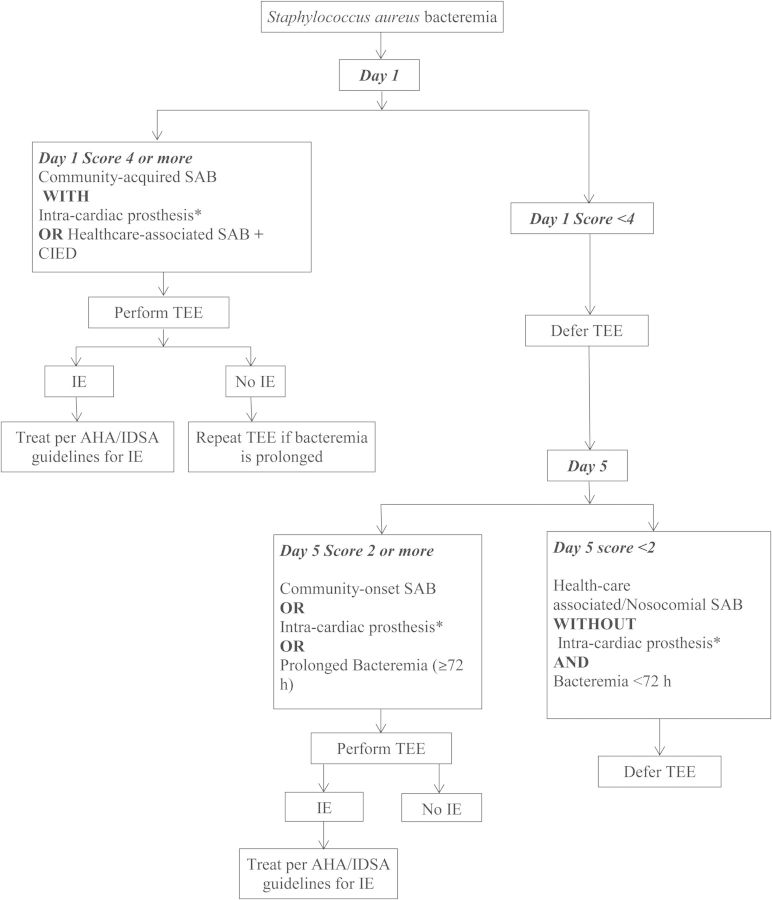
We propose a 2-stage screening strategy that combines the predictive values of day 1 and day 5 risk scores. The first stage (day 1 risk score) should be used to identify patients at highest risk of IE. Patients who have a high risk score on day 1 screening, such as ≥4, should undergo TEE soon after SAB is diagnosed as early detection of IE in high-risk patients may have therapeutic implications such as choice of antibiotic therapy (combination therapy if prosthetic valve IE is present) and need for surgical intervention (if indications for surgery, such as myocardial abscess, valve perforation, infected CIED, are present). This high-risk group includes patients with underlying implantable cardiac devices who develop community-onset SAB. If the initial TEE is negative for evidence of IE in this high-risk group, then they should be reevaluated with repeat TEE if bacteremia is prolonged. Repeat TEE was performed in 88 patients in our study cohort. In 5 patients whose initial TEE was negative, repeat TEE showed evidence of endocarditis. Patients with low risk score (eg, <4) on day 1 should be reassessed on day 5. For day 5 risk score, we favor a cutoff with very high negative predictive value, given the high mortality rate associated with IE, and defer TEE only in those with a very low likelihood of IE. For day 5, we suggest a risk score cutoff of <2 as it had a sensitivity of 98.8% and negative predictive value of 98.5%. Patients with either community-acquired SAB or prolonged bacteremia (≥72 hours) or those with underlying CIED will have day 5 scores ≥2 and should be evaluated with TEE. Although a significant risk factor in the modeling, prosthetic heart valve did not meet the more stringent criteria for inclusion in the scoring tool; this is possibly due to the small number of patients with prosthetic heart valves in our study cohort (6%). However, 2 of the 5 IE cases deemed as low risk by the scoring system had a prosthetic valve in place. Therefore, we have added patients with prosthetic heart valves to the high-risk category in our proposed decision-making algorithm (Figure 3).
Figure 3.
Proposed algorithm for optimal use of transesophageal echocardiogram (TEE) in patients with Staphylococcus aureus bacteremia (SAB). *Intracardiac prosthesis: Implantable cardioverter-defibrillator, permanent pacemaker, prosthetic heart valve. Abbreviations: AHA, American Heart Association; CIED, cardiovascular implantable electronic device; IE, infective endocarditis; IDSA, Infectious Diseases Society of America.
Strict application of the proposed scoring system to guide TEE use would have resulted in missing 5 IE cases in our SAB study cohort. Therefore, clinicians should not be discouraged from obtaining TEE if there is a strong clinical suspicion of IE in a given case, even if a patient does not meet scoring criteria. Table 5 includes the clinical characteristics of these 5 patients. As described above, 2 of the 5 patients had an underlying prosthetic heart valve, and we have added prosthetic valve to the high-risk category in our proposed algorithm. Moreover, all patients should have close clinical follow-up for at least 12 weeks to detect relapse or metastatic complications. In patients with recurrence or relapse of SAB, TEE should be done, regardless of initial clinical risk scoring and prior echocardiographic findings. Based on our classification scheme (Figures 2 and 3), more than one-third of SAB patients could be managed without performing TEE.
Table 5.
Clinical Characteristics of 5 Patients With Low Risk Score but Diagnosed With Infective Endocarditis
| Patient Number | Age (yr) | Sex | Day 5 Risk Score | Onset of SAB | Intracardiac Prosthesis | Duration of SAB (d) | Comments |
|---|---|---|---|---|---|---|---|
| 1 | 67 | M | 1 | Community onset, healthcare associated | None | 1 | TEE showed right atrial mass/thrombus; treated as possible infected thrombus with 42 d of intravenous antibiotics |
| 2 | 73 | M | 0 | Nosocomial | Prosthetic valve, recent mitral valve replacement | 2 | Acute prosthetic valve endocarditis; patient had skin manifestations of IE (prosthetic valve is included as high-risk category in our revised algorithm) |
| 3 | 88 | M | 1 | Community onset, healthcare associated | None | 2 | Recent hip surgery with acute septic arthritis and secondary bacteremia; TEE was positive for vegetation |
| 4 | 29 | F | 1 | Community onset, healthcare associated | Prosthetic valve; recent tricuspid valve replacement | 3 | Acute prosthetic valve endocarditis; patient had skin manifestations of IE |
| 5 | 61 | M | 1 | Community onset, healthcare associated | None | 1 | Chemotherapy-induced neutropenia; patient had SAB recurrence on day 49 of follow-up; TEE revealed possible vegetation on aortic valve |
Abbreviations: IE, infective endocarditis; SAB, Staphylococcus aureus bacteremia; TEE, transesophageal echocardiogram.
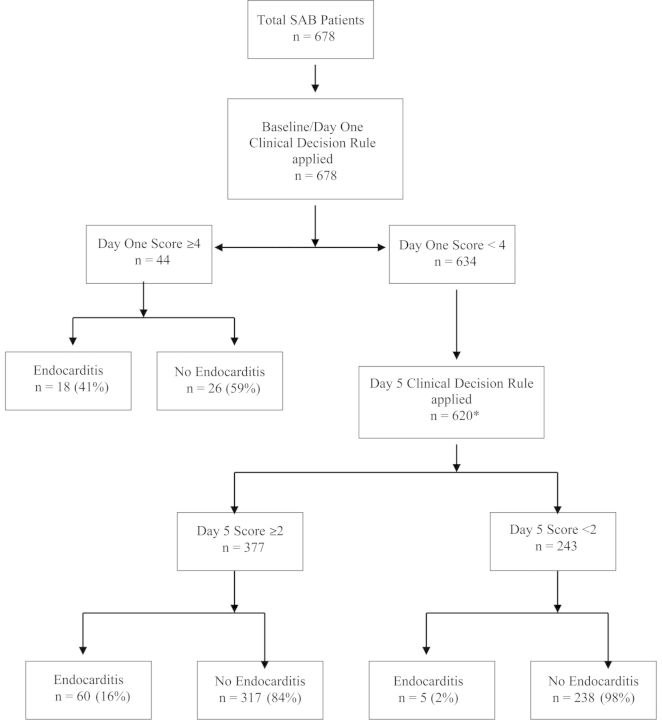
Figure 2.
A hypothetical application of 2-stage screening strategy on our study cohort. *Day 5 score was applied only on 620 patients in this hypothetical scenario. Fourteen patients either died or did not have follow up blood cultures. Abbreviation: SAB, Staphylococcus aureus bacteremia.
In our study, 131 patients underwent both TTE and TEE within 14 days after first positive blood culture, of whom 41 patients were diagnosed with IE. TEE was positive in 85% of IE (35/41) and TTE was positive in only 34% (14/41). These data are consistent with data from several earlier studies that suggest the sensitivity of TEE is much higher than that of TTE. TEE is the imaging modality of choice to evaluate for IE in patients with SAB.
Our study has several limitations, primarily due to its retrospective design. The decision to obtain TEE and its timing was at the discretion of the attending physician, and the median time to perform TEE was 4 days from the date of first positive blood culture. Therefore, it can be argued that some patients who underwent testing within 48 hours of SAB diagnosis but had prolonged bacteremia may have had a false-negative result for IE because testing at this stage may have missed evolving endocarditis. However, lack of relapse of SAB during the 12-week follow-up period suggests that these were likely true negative results. Because our cohort included few patients with IVDA as a risk factor for IE, our findings may not be applicable to this subset of higher-risk patients. The median duration of antibiotic therapy in those without IE was 28 days. The longer duration of antibiotics in non-IE patients is likely due to a higher complexity of cases in our tertiary referral center. It is possible that some patients with IE were undiagnosed but received longer antibiotic course. However, 71% of patients underwent TEE, hence the likelihood of unrecognized but treated IE is low. Also, due to variable duration of antimicrobial therapy in low-risk patients in our study cohort, it is unclear if a shorter course (2 weeks) of antimicrobial therapy is adequate, especially if an echocardiogram is deferred. Prospective studies are needed to further validate the scoring system and to address the question of antibiotic duration.
Overall, our study findings suggest that the risk of IE in individuals with SAB can be estimated using a simple scoring system that utilizes 2 elements of clinical history (onset of SAB and presence of CIED) and duration of bacteremia. We propose a 2-stage screening strategy to be applied on the day of SAB diagnosis (day1) and when results of day 3 blood cultures are available (day 5) to help guide the optimal use of TEE.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This study was supported by a research grant from the American Heart Association (12CRP12080058) to M. R. S. (principal investigator). Resources of the Mayo Clinic Center for Clinical and Translational Science, funded by the National Institutes of Health (Clinical and Translational Science Awards, UL1 RR024150), were used for data analysis and manuscript preparation. The study database was created and maintained using REDCap (grant UL1 TR000135).
Financial Disclosures. All <$10 000 unless specified otherwise. L. M. B. reports royalty payments (authorship) from UpToDate, Inc. (<$20 000) and editor-in-chief payments from the Massachusetts Medical Society (Journal Watch Infectious Diseases; <$20 000). M. R. S. reports receiving funds from TYRX Inc. and Medtronic for prior research unrelated to this study, administered according to a sponsored research agreement between Mayo Clinic and the study sponsor that prospectively defined the scope of the research effort and corresponding budget, and honoraria/consulting fees from Medtronic and Spectranetics.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kern WV. Management of Staphylococcus aureus bacteremia and endocarditis: progresses and challenges. Curr Opin Infect Dis 2010; 23:346–58. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–17. [DOI] [PubMed] [Google Scholar]
- 3.Petti CA, Fowler VG., Jr Staphylococcus aureus bacteremia and endocarditis. Cardiol Clin 2003; 21:219–33, vii. [DOI] [PubMed] [Google Scholar]
- 4.Roder BL, Wandall DA, Frimodt-Moller N, Espersen F, Skinhoj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 1999; 159:462–9. [DOI] [PubMed] [Google Scholar]
- 5.Sohail MR, Martin KR, Wilson WR, Baddour LM, Harmsen WS, Steckelberg JM. Medical versus surgical management of Staphylococcus aureus prosthetic valve endocarditis. Am J Med 2006; 119:147–54. [DOI] [PubMed] [Google Scholar]
- 6.Fowler VG, Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 7.Incani A, Hair C, Purnell P, et al. Staphylococcus aureus bacteraemia: evaluation of the role of transoesophageal echocardiography in identifying clinically unsuspected endocarditis. Eur J Clin Microbiol Infect Dis 2013; 32:1003–8. [DOI] [PubMed] [Google Scholar]
- 8.Fowler VG, Jr, Li J, Corey GR, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol 1997; 30:1072–8. [DOI] [PubMed] [Google Scholar]
- 9.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–434. [DOI] [PubMed] [Google Scholar]
- 10.Chu VH, Bayer AS. Use of echocardiography in the diagnosis and management of infective endocarditis. Curr Infect Dis Rep 2007; 9:283–90. [DOI] [PubMed] [Google Scholar]
- 11.Rosen AB, Fowler VG, Jr, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med 1999; 130:810–20. [DOI] [PubMed] [Google Scholar]
- 12.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 14.Sullenberger AL, Avedissian LS, Kent SM. Importance of transesophageal echocardiography in the evaluation of Staphylococcus aureus bacteremia. J Heart Valve Dis 2005; 14:23–8. [PubMed] [Google Scholar]
- 15.Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S. aureus and methicillin-resistant S. aureus bacteremia. Am Heart J 2004; 147:536–9. [DOI] [PubMed] [Google Scholar]
- 16.Fowler VG, Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–86. [DOI] [PubMed] [Google Scholar]
- 17.Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickerson EK, Hongsuwan M, Limmathurotsakul D, et al. Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One 2009; 4:e4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang FY, MacDonald BB, Peacock JE, Jr, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–32. [DOI] [PubMed] [Google Scholar]
- 20.Das I, O'Connell N, Lambert P. Epidemiology, clinical and laboratory characteristics of Staphylococcus aureus bacteraemia in a university hospital in UK. J Hosp Infect 2007; 65:117–23. [DOI] [PubMed] [Google Scholar]
- 21.Turnidge JD, Kotsanas D, Munckhof W, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust 2009; 191:368–73. [DOI] [PubMed] [Google Scholar]
- 22.Kaasch AJ, Barlow G, Edgeworth JD, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 2014; 68:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaasch AJ, Fowler VG, Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatib R, Sharma M. Echocardiography is dispensable in uncomplicated Staphylococcus aureus bacteremia. Medicine (Baltimore) 2013; 92:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph JP, Meddows TR, Webster DP, et al. Prioritizing echocardiography in Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2013; 68:444–9. [DOI] [PubMed] [Google Scholar]
- 26.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 27.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Hal SJ, Mathur G, Kelly J, Aronis C, Cranney GB, Jones PD. The role of transthoracic echocardiography in excluding left sided infective endocarditis in Staphylococcus aureus bacteraemia. J Infect 2005; 51:218–21. [DOI] [PubMed] [Google Scholar]
- 30.Hill EE, Vanderschueren S, Verhaegen J, et al. Risk factors for infective endocarditis and outcome of patients with Staphylococcus aureus bacteremia. Mayo Clin Proc 2007; 82:1165–9. [DOI] [PubMed] [Google Scholar]
- 31.El-Ahdab F, Benjamin DK, Jr, Wang A, et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med 2005; 118:225–9. [DOI] [PubMed] [Google Scholar]
- 32.Kelesidis T, Dien Bard J, Humphries R, Ward K, Lewinski MA, Uslan DZ. First report of treatment of Anaerobiospirillum succiniciproducens bloodstream infection with levofloxacin. J Clin Microbiol 2010; 48:1970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–75. [DOI] [PubMed] [Google Scholar]
- 34.Fowler VG, Jr, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703. [DOI] [PubMed] [Google Scholar]
- 35.Chang CF, Kuo BI, Chen TL, Yang WC, Lee SD, Lin CC. Infective endocarditis in maintenance hemodialysis patients: fifteen years’ experience in one medical center. J Nephrol 2004; 17:228–35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.