Abstract
Chyluria is the passage of chyle into urine, and develops as a result of communication between the lymphatic system and the urinary system. It is an unusual manifestation of lymphatic filariasis reported mainly from South Asian countries. We report the case of a 38-year-old man from an endemic area who presented with passage of milky urine. Physical examination did not reveal any lymphadenopathy or lymph oedema. Urine tests revealed nephrotic range proteinuria. A 99m technetium sulphur colloid lymphoscintigraphy confirmed connection between lymphatic vessels and the urinary tract. Predominant chyluria with no overt lymphatic filariasis remains an enigma.
Background
In India, lymphatic filariasis is an endemic disease. Lymphatic filariasis has a spectrum of manifestations, which still perplexes the epidemiologist. Tropical pulmonary eosinophilia and chyluria are unusual manifestations of lymphatic filariasis.1 Chyluria is usually associated with abnormal retrograde or collateral flow of lymph from intestinal lymphatics into lymphatics of the kidney ureter bladder (KUB) system.2 In this report, we describe a patient who presented with chyluria, nephrotic range proteinuria and hypoalbuminaemia. Chyluria as a presenting symptom with massive proteinuria and no obvious features of lymphatic filariasis is exceedingly rare. We also discuss the approach to a common tropical problem of ‘white urine’ along with a review of relevant literature.
Case presentation
A 38-year-old man, resident of Bihar, presented with a history of passage of white urine for the past 6 months. This was accompanied by occasional passage of fleshy particles. He also complained of significant weight loss. There was no dysuria, graveluria or fever. He did not have any history of lymphangitis, elephantiasis or passage of milky urine in the past. There was no history of diabetes mellitus, hypertension, renal stones or gross haematuria. He was on no medication and denied the use of alcohol, illicit drugs and tobacco. Physical examination revealed a thin and malnourished man weighing 52 kg. The lymph nodes were not enlarged. There was mild bipedal pitting oedema. His heart and lung examinations were normal. The abdominal examination did not reveal any organomegaly.
Investigations
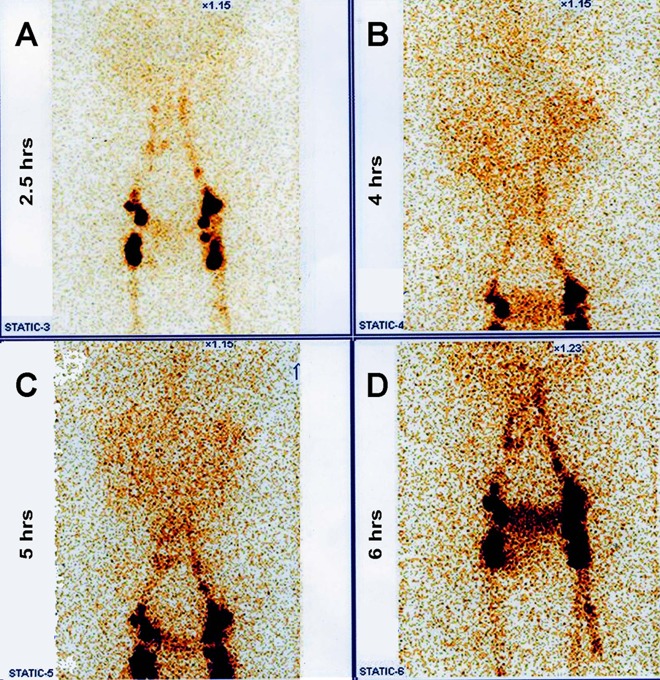
Laboratory investigations showed a normal peripheral leucocytic and eosinophil count. Biochemical profile revealed a serum albumin of 2.2 mg/dl. Urine examination showed uniformly milky urine (figure 1) with acidic reaction and urinary triglycerides of 167 mg/dl as against serum triglycerides of 84 mg/dl. A 24 h urine collection yielded 6.5 g of protein. Urine cleared significantly on addition of ether and no crystals were visible on microscopy. The bacterial culture was sterile. Urinary staining for acid-fast bacilli was negative and BACTEC culture for TB showed no growth. The filarial antigen detection test was negative. Significantly, the urine sample showed microfilaria of Wuchereria bancrofti (figure 2). Ultrasound examination showed a normal urinary tract and kidneys. Cystoscopy was planned to look for the origin of chyluria and milky white urine was seen effluxing from the right ureteral orifice (figure 3). A 99m technetium sulphur colloid lymphoscintigraphy was performed and there was evidence of communication of the lymphatic system with the upper ureter at the level of the lower abdomen near the bifurcation of the abdominal aorta. Delayed radiotracer activity was seen in the urinary bladder. There was no definitive scan evidence of lymphatic dysfunction of the lower limbs (figure 4). Venereal Disease Research Laboratory and serology tests for HIV, hepatitis B surface antigen and anti-hepatitis C virus (HCV) were negative. Kidney biopsy was reported as largely normal by light microscopy.
Figure 1.

Urine sample showing milkiness.
Figure 2.
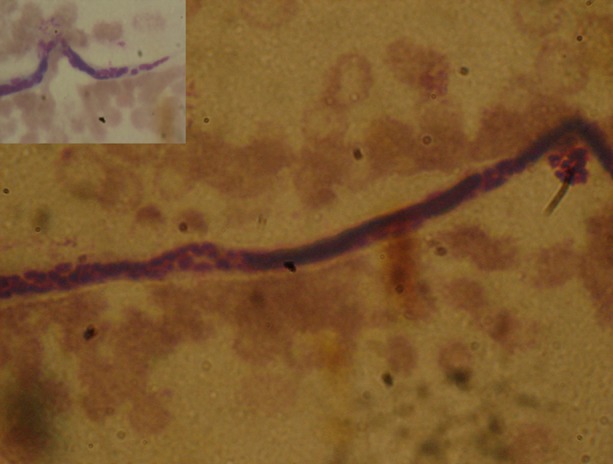
Bancroftian microfilaria in urine on a background of degenerated urothelial cells and lymphocytes (H and E, ×1000). Inset: tail tip free of nuclei with the pointed terminal end.
Figure 3.
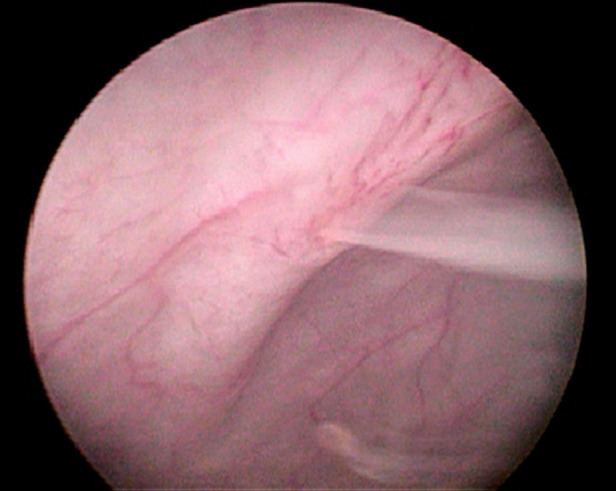
Cystoscopy showing milky white urine effluxing from the right ureteral orifice.
Figure 4.
(A) 99mTc sulphur colloid lymphoscintigraphy showed the symmetrical appearance of radioactivity in lymph vessels and nodes; radioactivity at (B) 4 h and (C) 5 h suggests a lymphouretic fistula on the right side; (D) delayed radiotracer activity (6 h) seen in the urinary bladder.
Differential diagnosis
Filarial chyluria
Cavitating renal tuberculosis
Nephrotic syndrome
Malignancies of the retroperitoneum.
Treatment
The patient was given diethyl carbamazine (DEC) for 14 days that failed to resolve the symptoms. He was on a high-protein diet exclusive of all fats except for medium-chain triglycerides (coconut oil). In view of persistent chyluria and malnutrition from excessive urinary losses of lipids and protein, a decision was made to treat the chyluria.Sclerosing therapy was performed using cystoscopy and a bladder wash. A ureteric catheter was placed in the right ureter, and several instillations of 0.5% povidone-iodine solution were carried out. The remaining povidone-iodine was left in the bladder for 4 h before the patient voided again.
Outcome and follow-up
Two days later the milky urine disappeared. Resolution of peripheral oedema with improvement in serum albumin occurred following disappearance of chyluria within a period of 7 days. The patient has been followed up for 6 months and is symptom free. Chyluria with nephrotic range proteinuria without any evidence of lymphatic dysfunction of the lower limbs in our patient is quite unusual.
Discussion
Our patient presented with a prominent history of passing white urine. Differential diagnosis of white urine includes chyluria, phosphaturia, hyperuricosuria, hyperoxaluria, proteinuria, pyuria, lipiduria secondary to fat embolism and caseous material from cavitating renal tuberculosis. Most significant of all chyluria can be differentiated by its disappearance or decrease on addition of ether. The microscopy shows small fat globules. More than 90% of fat is triglyceride and the milky appearance of urine is lost if the patient is given a fat-free diet. In filarial diseases, microfilaria may be recovered from blood and urine and filarial complement fixation and skin sensitivity may be positive. We discuss the aetiopathogenesis, diagnosis and management of chyluria in the context of our patient.
Filariasis is endemic in certain areas of Asia, particularly India, Sri Lanka, Bangladesh, Burma, Thailand, Malaysia, Indonesia, China, Philippines and Papua.1 The transmission of human filariae is confined to warm climates. Lymphatic filariasis can present with protean manifestations comprising asymptomatic amicrofilaraemia, asymptomatic microfilaraemia or overt clinical manifestations such as adenolymphangitis, hydrocele, lymphoedema, elephantiasis, chyluria and tropical eosinophilia. Chyluria is an uncommon manifestation of chronic lymphatic filariasis and occurs in 2% of filarial-affected patients in the filarial belt.1 In contrast to Bancroftian filariasis, hydrocele and other genital lesions are rare in areas endemic for Brugia malayi. Chyluria is also unusual with B malayi infection.3
Chyluria is defined as the passage of chyle into the urine comprising large quantities of dietary lipids, proteins and fat-soluble vitamins. Chyluria indicates the presence of an abnormal communication between intestinal lymphatics and the urinary tract. Renal lymphatics follow the renal vein, and through lateral aortic glands end up in the lumbar trunks. These drain into the cisterna chyli along with the intestinal trunks. Parasitic infestation leads to obliterative lymphangitis and impairment of the valvular mechanism. Chyle from either cisterna chyli or the intestinal trunk regurgitates into the lumbar trunk. The resultant variceal dilatation of distal lymphatics and eventual rupture of lymphatic vessels into the urinary tract create a lymphaticourinary fistula.2–4 The location of the lymphaticourinary fistula is most commonly at the calyceal fornix in the renal pelvis, but can also occur at the level of the ureter or urinary bladder as in our case.2 3
The aetiology of chyluria can be classified as either parasitic or non-parasitic (table 1).5 The primary cause of the parasitic form is filariasis and, unless proven otherwise, chyluria should be considered as filarial, especially in the filarial belt. Chyluria may be asymptomatic or may be associated with dysuria, haematuria, anaemia, loss of protein and fat causing hypoproteinaemia, weight loss, malnutrition and cachexia. Chylous proteinuria is episodic and increases with a fatty meal. Unlike patients with nephrotic syndrome, urinary sediment does not contain lipid-laden oval fat bodies or fatty casts. Instead, one may see large numbers of lymphocytes in the urine sediment, consistent with the leakage of lymphatic fluid into the urine.2 6 Prolonged massive chyluria may result in increasing IgG and IgA deficiency leading to various immunological deficits. Chyluric hypolipidaemia is an established state due to loss of lipids such as TG, phospholipids and cholesterol.7 8 Deficiency of fat-soluble vitamins may occur in patients with chyluria.9
Table 1.
Causes of chyluria
| Parasitic causes | |
| Filarial | Bancroftian and non-Brancroftian filariasis |
| Non-filarial | Ascariasis, malaria, Echinococcus, Cysticercus cellulose, Cercorrenas hominis, Tinea vera |
| Non-parasitic causes | |
| Congenital | Primary intestinal lymphangiectasia, congenital retroperitoneal lymphangiectasis, congenital lymphatic fistulas |
| Iatrogenic | Postnephrectomy, post-PCNL, post-traumatic, postcardiac catheterisation |
| Infections | Tuberculosis |
| Medical | Nephrotic syndrome, hypertriglyceridaemia |
| Neoplastic | Malignancies of the retroperitoneum, lymphangiomyomatosis, lymphangioleiomyomatosis, renal angiomyolipoma |
PCNL, percutaneous nephrolithotomy.
The important investigations for the work-up of chyluria are intravenous pyelography, cystoscopy, retrograde pyelography and lymphangiography. Retrograde pyelography is required to demonstrate the fistulous connection and dilated lymphatics. The most remarkable lymphographic feature of filarial chyluria is lymphopelvis fistulisation.2 In some patients, diagnostic contrast lymphangiography may prove to be therapeutic by causing closure of the lymphaticourinary fistula owing to its sclerosing effect on the lymphatic vessels.10 The procedure of lymphangiography is technically challenging, requiring a skilled operator, and is not without complications. Lymphoscintigraphy is a non-invasive and equally accurate procedure and has been increasingly utilised for the evaluation of chyluria. It allows the clear and precise analysis of the lymphatic system function in patients with filarial infection.4
The natural history of chyluria is still unclear. Because spontaneous remission of chyluria may occur in 50% of patients,11 patients may not require treatment if the chyluria enters a long interval of remission without nutritional complications. However, in patients with persistent chyluria, malnutrition occurs from excessive urinary losses of lipids and protein. A specifically designed low-fat, high-protein diet supplemented with medium-chain triglycerides has been demonstrated to decrease the proteinuria, lipiduria and haematuria.12 The underlying cause should be treated for non-filarial chyluria. Filaricidal drugs are usually not helpful as the infection is burnt out by the time chyluria develops. For patients with recurrent or persistent chyluria unresponsive to medical management, instillation of sclerosing solutions into the renal pelvis has an 80% success rate in achieving closure of the lymphaticopelvic communication.13 Silver nitrate injection is a successful modality executed by urologists in India for recurrent and refractory chyluria.14 One study found that the 3-day 8 h instillation was a more economical and less cumbersome regimen as compared with weekly instillation for 6weeks.14 Povidone-iodine 0.2% is also considered to be as effective as 1% silver nitrate therapy. The cumulative success rate after two courses of therapy was 82% (silver nitrate) and 83% (povidone).15 Surgical or retroperitoneoscopic pyelolymphatic disconnection is the treatment of choice for those who fail the renal pelvic instillation sclerotherapy.
We treated our patient with DEC, and endoscopic sclerotherapy with 0.5% povidone-iodine was performed. In our patient white urine cleared gradually and the 24 h urinary protein also decreased significantly. The patient's peripheral oedema and hypoalbuminaemia were resolved in due course. Until now there has been no evidence of fresh chyluria or proteinuria on follow-up. Surprisingly, our patient presented with chyluria without any other stigma of a chronic filarial infection and presence of microfilaria in the urine sample is a very rare finding. Moreover, the lymphaticourinary communication was on the right side and at the level of the ureter, which are found in a minority of patients.
Learning points.
The patient may present with chyluria without any other stigma of chronic filarial infection.
Nephrotic range proteinuria can happen along with chyluria in the absence of a significant kidney lesion.
A urine examination for microfilaria may be rewarding when filarial serology is negative.
Lymphoscintigraphy can be used as an alternative to lymphangiography for the evaluation of chyluria.
Antihelminthic treatment should be considered besides providing definitive treatment for chyluria when microfilaria is found in urine samples.
Acknowledgments
The authors are thankful to Dr. Saikat Choudhury (Nuclear medicine physician, Gamma SPECT- Imaging and Diagnostic Centre Pvt. Ltd.) for his technical support and performing the lymphoscintigraphy.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Simonsen PE. Filariases. In: Cook GC, Zumla AI. eds. Manson's tropical diseases. 21st edn. Philadelphia: W. B. Saunders, 2003:1488–94. [Google Scholar]
- 2.Akisada M, Tani S. Filarial chyluria in Japan. Lymphography, etiology and treatment in 30 cases. Radiology 1968;90:311–17. [Google Scholar]
- 3.Diamond E, Schapira HE. Chyluria—a review of the literature. Urology 1985;26:427–31. [DOI] [PubMed] [Google Scholar]
- 4.Lymphatic filariasis: the disease and its control. Fifth report of the WHO expert committee on Filariasis. World Health Organ Tech Rep Ser 1992;821:1–71. [PubMed] [Google Scholar]
- 5.Koga S, Arakaki Y, Matsuoka M, et al. Remission of filarial chyluria after treatment of coexistent conditions. Lymphology 1990;23:164–6. [PubMed] [Google Scholar]
- 6.Koo CG, Van Langenberg A. Chyluria: a clinical study. J R Coll Surg Edinb 1969;14:31–41. [PubMed] [Google Scholar]
- 7.Tripathy VNP. Chyluria: a chronic filarial legacy. Indian J Prev Soc Med 1975;6:169–70. [Google Scholar]
- 8.Cahill KM. Filarial Chyluria: a biochemical and radiological study of five patients. J Trop Med Hyg 1965;68:27–31. [PubMed] [Google Scholar]
- 9.Sridhar M, Pal DK, Saxena ID, et al. Fat soluble vitamin profile in filarial chyluria—a preliminary study. Indian J Urol 1999;16:18–20. [Google Scholar]
- 10.Nunez Mora C, Carcamo Valore P, deCabo Ripoll M, et al. [Recurrent nonparasitic chyluria]. Arch Esp Urol 1998;51:932–4. [PubMed] [Google Scholar]
- 11.Ohyama C, Saita H, Miyasato N. Spontaneous remission of chyluria. J Urol 1979;121:316–17. [DOI] [PubMed] [Google Scholar]
- 12.Hashim S, Roholt HB, Babyyan VK, et al. Treatment of chyluria and chylothorax with medium-chain triglyceride. N Engl J Med 1964;270:756–61. [DOI] [PubMed] [Google Scholar]
- 13.Dalela D, Rastogi M, Goel A, et al. Silver nitrate sclerotherapy for ‘clinically significant’ chyluria: a prospective evaluation of duration of therapy. Urol Int 2004;72:335–40. [DOI] [PubMed] [Google Scholar]
- 14.Suri A, Kumar A. Chyluria—SGPGI experience. Indian J Urol 2005;21:59–62. [Google Scholar]
- 15.Goel S, Mandhani A, Srivastava A, et al. Is povidone iodine an alternative to silver nitrate for renal pelvic instillation sclerotherapy in chyluria? BJU Int 2004;94:1082–5. [DOI] [PubMed] [Google Scholar]