The San Diego Early Test risk score provides an easy-to-use scoring modality that may help to prioritize resources and target interventions such as preexposure prophylaxis to men who have sex with men at greatest risk of acquiring human immunodeficiency virus infection.
Keywords: acute and early HIV, MSM, risk behavior, risk score
Abstract
Background. Although men who have sex with men (MSM) represent a dominant risk group for human immunodeficiency virus (HIV), the risk of HIV infection within this population is not uniform. The objective of this study was to develop and validate a score to estimate incident HIV infection risk.
Methods. Adult MSM who were tested for acute and early HIV (AEH) between 2008 and 2014 were retrospectively randomized 2:1 to a derivation and validation dataset, respectively. Using the derivation dataset, each predictor associated with an AEH outcome in the multivariate prediction model was assigned a point value that corresponded to its odds ratio. The score was validated on the validation dataset using C-statistics.
Results. Data collected at a single HIV testing encounter from 8326 unique MSM were analyzed, including 200 with AEH (2.4%). Four risk behavior variables were significantly associated with an AEH diagnosis (ie, incident infection) in multivariable analysis and were used to derive the San Diego Early Test (SDET) score: condomless receptive anal intercourse (CRAI) with an HIV-positive MSM (3 points), the combination of CRAI plus ≥5 male partners (3 points), ≥10 male partners (2 points), and diagnosis of bacterial sexually transmitted infection (2 points)—all as reported for the prior 12 months. The C-statistic for this risk score was >0.7 in both data sets.
Conclusions. The SDET risk score may help to prioritize resources and target interventions, such as preexposure prophylaxis, to MSM at greatest risk of acquiring HIV infection. The SDET risk score is deployed as a freely available tool at http://sdet.ucsd.edu.
Men who have sex with men (MSM) bear the greatest burden of human immunodeficiency virus (HIV) infection in the United States and many other nations [1, 2]. MSM represent a dominant risk group for HIV; however, the risk of HIV infection within this population is not uniform [3–5]. Characterizing and identifying the MSM at greatest risk for incident HIV infection might permit more focused delivery of both prevention resources and selection of appropriate interventions, such as intensive counseling, regular HIV screening with methods that detect acute infection (ie, nucleic acid amplification test [NAAT]), and antiretroviral preexposure prophylaxis (PrEP) [6].
Although there are a number of symptom-based scores correlated with risk of acute and early HIV infection (AEH), few of these scores actually predict HIV acquisition risk [7, 8]. One of these, the Denver HIV risk score, focuses on the overall population at risk for HIV infection [9, 10]. Demographic characteristics such as male sex, younger age, and being an MSM are the main drivers of this score [9]; therefore, it would be difficult to discern the relative risk of incident HIV infection in populations that share some or all of these characteristics (ie, MSM). To date, 2 scores have been developed based on data from MSM repeat HIV testers: the Menza score [5] and the Smith score [11]. Both of these risk scores focus mainly on risk behavior during the 6 months before HIV diagnosis. Each of these scores has issues, however, that may contribute to suboptimal performance in real-world settings.
First, there are issues with the derivation and validation cohorts used to estimate these scores. The Menza score focused on HIV acquisition in general (ie, acute and chronic infection at the time of diagnosis) in sexually transmitted infection (STI) clinic patients. Because the population used for development of the score sought HIV testing at a median of every 1.6 years (range, 30 days–6.7 years) [5] the behavior reported for the 6 months before diagnosis may not have included the risk behavior at the time of HIV acquisition. The Smith score was derived using a clinical vaccine trial population (enrolled 1998–1999) [11]. Both of the scores were validated using subjects of Project Explore (a HIV prevention trial conducted between 1999 and 2001 [12]). Thus, both scoring methods relied on behavioral risk data collected more than a decade ago in a clinical trial population that may not accurately represent the behavioral risks associated with HIV acquisition risk in a real-world setting today. Finally, the use of methamphetamine or inhaled nitrites in the prior 6 months is weighted in both scores, whereas other drugs are not [5, 11]. The Menza score weighted the use of methamphetamine or inhaled nitrites as the most important variable (11 points), whereas all other risk variables together accounted for a maximum total of 8 points [5]. The fact that behaviors directly associated with HIV acquisition, such as condomless anal intercourse (1 point), have been weighted as significantly less important restricts the use of the score to settings where methamphetamines and inhaled nitrites are the primary drivers of the HIV epidemic (ie, the score may not be applicable to settings where other drugs such as ketamine, γ-hydroxybutyric acid [GHB], cocaine, or ecstasy are significant drivers of HIV risk). An abbreviated version of the Smith score—with similar limitations (ie, data were collected more than a decade ago in a clinical trial population using behavior reported for the 6 months before diagnosis, with inclusion of methamphetamine but exclusion of other drugs)—is currently recommended as a tool to target PrEP among MSM by the US Public Health Service [13].
It may be possible to derive a more robust model that predicts incident HIV acquisition risk by assessing contemporary risk behaviors reported in the period prior to diagnosis with AEH, not chronic infection. Here we aimed (1) to estimate the risk of AEH among MSM, designated the San Diego Early Test (SDET) score, and (2) to validate the SDET score and compare to the 2 previously published risk scores in a real-world population of MSM who underwent HIV testing between 2008 and 2014.
MATERIALS AND METHODS
This study represents an analysis of risk behavior reported for the 12 months prior to HIV screening in individuals who enrolled in the “Early Test” during the 2008–2014 study period. The Early Test is a community-based, confidential AEH screening program in San Diego, California, that provides point-of-care rapid HIV testing followed by reflex HIV NAAT in all antibody (Ab)–negative persons [14, 15]. Eligible participants included MSM diagnosed with AEH (acute: HIV NAAT+/Ab– and early: HIV Ab+/detuned HIV Ab consistent with infection <70 days [16, 17]) and those who were HIV uninfected. In repeat testers, data reported at the most recent Early Test encounter were used. Eligible participants were retrospectively randomized 2:1 to create a derivation and validation dataset, respectively. Risk behavior data reported for the 12 months prior to the Early Test encounter were used to calculate the SDET score.
Risk Score Development
For the risk model, we selected 7 binary variables based on simplicity and published epidemiological data that supported inclusion in the predictive model. The variables selected were (1) ≥10 partners within the last year [5, 18, 19], (2) self-reported bacterial STI during the last 12 months (syphilis, gonorrhea, or chlamydia) [20, 21], (3) the combination of condomless receptive anal intercourse (CRAI) and ≥5 male partners [22–24], (4) CRAI with an HIV-positive male [9, 18, 19], (5) CRAI with a person who injects drugs [9, 25], (6) injection drug use with shared needles [22, 26], and (7) noninjection stimulant drug use (NIDU, defined as use of methamphetamine, ketamine, cocaine, inhaled nitrites, Ecstasy, or GHB) [27, 28].
As recreational drugs used by MSM are somewhat unique by geographic location and time [29], a combined variable for NIDU was chosen. Published data suggest that methamphetamines and inhaled nitrites increase risk of HIV acquisition more than other drugs [19, 30]; thus, we also generated alternative models by substituting our combined variable (any stimulant NIDU) with reported use of either methamphetamines or inhaled nitrites.
Univariate and multivariate binary logistic regression analyses of the derivation dataset were conducted for the 7 risk variables, with AEH diagnosis used as the outcome. Odds ratios (ORs) including 95% confidence intervals (CIs) were calculated. Variables in the final model were selected with a forward stepwise procedure. Model discrimination was assessed by the goodness-of-fit Hosmer–Lemeshow statistic, and its predictive performance was assessed using receiver operating characteristic (ROC) analysis. Each significantly associated predictor in the multivariable model (P < .05, not adjusted for multiple comparisons) was assigned a point value that corresponded to its OR rounded to the nearest whole integer. Integer scores were subsequently summed to give the SDET score for each patient.
Risk Score Validation
To test the validity of this new scoring system, we calculated the predictive potential of our new risk score for AEH in the validation dataset. Score performance was assessed by ROC analysis and area under the curve (AUC) values with 95% CIs. The same validation dataset was then used to compare the performance of the SDET score with scores derived using previously published risk score models [5, 11]. Slight modifications of both scores were necessary to fit our data (variables of all 3 scores as well as modifications are depicted in Table 1). AUCs were compared according to Hanley and McNeil's method [31]. Cutoff values were determined using the Youden index. Different cutoffs were compared using the diagnostic odds ratio (DOR) method.
Table 1.
Comparison of the San Diego Early Test Score With the Smith Score and the Menza Score
| SDET Score |
Smith Score |
Menza Score |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables reported for previous 12 mo (weight) |
|
|
|
||||||||||||
| Cutoffs for AEH | Sens | Spec | PPV | NPV | DOR (95% CI) | Sens | Spec | PPV | NPV | DOR (95% CI) | Sens | Spec | PPV | NPV | DOR (95% CI) |
| Score ≥1 | 81% | 49% | 4% | 99% | 4.06 (2.82–5.85) | 99% | 4% | 2% | 99% | 2.73 (.38–19.65) | 73% | 51% | 3% | 99% | 2.81 (2.00–3.95) |
| Youden index (≥4 for SDET, ≥18 for Smith scored, ≥2 Menza scored) | 58% | 76% | 6% | 99% | 4.56 (3.40–6.11) | 69% | 60% | 4% | 99% | 3.40 (2.45–4.73) | 67% | 54% | 3% | 99% | 2.42 (1.76–3.35) |
| Cutoff for a PPV approximately 10% (≥6 for SDET, ≥36 for Smith score, ≥19 Menza score) | 34% | 92% | 10% | 98% | 6.16 (4.52–8.41) | 7% | 99% | 10% | 98% | 5.39 (2.91–10.00) | 3% | 99% | 9% | 98% | 4.37 (1.72–11.07) |
| Highest cutoff for a sensitivity approximately 20% (≥8 for SDET, ≥30 for Smith score, ≥12 Menza score | 23% | 96% | 12% | 98% | 7.27 (5.09–10.37) | 20% | 94% | 7% | 98% | 4.14 (2.81–6.11) | 22% | 88% | 4% | 98% | 2.10 (1.45–3.05) |
Abbreviations: AEH, acute and early HIV infection; CAI, condomless anal intercourse; CI, confidence interval; CIAI, condomless insertive anal intercourse; CRAI, condomless receptive anal intercourse; DOR, diagnostic odds ratio; HIV, human immunodeficiency virus; NPV, negative predictive value; PPV, positive predictive value; SDET, San Diego Early Test; Sens, sensitivity; Spec, specificity; STI, sexually transmitted infection.
a Time period modified from previous 6 months (original score) to previous 12 months.
b Modification from original score necessary as number of HIV-infected male partners was not available (ie, median; originally 1 HIV-infected male partner was weighted with 4 points and ≥2 male partners with 8 points).
c Modification from original score necessary as number of HIV-infected male partners was not available (ie, median; originally CIAI with ≥5 HIV-infected male partners was weighted with 6 points and CIAI with less than 5 partners gave no score).
In addition, performance of all 3 scores was compared using the whole study population (derivation and validation cohort), and for prediction of transmitted HIV drug resistance (TDR). Blood specimens were collected at the time of AEH diagnosis for drug resistance evaluation. Population sequencing of the partial HIV-1 pol coding region and genotypic analysis were performed, as previously described [15, 32]. Major drug resistance mutations were identified using the Stanford HIV Database Calibrated Population Resistance Tool version 6.0 (available at http://cpr.stanford.edu/cpr/index.html) [33]. The presence of 1 or more major resistance mutations in any drug class was considered to be TDR [34].
Finally, we developed an online assessment tool based on the SDET score categories.
For statistical analysis, SPSS 21 (SPSS Inc, Chicago, Illinois) was used. The University of California, San Diego Human Research Protections Program approved the study protocol, consent, and all study-related procedures. All study participants provided voluntary, written informed consent before any study procedures were undertaken.
RESULTS
A total of 8531 unique MSM underwent HIV screening during the study period. After exclusion of 205 newly diagnosed, chronically infected MSM (duration of infection >70 days), 8326 evaluable MSM were included in the analysis (200 with AEH [2.4%] and 8126 HIV-uninfected [97.6%]). The majority self-identified as white (67%), Asian (8%), and black (6%); 27% reported Hispanic ethnicity. Although there was no significant difference in race and number of previous tests, individuals with AEH were significantly younger (median, 30 [interquartile range {IQR}, 25–40] years vs 33 [IQR, 27–43] years, P = .001) than those who remained HIV uninfected. Data derived from evaluable participants were then randomly split (2:1) into scoring derivation (n = 5568) and validation (n = 2758) datasets.
Derivation Dataset
A total of 137 men with AEH were included in the derivation dataset of 5568 MSM. Each of the 7 selected risk variables (Table 2) were significantly associated with AEH in univariate analysis (Table 2). Results of the multivariable binary logistic regression model are shown in Table 2. The Hosmer–Lemeshow χ2 was 2.176 (P = .703), and the AUC was 0.741 (95% CI, .697–.786) for the model.
Table 2.
Model of Risk Variables Associated With Acute and Early Human Immunodeficiency Virus Infection in Men Who Have Sex With Mena
| Risk Variableb | Univariate Analysis |
Multivariable Binary Logistic Regression Model |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | Coefficient β | Weight for Risk Scorec | |
| ≥10 male partners | 2.615 | 1.857–3.682 | <.001 | 1.568 | 1.058–2.323 | .025 | 0.450 | 2 |
| CRAI and ≥5 male partners | 4.144 | 2.903–5.914 | <.001 | 2.725 | 1.796–4.137 | <.001 | 1.003 | 3 |
| CRAI with HIV-infected partnerd | 4.961 | 3.384–7.272 | <.001 | 3.230 | 2.156–4.841 | <.001 | 1.173 | 3 |
| Bacterial STI | 2.422 | 1.592–3.683 | <.001 | 1.695 | 1.087–2.645 | .020 | 0.528 | 2 |
| CRAI with PWID | 4.810 | 2.580–8.966 | <.001 | .241 | ||||
| NIDUe | 1.826 | 1.291–2.382 | .001 | .804 | … | |||
| IDU with shared needles | 3.358 | 1.194–9.446 | .022 | .444 | … | |||
Abbreviations: CI, confidence interval; CRAI, condomless receptive anal intercourse; GHB, γ-hydroxybutyric acid; HIV, human immunodeficiency virus; IDU, injection drug use; NIDU, noninjection stimulant drug use; OR, odds ratio; PWID, person who injects drugs; STI, sexually transmitted infection.
a Obtained by using data for individuals with no missing covariate values (ie, 98.1% of individuals included in derivation cohort).
b Within the 12 months prior to HIV test encounter.
c Calculated for significant predictors by assigning a point value that corresponded to the OR rounded to the nearest whole integer.
d Defined as either reporting CRAI with an HIV-infected partner or failing to report condom use during receptive anal intercourse with an HIV-infected partner.
e Methamphetamine, cocaine, poppers, GHB, ketamine, Ecstasy.
Four different risk behavior variables (Table 2) were identified as independent predictors of AEH and therefore assigned a point value that corresponded to their OR rounded to the nearest whole integer. The corresponding integer score assignments are displayed in Table 2. The AUC for the score for prediction of AEH was 0.740 (95% CI, .696–.785).
The derived risk score remained unchanged when replacing the combined NIDU variable with methamphetamine use and/or inhaled nitrites alone (data not shown). None of these replacement variables was a significant predictor of AEH in the multivariable analysis. Therefore, the alternative models were discarded.
Validation of SDET Score
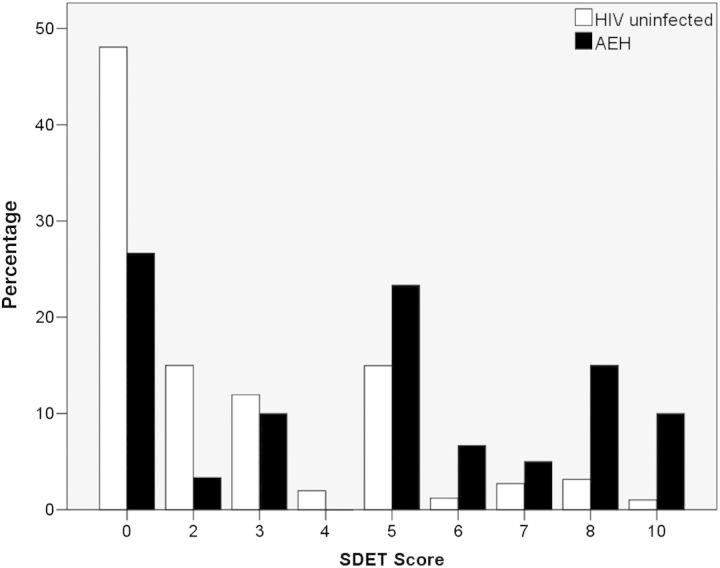
The dataset derived from the remaining 2758 of 8326 (33%) men, including 63 with AEH (31.5% of all AEH), were used for model validation. The median SDET scores for those with AEH were 5 (IQR, 0–8) compared with 2 (IQR, 0–4) for HIV-uninfected persons (P < .001, Mann–Whitney U test). Distribution of SDET scores in those with AEH and those without AEH are depicted in Figure 1. The prevalence of AEH was highly correlated with the SDET score (Table 3). In particular, MSM with a score of ≥5 had a 5 times higher prevalence of AEH (prevalence of 1.2% in those with a score between 0 and 4 vs 5.7% in those with a score of ≥5). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and DOR for 6 SDET score cutoffs are depicted in Table 3. The ROC curve analysis revealed an AUC of 0.703 (95% CI, .625–.781) for AEH prediction in the validation cohort.
Figure 1.
Distribution of San Diego Early Test (SDET) score in the validation cohort in human immunodeficiency virus (HIV)–uninfected individuals (white bars) and those with acute and early HIV (AEH) infection (black bars).
Table 3.
Application of the San Diego Early Test Score to the Validation Cohorta
| SDET Score | Men, No. (%) | Incident HIV Infection, No. (%) | Incident HIV Infection Prevalence | Prevalence of Incident Infection in Derivation Cohort |
|
|---|---|---|---|---|---|
| Total | 2640 | 60 | 2.27% | 2.44% | |
| 0–2 | 1645 (62) | 18 (30) | 1.09% | 1.07% | |
| 3–4 | 365 (14) | 6 (19) | 1.64% | 2.90% | |
| 5 | 400 (15) | 14 (23) | 3.50% | 4.00% | |
| 6–7 | 108 (4) | 7 (12) | 6.48% | 6.70% | |
| 8 | 90 (3) | 9 (15) | 10.00% | 12.57% | |
| 10 | 32 (1) | 6 (10) | 18.75% | 12.24% | |
| Cutoffs for AEH in the Validation Cohort, Score | Sensitivity | Specificity | PPV | NPV | DOR (95% CI) |
| ≥1 | 73% | 48% | 3% | 99% | 2.54 (1.42–4.53) |
| ≥3 | 70% | 63% | 4% | 99% | 3.98 (2.28–6.96) |
| ≥5 | 60% | 77% | 6% | 99% | 5.02 (2.97–8.47) |
| ≥6 | 37% | 92% | 10% | 98% | 6.60 (3.83–11.37) |
| ≥8 | 25% | 96% | 12% | 98% | 7.70 (4.16–14.26) |
| ≥10 | 10% | 99% | 19% | 98% | 10.91 (4.32–27.6) |
Abbreviations: AEH, acute and early HIV infection; CI, confidence interval; DOR, diagnostic odds ratio; HIV, human immunodeficiency virus; NPV, negative predictive value; PPV, positive predictive value; SDET, San Diego Early Test.
a Applied to individuals with no missing variables of the SDET risk score (ie, 97.9% of individuals included in validation cohort).
Comparisons of the SDET Score With Other Scores
The same validation dataset was applied to 2 previously published risk score models, and ROC curve analysis found AUCs of 0.629 (95% CI, .547–.710) for the Menza score [5], and 0.731 (95% CI, .662–.801) for the Smith score [11]. The difference between the AUC of the SDET score and the 2 other scores was not significant in the validation cohort.
When comparing performance of scores in the whole study population (ie, derivation and validation cohort), the SDET score (AUC, 0.728 [95% CI, .689–.767]; P < .001) and the score by Smith (AUC, 0.703 [95% CI, .665–.741]; P = .008) were both significantly more discriminative than the Menza score (AUC, 0.634 [95% CI, .593–.676]). Sensitivities, specificities, PPVs, NPVs, and DORs of the 3 scores are depicted in Table 1.
TDR was detected in 15 of 131 (11.5%) AEH cases. SDET scores were higher for those with TDR than for those without TDR (median, 7 [IQR, 5–8] vs 5 [IQR, 2–7]; P = .006). There was no significant difference found for the other 2 scores. Sensitivity, specificity, PPV, NPV, and DOR for an SDET score of ≥6 (determined by Youden index) for prediction of TDR were 67%, 70%, 23%, 94%, and 4.72 (95% CI, 1.5–14.9), respectively. ROC curve analysis revealed an AUC of 0.717 (95% CI, .586–.848) for prediction of TDR in AEH cases.
Figure 2 shows our online tool, which is freely available at http://sdet.ucsd.edu and can be used by providers and MSM to assess HIV risk.
Figure 2.
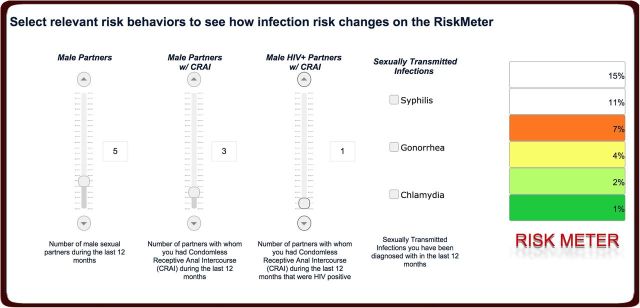
The San Diego Early Test (SDET) score online tool with intuitive sliders (track bars) that allow users to easily select relevant risk behaviors across the 4 dimensions central to the SDET score. After answering some basic demographic questions (used to anonymously map reported risk behavior to population-level data), users will select their risk behaviors. Once this is set, they will receive immediate feedback in terms of their current human immunodeficiency virus (HIV) risk, as indicated by the colored bars on the “risk meter.” After users receive their risk score, the online SDET tool invites them to explore how their risk changes when they change behavior. By moving the scroll bars, users see the direct impact of behavior change on their HIV risk score. If users agree, the tool automatically sends each user a reminder to participate again 12 months later, which will allow exploration of linkage between increased risk awareness and effective behavior change. Available at http://sdet.ucsd.edu.
DISCUSSION
We used clinical and behavioral data collected with an AEH screening program during a 6-year period to construct and validate a simple multivariable risk behavior score predictive of AEH among MSM. The SDET score excludes demographics and focuses instead on relevant current risk variables directly associated with HIV acquisition among MSM: CRAI, number of male partners within the previous 12 months, and bacterial STIs (Table 2). In contrast to previously published scores [5, 11, 13], NIDU, methamphetamine, and inhaled nitrite use were nonsignificant predictors of AEH in multivariate analysis. Although it has been demonstrated that recreational drugs such as methamphetamine or nitrites may increase sexual risk behavior such as CRAI [30, 35], which is captured in the SDET score, their usage rates change depending on geographic location [29, 36, 37]. In addition, methamphetamine use has recently been decreasing in many settings whereas sexual risk behaviors are steadily increasing [37, 38]. The fact that the SDET score focuses on sexual risk behavior instead of substance use therefore may be considered a strength, as the score is independent of regional drug use behavior and thus may be more broadly applicable to different MSM populations (as changes in sexual risk behavior associated with NIDU will still be captured). In addition, the predictors of AEH that we identified are consistent with the biology and transmission of HIV infection. The associations of AEH with multiple sexual partners, CRAI with and without high-risk partners, and concurrent bacterial STI have all previously been shown to correspond to an increased likelihood of recent HIV exposure [5, 18, 21, 24]. We therefore propose the SDET score as a straightforward and easy-to-use scoring modality that might not only help to better focus and prioritize prevention resources (such as NAAT screening or PrEP; an SDET score of ≥5 was associated with an AEH prevalence of 5.7% and a sensitivity of 60% for AEH) in similar metropolitan populations of MSM around the world, but may also help researchers identify MSM at high risk of HIV infection for prevention trials with HIV acquisition as the primary outcome or SDET score as an outcome of harm reduction interventions in HIV-uninfected populations.
Overall, the SDET score showed a fair potential not only for predicting AEH but also for predicting TDR among those with AEH. At a cutoff of 6, the score exhibited an NPV of 94% for TDR among AEH cases. The association of TDR in persons with higher AEH risk may reflect exposure to sexual partners more likely to be HIV infected and failure of (or previous) antiretroviral therapy (ART). The SDET score may therefore help guiding immediate ART (ie, before baseline resistance testing results are available) in those with AEH, which is important in terms of preserving immune function, minimizing latent HIV reservoir, and preventing further transmissions [15, 39].
We also validated 2 previously published risk scores (validated in clinical trial cohorts from the 1990s) for the first time in a real-world population of MSM self-selecting to receive HIV screening. The SDET algorithm performed better than the Menza score for prediction of AEH [5], and was comparable to the Smith score [11]. We confirmed that a slightly modified version of the score by Smith was still useful more than a decade later (the AUC for HIV acquisition was 0.703 for our cohort vs 0.721 for the original clinical trial validation cohort). However, in contrast to the more complex Smith score, which contains 11 score categories, the SDET score might be considered simpler (only 4 score categories), suggesting that the SDET score has identified the most critical drivers that predict AEH. The SDET score may thus be more broadly applicable (independent of regional drug use behavior, no demographic variables) to MSM populations in urban settings. There were also important differences between the 2 previously published scores: In the Menza score, methamphetamine and inhaled nitrites were weighted as the most important variables, whereas in the score by Smith, their weight was significantly reduced. This may have contributed to the better performance of the Smith score compared with the Menza score for AEH. In contrast to the SDET score, neither one of the previously published scores was able to predict TDR.
Our study is subject to important limitations including its single-center and retrospective design, and the relatively small number of AEH cases. Optimally, the SDET score should undergo additional validation in other US and international populations to confirm its accuracy. Also, our development and validation samples were composed mostly of white and Hispanic MSM; thus, the results may be less generalizable to populations consisting of persons with different racial and ethnic demographics. Finally, some modifications of the 2 previous risk scores were necessary to fit our data and our analyses, potentially influencing performance of these scoring models.
In conclusion, our SDET score provides an easy-to-use scoring modality predictive of AEH and TDR in MSM populations, which may be less subject to regional patterns of illicit drug use than previously published scoring estimates. This scoring algorithm and its availability as an online scoring tool may be useful to clinicians and others in counseling MSM about their risk of HIV infection. By using a cutoff of 5, the score may help to identify persons who require more intensive prevention interventions such as PrEP or more frequent NAAT testing, while a cutoff of 6 may be helpful in guiding immediate ART in those with AEH.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by funds from the Max Kade Foundation, New York (Max Kade Postdoctoral Research grant); the Department of Veterans Affairs; the National Institutes of Health (grant numbers AI093163, AI100665, DA034978, AI036214, AI106039); Centers for AIDS Research program; and the James B. Pendleton Charitable Trust.
Potential conflicts of interest. M. H. has served on the speakers' bureau of Merck. D. M. S. has received grant funding from ViiV Healthcare and has served as a consultant for Genprobe and Testing Talent Services. S. J. L. reported grant funding from Gilead Sciences, Inc. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fisher HH, Habarta N, Hardnett F, et al. Characteristics of first-time and repeat HIV tests among men who have sex with men who test at CDC-supported sites, 2007. AIDS Educ Prev 2011; 23(3 suppl):17–29. [DOI] [PubMed] [Google Scholar]
- 2.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med 2007; 4:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS 2006; 20:731–9. [DOI] [PubMed] [Google Scholar]
- 4.MacKellar DA, Valleroy LA, Secura GM, et al. Repeat HIV testing, risk behaviors, and HIV seroconversion among young men who have sex with men: a call to monitor and improve the practice of prevention. J Acquir Immune Defic Syndr 2002; 29:76–85. [DOI] [PubMed] [Google Scholar]
- 5.Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis 2009; 36:547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrazzo JM, del Rio C, Holtgrave DR, et al. HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society-USA panel. JAMA 2014; 312:390–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahome E, Fegan G, Okuku HS, et al. Evaluation of an empiric risk screening score to identify acute and early HIV-1 infection among MSM in coastal Kenya. AIDS 2013; 27:2163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS 2007; 21:2237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haukoos JS, Lyons MS, Lindsell CJ, et al. Derivation and validation of the Denver human immunodeficiency virus (HIV) risk score for targeted HIV screening. Am J Epidemiol 2012; 175:838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh YH, Haukoos JS, Rothman RE. Validation of an abbreviated version of the Denver HIV risk score for prediction of HIV infection in an urban ED. Am J Emerg Med 2014; 32:775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DK, Pals SL, Herbst JH, Shinde S, Carey JW. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2012; 60:421–7. [DOI] [PubMed] [Google Scholar]
- 12.Koblin B, Chesney M, Coates T; EXPLORE Study Team. Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet 2004; 364:41–50. [DOI] [PubMed] [Google Scholar]
- 13.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014. Clinical providers’ supplement, 2014. Available at: http://www.cdc.gov/hiv/pdf/guidelines/PrEPProviderSupplement2014.pdf Accessed 8 April 2015.
- 14.Morris SR, Little SJ, Cunningham T, Garfein RS, Richman DD, Smith DM. Evaluation of an HIV nucleic acid testing program with automated internet and voicemail systems to deliver results. Ann Intern Med 2010; 152:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare CB, Pappalardo BL, Busch MP, et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis 2006; 42:700–8. [DOI] [PubMed] [Google Scholar]
- 17.Hurt CB, McCoy SI, Kuruc J, et al. Transmitted antiretroviral drug resistance among acute and recent HIV infections in North Carolina from 1998 to 2007. Antivir Ther 2009; 14:673–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Drabkin AS, Sikkema KJ, Wilson PA, et al. Risk patterns preceding diagnosis among newly HIV-diagnosed men who have sex with men in New York City. AIDS Patient Care STDS 2013; 27:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchbinder SP, Vittinghoff E, Heagerty PJ, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. J Acquir Immune Defic Syndr 2005; 39:82–9. [DOI] [PubMed] [Google Scholar]
- 20.Zetola NM, Bernstein KT, Wong E, Louie B, Klausner JD. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. J Acquir Immune Defic Syndr 2009; 50:546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrer L, Furegato M, Foschia JP, et al. Undiagnosed HIV infection in a population of MSM from six European cities: results from the Sialon Project. Eur J Public Health 2014; doi:10.1093/eurpub/cku139. [DOI] [PubMed] [Google Scholar]
- 22.Ostrow DG, Plankey MW, Cox C, et al. Specific sex drug combinations contribute to the majority of recent HIV seroconversions among MSM in the MACS. J Acquir Immune Defic Syndr 2009; 51:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarcz S, Scheer S, McFarland W, et al. Prevalence of HIV infection and predictors of high-transmission sexual risk behaviors among men who have sex with men. Am J Public Health 2007; 97:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 2007; 45:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YH, McFarland W, Raymond HF. Risk behaviors for HIV in sexual partnerships of San Francisco injection drug users. AIDS Care 2014; 26:554–8. [DOI] [PubMed] [Google Scholar]
- 26.Salzer HJ, Hoenigl M, Kessler HH, et al. Lack of risk-awareness and reporting behavior towards HIV infection through needlestick injury among European medical students. Int J Hyg Environ Health 2011; 214:407–10. [DOI] [PubMed] [Google Scholar]
- 27.Thiede H, Jenkins RA, Carey JW, et al. Determinants of recent HIV infection among Seattle-area men who have sex with men. Am J Public Health 2009; 99(suppl 1):S157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drumright LN, Gorbach PM, Little SJ, Strathdee SA. Associations between substance use, erectile dysfunction medication and recent HIV infection among men who have sex with men. AIDS Behav 2009; 13:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on drug use: 2012 overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan, 2013. [Google Scholar]
- 30.Freeman P, Walker BC, Harris DR, et al. Methamphetamine use and risk for HIV among young men who have sex with men in 8 US cities. Arch Pediatr Adolesc Med 2011; 165:736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148:839–43. [DOI] [PubMed] [Google Scholar]
- 32.Little SJ, Kosakovsky Pond SL, Anderson CM, et al. Using HIV networks to inform real time prevention interventions. PLoS One 2014; 9:e98443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gifford RJ, Liu TF, Rhee SY, et al. The calibrated population resistance tool: standardized genotypic estimation of transmitted HIV-1 drug resistance. Bioinformatics 2009; 25:1197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DE, Camacho RJ, Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009; 4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoptaw S, Reback CJ. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. J Urban Health 2006; 83:1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsolek MR, White NC, Litovitz TL. Inhalant abuse: Monitoring trends by using poison control data, 1993–2008. Pediatrics 2010; 125:906–13. [DOI] [PubMed] [Google Scholar]
- 37.Raymond HF, Chen YH, Ick T, et al. A new trend in the HIV epidemic among men who have sex with men, San Francisco, 2004–2011. J Acquir Immune Defic Syndr 2013; 62:584–9. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC). HIV testing and risk behaviors among gay, bisexual, and other men who have sex with men—United States. MMWR Morb Mortal Wkly Rep 2013; 62:958–62. [PMC free article] [PubMed] [Google Scholar]
- 39.Touloumi G, Pantazis N, Pillay D, et al. Impact of HIV-1 subtype on CD4 count at HIV seroconversion, rate of decline, and viral load set point in European seroconverter cohorts. Clin Infect Dis 2013; 56:888–97. [DOI] [PubMed] [Google Scholar]